Anti-Virulence Strategy against the Honey Bee Pathogenic Bacterium Paenibacillus larvae via Small Molecule Inhibitors of the Bacterial Toxin Plx2A
Abstract
:1. Introduction
2. Results
2.1. Inhibition of Plx2A GH Activity by Synthetic and Plant-Derived Small Molecules
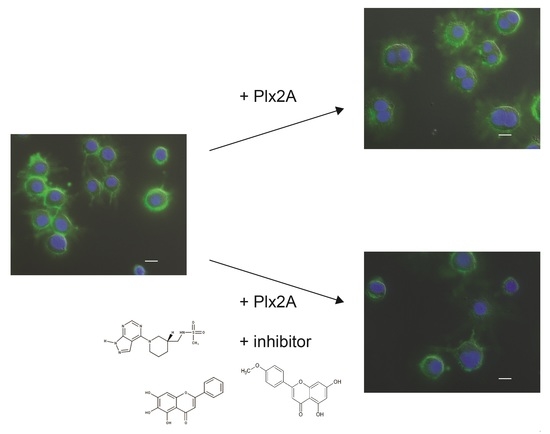
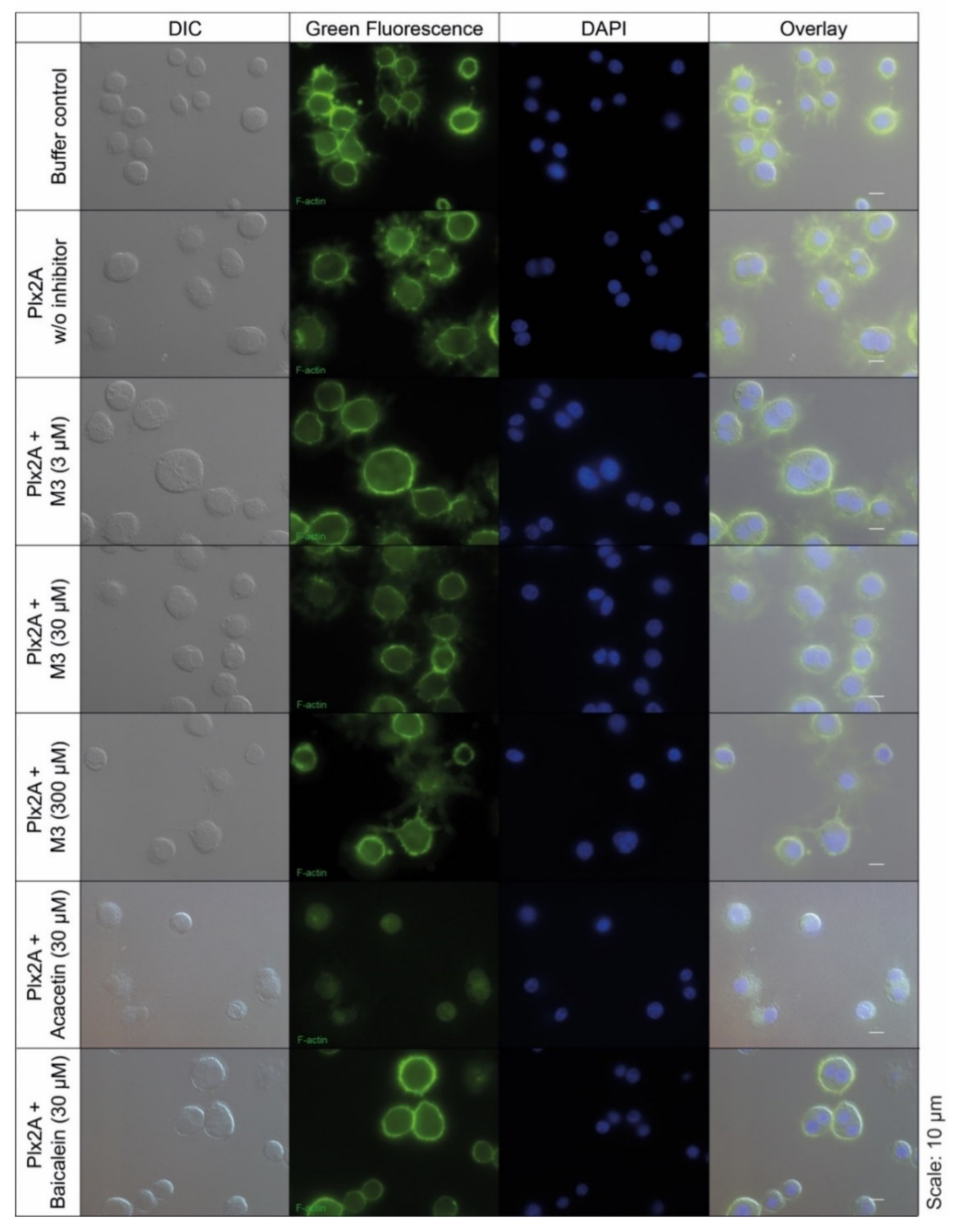
2.2. Inhibition of Plx2A Effect in an Insect Cell Culture Model
2.3. Testing the Synthetic Small Molecule Inhibitor M3 and the Plant-Derived Small Molecules Acacetin and Baicalein in Honey Bee Larvae Experimentally Infected with P. larvae
3. Discussion
4. Materials and Methods
4.1. Recombinant Plx2A and Small Molecule Inhibitors
4.2. Glycohydrolase (GH) Activity, Inhibitor Testing and Generation of IC50 Data
4.3. Insect Cell Culture and Actin and DAPI Staining
4.4. Bacterial Strain and Culture Conditions
4.5. Exposure Bioassays with Inhibitor Feeding
4.6. DMSO Effect on Vegetative P. larvae and P. larvae Spores
4.7. DMSO Effect on Honey Bee Larvae
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.; Garibaldi, L.; Cunningham, S.; Klein, A. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Curr. Biol. 2008, 18, 1572–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherubin, P.; Garcia, M.C.; Curtis, D.; Britt, C.B.T.; Craft, J.W., Jr.; Burress, H.; Berndt, C.; Reddy, S.; Guyette, J.; Zheng, T.; et al. Inhibition of cholera toxin and other ab toxins by polyphenolic compounds. PLoS ONE 2016, 11, e0166477. [Google Scholar] [CrossRef] [Green Version]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.-M.; Boreux, V.; Fornoff, F.; Mupepele, A.-C.; Pufal, G. Relevance of wild and managed bees for human well-being. Curr. Opin. Insect Sci. 2018, 26, 82–88. [Google Scholar] [CrossRef]
- Irving, G.W.; Fontaine, T.D.; Doolittle, S.P. Partial antibiotic spectrum of tomatin, an antibiotic agent from the tomato plant. J. Bacteriol. 1946, 52, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Genersch, E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Genersch, E.; Aubert, M. Emerging and re-emerging viruses of the honey bee (Apis mellifera). Vet. Res. 2010, 41, 54. [Google Scholar] [CrossRef] [Green Version]
- Slater, L.H.; Hett, E.C.; Mark, K.; Chumbler, N.M.; Patel, D.; Lacy, D.B.; Collier, R.J.; Hung, D.T. Identification of novel host-targeted compounds that protect from anthrax lethal toxin-induced cell death. ACS Chem. Biol. 2013, 8, 812–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, I. Nosema apis—A parasite in the honey bee colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Zander, E. Tierische Parasiten als Krankheitserreger bei der Biene. Münchener Bienenzeitung 1909, 31, 196–204. [Google Scholar]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n sp (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Fries, I.; Martin, R.; Meana, A.; Garcia-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apicult. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the Western honey bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Forsgren, E. European foulbrood in honey bees. J. Invertebr. Pathol. 2010, 103, S5–S9. [Google Scholar] [CrossRef]
