Analytical Validation of a Direct Competitive ELISA for Multiple Mycotoxin Detection in Human Serum
Abstract
1. Introduction
- Mycotoxin in food does not always imply exposure as their bioavailability, food treatment or composition, and individual-to-individual differences are diverse [8].
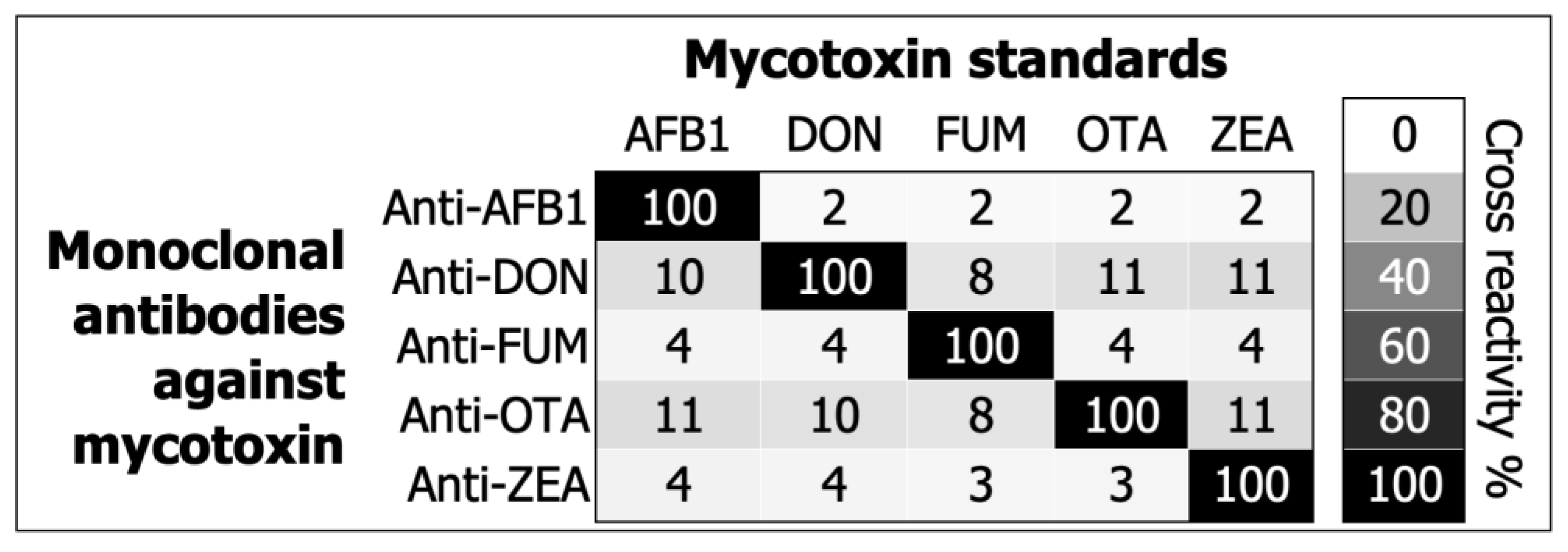
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Equipment
5.2. Development of a Direct Competitive ELISA
5.3. Construction of Calibration Curves through Competition
5.4. Pretreatment Method to Determine Recovery and Matrix Effects
5.5. Characterizing Assay Specificity
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulyok, M.; Krska, R.; Schuhmacher, R. A Liquid Chromatography/Tandem Mass Spectrometric Multi-Mycotoxin Method for the Quantification of 87 Analytes and Its Application to Semi-Quantitative Screening of Moldy Food Samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.M. Managing the Risk of Mycotoxins in Modern Feed Production. Anim. Feed. Sci. Tech. 2007, 133, 149–166. [Google Scholar] [CrossRef]
- European Commission Decision of 12 August 2002, Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (2002/657/EC). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:221:0008:0036:EN:PDF (accessed on 27 October 2021).
- European Commission Recommendation 401/2006 of 23 February 2006, Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R0401:20100313:EN:PDF (accessed on 28 August 2022).
- Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 27 October 2021).
- Guideline on Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 27 October 2021).