- Bailey, L. Melissococcus pluton, the cause of European foulbrood of honeybees (Apis ssp.). J. Appl. Bacteriol. 1983, 55, 65–69. [Google Scholar] [CrossRef]
- Bailey, L. Aetiology of European foulbrood—Disease of the larval honeybee. Nature 1956, 178, 1130. [Google Scholar] [CrossRef]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsppulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, G.F. The Bacteria of the Apiary with Special Reference to Bee Disease; Technical Series; USDA, Bureau of Entomology: Wahington, DC, USA, 1906; Volume 14, pp. 1–50. [Google Scholar]
- Ebeling, J.; Knispel, H.; Hertlein, G.; Fünfhaus, A.; Genersch, E. Biology of Paenibacillus larvae, a deadly pathogen of honey bee larvae. Appl. Microbiol. Biotechnol. 2016, 100, 7387–7395. [Google Scholar] [CrossRef]
- Fünfhaus, A.; Ebeling, J.; Genersch, E. Bacterial pathogens of bees. Curr. Opin. Insect Sci. 2018, 26, 89–96. [Google Scholar] [CrossRef]
- Genersch, E. American foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 2010, 103, S10–S19. [Google Scholar] [CrossRef]
- Tarr, H.L.A. Studies on American foulbrood of bees. I. The relative pathogenicity of vegetative cells and endospores of Bacillus larvae for the brood of the bee. Ann. Appl. Biol. 1937, 24, 377–384. [Google Scholar] [CrossRef]
- Hasemann, L. How long can spores of American foulbrood live? Am. Bee J. 1961, 101, 298–299. [Google Scholar]
- Hornitzky, M. The pathogenicity of Paenibacillus larvae subsp. larvae spores and vegetative cells to honey bee (Apis mellifera) colonies and their susceptibility to royal jelly. J. Apicult. Res. 1998, 37, 267–271. [Google Scholar]
- Dobbelaere, W.; de Graaf, D.C.; Reybroeck, W.; Desmedt, E.; Peeters, J.E.; Jacobs, F.J. Disinfection of wooden structures contaminated with Paenibacillus larvae subsp. larvae spores. J. Appl. Microbiol. 2001, 91, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Woodrow, A.W. Susceptibility of honeybee larvae to individual inoculations with spores of Bacillus larvae. J. Econ. Entomol. 1942, 35, 892–895. [Google Scholar] [CrossRef]
- Hoage, T.R.; Rothenbuhler, W.C. Larval honey bee response to various doses of Bacillus larvae spores. J. Econ. Entomol. 1966, 59, 42–45. [Google Scholar] [CrossRef]
- Brodsgaard, C.J.; Ritter, W.; Hansen, H. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 1998, 29, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Yue, D.; Nordhoff, M.; Wieler, L.H.; Genersch, E. Fluorescence in situ-hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environ. Microbiol. 2008, 10, 1612–1620. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA-sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; Ashiralieva, A.; Fries, I. Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp. larvae, the causative agent of American foulbrood disease in honey bees. Appl. Environ. Microbiol. 2005, 71, 7551–7555. [Google Scholar]