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, Metabolism, and Impact of Mycotoxins on Humans and Animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Vettorazzi, A.; González-Peñas, E. Human Biomonitoring of Mycotoxins in Blood, Plasma and Serum in Recent Years: A Review. Toxins 2020, 12, 147. [Google Scholar] [CrossRef]
- Humans, I.W.G. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; Cancer, I.A., Ed.; International Agency for Research on Cancer: Lyon, France, 1993; Volume 56, ISBN 978-92-832-1256-0. [Google Scholar]
- Fink-Gremmels, J.; Jahn, A.; Blom, M.J. Toxicity and Metabolism of Ochratoxin A. Nat. Toxins 1995, 3, 214–220. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- McCullough, A.K.; Lloyd, R.S. Mechanisms Underlying Aflatoxin-Associated Mutagenesis—Implications in Carcinogenesis. DNA Repair 2019, 77, 76–86. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of Action, Human Exposure, and Toxicological Relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and Potential Effects on Humans. J. Toxicol. Environ. Health Part B 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.J.; Crane, H.M.; Xia, J.; Liotta, D.C.; Merrill, A.H. Disruption of Sphingolipid Metabolism and Stimulation of DNA Synthesis by Fumonisin B1. A Molecular Mechanism for Carcinogenesis Associated with Fusarium Moniliforme. J. Biol. Chem. 1994, 269, 3475–3481. [Google Scholar] [CrossRef]
- Reddy, L.; Bhoola, K. Ochratoxins—Food Contaminants: Impact on Human Health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Tech. 2018, 84, 38–40. [Google Scholar] [CrossRef]
- Rychlik, M.; Humpf, H.-U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a Comprehensive Definition of Modified and Other Forms of Mycotoxins Including “Masked” Mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; Henauw, S.D.; Saeger, S.D. Human Biomonitoring of Multiple Mycotoxins in the Belgian Population: Results of the BIOMYCO Study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef]
- Turner, P.C.; Flannery, B.; Isitt, C.; Ali, M.; Pestka, J. The Role of Biomarkers in Evaluating Human Health Concerns from Fungal Contaminants in Food. Nutr. Res. Rev. 2012, 25, 162–179. [Google Scholar] [CrossRef]
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef]
- Slobodchikova, I.; Vuckovic, D. Liquid chromatography—High resolution mass spectrometry method for monitoring of 17 mycotoxins in human plasma for exposure studies. J. Chromatogr. A 2018, 1548, 51–63. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Lattanzio, V.M.; Powers, S.; Visconti, A. Simultaneous LC-MS/MS determination of aflatoxin M1, ochratoxin A, deoxynivalenol, de-epoxydeoxynivalenol, α and β-zearalenols and fumonisin B1 in urine as a multi-biomarker method to assess exposure to mycotoxins. Anal. Bioanal. Chem. 2011, 401, 2831–2841. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, X.; Jiao, Y.; Duan, X.; Yu, L.; Zheng, F.; Wang, X.; Wang, L.; Wang, J.-S.; Zhao, X.; et al. Exposure Assessment of Aflatoxins and Zearalenone in Edible Vegetable Oils in Shandong, China: Health Risks Posed by Mycotoxin Immunotoxicity and Reproductive Toxicity in Children. Environ. Sci. Pollut. Res. Int. 2022, 1–16. [Google Scholar] [CrossRef]
- Jubeen, F.; Zahra, N.; Nazli, Z.-H.; Saleemi, M.K.; Aslam, F.; Naz, I.; Farhat, L.B.; Saleh, A.; Alshawwa, S.Z.; Iqbal, M. Risk Assessment of Hepatocellular Carcinoma with Aflatoxin B1 Exposure in Edible Oils. Toxins 2022, 14, 547. [Google Scholar] [CrossRef]