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative correlation between individual-insect-level virulence and colony-level virulence of Paenibacillus larvae, the etiological agent of American foulbrood of honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, B.J.; Helgason, T.; Poppinga, L.; Fünfhaus, A.; Genersch, E.; Budge, G.E. Biogeography of Paenibacillus larvae, the causative agent of American foulbrood, using a new multilocus sequence typing scheme. Environ. Microbiol. 2015, 17, 1414–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djukic, M.; Brzuszkiewicz, E.; Fünfhaus, A.; Voss, J.; Gollnow, K.; Poppinga, L.; Liesegang, H.; Garcia-Gonzalez, E.; Genersch, E.; Daniel, R. How to kill the honey bee larva: Genomic potential and virulence mechanisms of Paenibacillus larvae. PLoS ONE 2014, 9, e90914. [Google Scholar] [CrossRef]
- Poppinga, L.; Genersch, E. Molecular pathogenesis of American foulbrood: How Paenibacillus larvae kills honey bee larvae. Curr. Opin. Insect Sci. 2015, 10, 29–36. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Poppinga, L.; Fünfhaus, A.; Hertlein, G.; Hedtke, K.; Jakubowska, A.; Genersch, E. Paenibacillus larvae chitin-degrading protein PlCBP49 is a key virulence factor in American foulbrood of honey bees. PLoS Path. 2014, 10, e1004284. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, E.; Genersch, E. Honey bee larval peritrophic matrix degradation during infection with Paenibacillus larvae, the aetiological agent of American foulbrood of honey bees, is a key step in pathogenesis. Environ. Microbiol. 2013, 15, 2894–2901. [Google Scholar]
- Ebeling, J.; Fünfhaus, A.; Knispel, H.; Krska, D.; Ravulapalli, R.; Heney, K.A.; Lugo, M.R.; Merrill, A.R.; Genersch, E. Characterization of the toxin Plx2A, a RhoA-targeting ADP-ribosyltransferase produced by the honey bee pathogen Paenibacillus larvae. Environ. Microbiol. 2017, 19, 5100–5116. [Google Scholar] [CrossRef]
- Fünfhaus, A.; Poppinga, L.; Genersch, E. Identification and characterization of two novel toxins expressed by the lethal honey bee pathogen Paenibacillus larvae, the causative agent of American foulbrood. Environ. Microbiol. 2013, 15, 2951–2965. [Google Scholar]
- Fünfhaus, A.; Genersch, E. Proteome analysis of Paenibacillus larvae reveals the existence of a putative S-layer protein. Environ. Microbiol. Rep. 2012, 4, 194–202. [Google Scholar] [CrossRef]
- Poppinga, L.; Janesch, B.; Fünfhaus, A.; Sekot, G.; Garcia-Gonzalez, E.; Hertlein, G.; Hedtke, K.; Schäffer, C.; Genersch, E. Identification and functional analysis of the S-layer protein SplA of Paenibacillus larvae, the causative agent of American foulbrood of honey bees. PLoS Path. 2012, 8, e1002716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.Y.S.; Mussen, E.C.; Fong, A.; Cheng, P.; Wong, G.; Montague, M.A. Laboratory and field studies on the effect of the antibiotic tylosin on honey bee Apis mellifera l. (Hymenoptera: Apidae) development and prevention of American foulbrood disease. J. Invertebr. Pathol. 1996, 67, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Peng, C.Y.S.; Chuang, R.Y.; Mussen, E.C.; Spivak, M.S.; Doi, R.H. Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the united states. J. Invertebr. Pathol. 2000, 75, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Mussen, E.C. Antibiotic-resistant American foulbrood. Am. Bee J. 2000, 140, 300–301. [Google Scholar]
- Kochansky, A.; Pettis, J. Screening additional antibiotics for efficacy against American foulbrood. J. Apicult. Res. 2005, 44, 24–28. [Google Scholar] [CrossRef]
- Kochansky, J.; Knox, D.A.; Feldlaufer, M.; Pettis, J.S. Screening alternative antibiotics against oxytetracycline-susceptible and -resistant Paenibacillus larvae. Apidologie 2001, 32, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.C.; Alonso-Salces, R.M.; Umpierrez, M.L.; Rossini, C.; Fuselli, S.R. Chemical composition, antimicrobial activity, and mode of action of essential oils against Paenibacillus larvae, etiological agent of American foulbrood on Apis mellifera. Chem. Biodivers. 2017, 14, e1600382. [Google Scholar] [CrossRef]
- Damiani, N.; Fernandez, N.J.; Porrini, M.P.; Gende, L.B.; Alvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Beoletto, V.G.; Agnese, A.M.; Audisio, M.C.; Marioli, J.M. Purification of substances from Achyrocline satureioides with inhibitory activity against Paenibacillus larvae, the causal agent of American foulbrood in honeybees’ larvae. Appl. Biochem. Biotechnol. 2015, 175, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Piana, M.; de Brum, T.F.; Boligon, A.A.; Alves, C.F.; de Freitas, R.B.; Nunes, L.T.; Mossmann, N.J.; Janovik, V.; Jesus, R.S.; Vaucher, R.A.; et al. In vitro growth-inhibitory effect of Brazilian plants extracts against Paenibacillus larvae and toxicity in bees. An. Acad. Bras. Cienc. 2015, 87, 1041–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaimanee, V.; Thongtue, U.; Sornmai, N.; Songsri, S.; Pettis, J.S. Antimicrobial activity of plant extracts against the honeybee pathogens, Paenibacillus larvae and Ascosphaera apis and their topical toxicity to Apis mellifera adults. J. Appl. Microbiol. 2017, 123, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Buczek, K.; Segiet, A.; Zambrowski, G.; Swiecicka, I. Activity of selected plant extracts against honey bee pathogen Paenibacillus larvae. Apidologie 2018, 49, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, I.; Margotta, J.W.; Aoki, M.M.; Flores, F.; Agudelo, F.; Michel, G.; Elekonich, M.M.; Abel-Santos, E. Inhibitory effect of indole analogs against Paenibacillus larvae, the causal agent of American foulbrood disease. J. Insect Sci. 2017, 17, 104. [Google Scholar] [CrossRef]
- Beims, H.; Wittmann, J.; Bunk, B.; Sproer, C.; Rohde, C.; Gunther, G.; Rohde, M.; von der Ohe, W.; Steinert, M. Paenibacillus larvae-directed bacteriophage HB10c2 and its application in American foulbrood-affected honey bee larvae. Appl. Environ. Microbiol. 2015, 81, 5411–5419. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani-Nezami, S.; LeBlanc, L.; Yost, D.G.; Amy, P.S. Phage therapy is effective in protecting honeybee larvae from American foulbrood disease. J. Insect Sci. 2015, 15, 84. [Google Scholar] [CrossRef] [Green Version]
- Yost, D.G.; Tsourkas, P.; Amy, P.S. Experimental bacteriophage treatment of honey bees (Apis mellifera) infected with Paenibacillus larvae, the causative agent of American foulbrood disease. Bacteriophage 2016, 6, e1122698. [Google Scholar] [CrossRef] [Green Version]
- Brady, T.S.; Fajardo, C.P.; Merrill, B.D.; Hilton, J.A.; Graves, K.A.; Eggett, D.L.; Hope, S. Bystander phage therapy: Inducing host-associated bacteria to produce antimicrobial toxins against the pathogen using phages. Antibiotics 2018, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Brady, T.S.; Merrill, B.D.; Hilton, J.A.; Payne, A.M.; Stephenson, M.B.; Hope, S. Bacteriophages as an alternative to conventional antibiotic use for the prevention or treatment of Paenibacillus larvae in honeybee hives. J. Invertebr. Pathol. 2017, 150, 94–100. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, L.; Nezami, S.; Yost, D.; Tsourkas, P.; Amy, P.S. Isolation and characterization of a novel phage lysin active against Paenibacillus larvae, a honeybee pathogen. Bacteriophage 2015, 5, e1080787. [Google Scholar] [CrossRef]
- Spivak, M.S.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef] [Green Version]
- Locke, B.; Low, M.; Forsgren, E. An integrated management strategy to prevent outbreaks and eliminate infection pressure of American foulbrood disease in a commercial beekeeping operation. Prev. Vet. Med. 2019, 167, 48–52. [Google Scholar] [CrossRef]
- Daisley, B.A.; Pitek, A.P.; Chmiel, J.A.; Al, K.F.; Chernyshova, A.M.; Faragalla, K.M.; Burton, J.P.; Thompson, G.J.; Reid, G. Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J. 2020, 14, 476–491. [Google Scholar] [CrossRef] [Green Version]
- Clatworthy, A.E.; Pierson, E.; Hung, D.T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 2007, 3, 541–548. [Google Scholar] [CrossRef]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.R.; Merrill, A.R. Development of anti-virulence therapeutics against mono-ADP-ribosyltransferase toxins. Toxins 2020, 13, 16. [Google Scholar] [CrossRef]
- Mühlen, S.; Dersch, P. Anti-virulence strategies to target bacterial infections. Curr. Top. Microbiol. Immunol. 2016, 398, 147–183. [Google Scholar] [PubMed]
- Armstrong, S.; Li, J.H.; Zhang, J.; Merrill, A.R. Characterization of competitive inhibitors for the transferase activity of Pseudomonas aeruginosa exotoxin A. J. Enzyme Inhib. Med. Chem. 2002, 17, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, S.P.; Taylor, P.L.; Jørgensen, R.; Ferraris, D.; Zhang, J.; Andersen, G.R.; Merrill, A.R. Structure-function analysis of water-soluble inhibitors of the catalytic domain of exotoxin a from Pseudomonas aeruginosa. Biochem. J. 2005, 385, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Turgeon, Z.; Jørgensen, R.; Visschedyk, D.; Edwards, P.R.; Legree, S.; McGregor, C.; Fieldhouse, R.J.; Mangroo, D.; Schapira, M.; Merrill, A.R. Newly discovered and characterized antivirulence compounds inhibit bacterial mono-ADP-ribosyltransferase toxins. Antimicrob. Agents Chemother. 2011, 55, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Visschedyk, D.; Rochon, A.; Tempel, W.; Dimov, S.; Park, H.W.; Merrill, A.R. Certhrax toxin, an anthrax-related ADP-ribosyltransferase from Bacillus cereus. J. Biol. Chem. 2012, 287, 41089–41102. [Google Scholar] [CrossRef] [Green Version]
- Ravulapalli, R.; Lugo, M.R.; Pfoh, R.; Visschedyk, D.; Poole, A.; Fieldhouse, R.J.; Pai, E.F.; Merrill, A.R. Characterization of Vis toxin, a novel ADP-ribosyltransferase from Vibrio splendidus. Biochemistry 2015, 54, 5920–5936. [Google Scholar] [CrossRef] [PubMed]
- Krska, D.; Ravulapalli, R.; Fieldhouse, R.J.; Lugo, M.R.; Merrill, A.R. C3larvin toxin, an ADP-ribosyltransferase from Paenibacillus larvae. J. Biol. Chem. 2015, 290, 1639–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Trill, J.; Gibbons, S.; Viljoen, A. Acacetin—A simple flavone exhibiting diverse pharmacological activities. Phytochemistry Letters 2019, 32, 56–65. [Google Scholar] [CrossRef]
- Bie, B.; Sun, J.; Li, J.; Guo, Y.; Jiang, W.; Huang, C.; Yang, J.; Li, Z. Baicalein, a natural anti-cancer compound, alters microRNA expression profiles in Bel-7402 human hepatocellular carcinoma cells. Cell. Physiol. Biochem. 2017, 41, 1519–1531. [Google Scholar] [CrossRef]
- Rasko, D.A.; Sperandio, V. Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 2010, 117, 117–128. [Google Scholar] [CrossRef]
- Ebeling, J.; Knispel, H.; Fünfhaus, A.; Genersch, E. The biological role of the enigmatic C3larvinAB toxin of the honey bee pathogenic bacterium Paenibacillus larvae. Environ. Microbiol. 2019, 21, 3091–3106. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Tremblay, O.; Heney, K.A.; Lugo, M.R.; Ebeling, J.; Genersch, E.; Merrill, A.R. Characterization of C3larvinA, a novel RhoA-targeting ADP-ribosyltransferase toxin produced by the honey bee pathogen, Paenibacillus larvae. Biosci. Rep. 2020, 40, BSR20193405. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [PubMed]
- Harris, H.A. Antibacterial activity of seedling extracts of cultivated plants. Bull. Torrey Botan. Club 1949, 76, 244–254. [Google Scholar] [CrossRef]
- Little, J.E.; Grubaugh, K.K. Antibiotic activity of some crude plant juices. J. Bacteriol. 1946, 52, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Gonzalez, E.; Müller, S.; Hertlein, G.; Heid, N.C.; Süssmuth, R.D.; Genersch, E. Biological effects of paenilamicin, a secondary metabolite antibiotic produced by the honey bee pathogenic bacterium Paenibacillus larvae. MicrobiologyOpen 2014, 3, 642–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrio, J.R.; Secrist, J.A.I.; Leonard, N.J. A fluorescent analog of nicotinamide adenine dinucleotide. Proc. Natl. Acad. Sci. USA 1972, 69, 2039–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, S.; Merrill, A.R. Application of a fluorometric assay for characterization of the catalytic competency of a domain III fragment of Pseudomonas aeruginosa exotoxin A. Anal. Biochem. 2001, 292, 26–33. [Google Scholar] [CrossRef]
- Neuendorf, S.; Hedtke, K.; Tangen, G.; Genersch, E. Biochemical characterization of different genotypes of Paenibacillus larvae subs. plarvae, a honey bee bacterial pathogen. Microbiology 2004, 150, 2381–2390. [Google Scholar] [CrossRef] [Green Version]
- Crailsheim, K.; Brodschneider, R.; Aupinel, P.; Behrens, D.; Genersch, E.; Vollmann, J.; Riessberger-Gallé, U. Standard methods for artificial rearing of Apis mellifera larvae. J. Apicult. Res. 2013, 52, 1–15. [Google Scholar] [CrossRef]
- Brayton, C.F. Dimethyl sulfoxide (DMSO): A review. Cornell Vet. 1986, 76, 61–90. [Google Scholar] [PubMed]
- Jacob, S.W.; Herschler, R. Pharmacology of DMSO. Cryobiology 1986, 23, 14–27. [Google Scholar] [CrossRef]
- Piazza, G.A.; Rahm, A.L.; Krutzsch, M.; Sperl, G.; Paranka, N.S.; Gross, P.H.; Brendel, K.; Burt, R.W.; Alberts, D.S.; Pamukcu, R.; et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995, 55, 3110–3116. [Google Scholar]
- Spindler-Barth, M.; Spindler, K.-D.; Londershausen, M.; Thomas, H.; Ag, B. Inhibition of chitin synthesis in an insect cell-line. Pesticide Sci. 1989, 25, 115–121. [Google Scholar] [CrossRef]
- Liao, L.H.; Wu, W.Y.; Berenbaum, M.R. Behavioral responses of honey bees (Apis mellifera) to natural and synthetic xenobiotics in food. Sci Rep. 2017, 7, 15924. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.M.; Baker, D.D.; Wright, G.A. Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invert. Neurosci. 2013, 13, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milchreit, K.; Ruhnke, H.; Wegener, J.; Bienefeld, K. Effects of an insect growth regulator and a solvent on honeybee (Apis mellifera L.) brood development and queen viability. Ecotoxicology 2016, 25, 530–537. [Google Scholar] [CrossRef]
- Yang, E.C.; Chuang, Y.C.; Chen, Y.L.; Chang, L.H. Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2008, 101, 1743–1748. [Google Scholar] [CrossRef]
- Lambin, M.; Armengaud, C.; Raymond, S.; Gauthier, M. Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honeybee. Arch. Insect Biochem. Physiol. 2001, 48, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Da Violante, G.; Zerrouk, N.; Richard, I.; Provot, G.; Chaumeil, J.C.; Arnaud, P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol. Pharmaceut. Bull. 2002, 25, 1600–1603. [Google Scholar] [CrossRef] [Green Version]
- Eter, N.; Spitznas, M. DMSO mimics inhibitory effect of thalidomide on choriocapillary endothelial cell proliferation in culture. Br. J. Ophthalmol. 2002, 86, 1303. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Pedenovi, M.; Niewiarowski, A.; Casali, B.; Donati, M.B.; Corbascio, G.C.; Marchisio, P.C. Effects of dimethyl sulfoxide (DMSO) on microfilament organization, cellular adhesion, and growth of cultured mouse B16 melanoma cells. Exp. Cell Res. 1987, 172, 385–396. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebeling, J.; Pieper, F.; Göbel, J.; Knispel, H.; McCarthy, M.; Goncalves, M.; Turner, M.; Merrill, A.R.; Genersch, E. Anti-Virulence Strategy against the Honey Bee Pathogenic Bacterium Paenibacillus larvae via Small Molecule Inhibitors of the Bacterial Toxin Plx2A. Toxins 2021, 13, 607. https://doi.org/10.3390/toxins13090607
Ebeling J, Pieper F, Göbel J, Knispel H, McCarthy M, Goncalves M, Turner M, Merrill AR, Genersch E. Anti-Virulence Strategy against the Honey Bee Pathogenic Bacterium Paenibacillus larvae via Small Molecule Inhibitors of the Bacterial Toxin Plx2A. Toxins. 2021; 13(9):607. https://doi.org/10.3390/toxins13090607
Chicago/Turabian StyleEbeling, Julia, Franziska Pieper, Josefine Göbel, Henriette Knispel, Michael McCarthy, Monica Goncalves, Madison Turner, Allan Rod Merrill, and Elke Genersch. 2021. "Anti-Virulence Strategy against the Honey Bee Pathogenic Bacterium Paenibacillus larvae via Small Molecule Inhibitors of the Bacterial Toxin Plx2A" Toxins 13, no. 9: 607. https://doi.org/10.3390/toxins13090607
APA StyleEbeling, J., Pieper, F., Göbel, J., Knispel, H., McCarthy, M., Goncalves, M., Turner, M., Merrill, A. R., & Genersch, E. (2021). Anti-Virulence Strategy against the Honey Bee Pathogenic Bacterium Paenibacillus larvae via Small Molecule Inhibitors of the Bacterial Toxin Plx2A. Toxins, 13(9), 607. https://doi.org/10.3390/toxins13090607