- Andreasson, U.; Perret-Liaudet, A.; Doorn, L.J.; van Blennow, K.; Chiasserini, D.; Engelborghs, S.; Fladby, T.; Genc, S.; Kruse, N.; Kuiperij, B.H.; et al. A Practical Guide to Immunoassay Method Validation. Front. Neurol. 2015, 6, 179. [Google Scholar] [CrossRef]
- Hernandez-Vargas, H.; Castelino, J.; Silver, M.J.; Dominguez-Salas, P.; Cros, M.-P.; Durand, G.; Calvez-Kelm, F.L.; Prentice, A.M.; Wild, C.P.; Moore, S.E.; et al. Exposure to Aflatoxin B1 in Utero Is Associated with DNA Methylation in White Blood Cells of Infants in The Gambia. Int. J. Epidemiol. 2015, 44, 1238–1248. [Google Scholar] [CrossRef]
- Seetha, A.; Monyo, E.S.; Tsusaka, T.W.; Msere, H.W.; Madinda, F.; Chilunjika, T.; Sichone, E.; Mbughi, D.; Chilima, B.; Matumba, L. Aflatoxin-Lysine Adducts in Blood Serum of the Malawian Rural Population and Aflatoxin Contamination in Foods (Groundnuts, Maize) in the Corresponding Areas. Mycotoxin Res. 2018, 34, 195–204. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Huang, H.; Chen, X.; Leng, Y.; Lai, W.; Huang, X.; Xiong, Y. Engineered Gold Nanoparticles as Multicolor Labels for Simultaneous Multi-Mycotoxin Detection on the Immunochromatographic Test Strip Nanosensor. Sens. Actuators B Chem. 2020, 316, 128107. [Google Scholar] [CrossRef]
- Xing, C.; Dong, X.; Xu, T.; Yuan, J.; Yan, W.; Sui, X.; Zhao, X. Analysis of Multiple Mycotoxins-Contaminated Wheat by a Smart Analysis Platform. Anal. Biochem. 2020, 610, 113928. [Google Scholar] [CrossRef]
- Charlermroj, R.; Phuengwas, S.; Makornwattana, M.; Sooksimuang, T.; Sahasithiwat, S.; Panchan, W.; Sukbangnop, W.; Elliott, C.T.; Karoonuthaisiri, N. Development of a Microarray Lateral Flow Strip Test Using a Luminescent Organic Compound for Multiplex Detection of Five Mycotoxins. Talanta 2021, 233, 122540. [Google Scholar] [CrossRef]
- Joshi, S.; Segarra-Fas, A.; Peters, J.; Zuilhof, H.; Beek, T.A.; van Nielen, M.W.F. Multiplex Surface Plasmon Resonance Biosensing and Its Transferability towards Imaging Nanoplasmonics for Detection of Mycotoxins in Barley. Analyst 2016, 141, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ren, P.; Huang, L.; Ouyang, Z.; Wang, Z.; Kong, X.; Li, T.; Yin, Y.; Wu, Y.; He, Q. Simultaneous Detection of Aflatoxin B1, Ochratoxin A, Zearalenone and Deoxynivalenol in Corn and Wheat Using Surface Plasmon Resonance. Food Chem. 2019, 300, 125176. [Google Scholar] [CrossRef]
- Cusabio Mycotoxin ELISA Kit. Available online: https://www.cusabio.com/mycotoxin-ELISA.html (accessed on 5 September 2022).
- Kohl, T.O.; Ascoli, C.A. Direct Competitive Enzyme-Linked Immunosorbent Assay (ELISA). Cold Spring Harb. Protoc. 2017, 2017, pdb.prot093740. [Google Scholar] [CrossRef]
- Elabscience Elabscience AFB1 (Aflatoxin B1) ELISA Kit. Available online: https://www.elabscience.com/p-afb1_aflatoxin_b1_elisa_kit-40683.html (accessed on 5 September 2022).
- Elabscience Elabscience DON (Deoxynivalenol) ELISA Kit. Available online: https://www.elabscience.com/p-don_deoxynivalenol_elisa_kit-40678.html (accessed on 5 September 2022).
- Elabscience Elabscience FB1 (Fumonisin B1) ELISA Kit. Available online: https://www.elabscience.com/p-fb1_fumonisin_b1_elisa_kit-343132.html (accessed on 5 September 2022).
- Elabscience Elabscience OTA (Ochratoxin A) ELISA Kit. Available online: https://www.elabscience.com/p-ota_ochratoxin_a_elisa_kit-40687.html (accessed on 5 September 2022).
- Elabscience Elabscience ZEN (Zearalenone) ELISA Kit. Available online: https://www.elabscience.com/p-zen_zearalenone_elisa_kit-40677.html (accessed on 5 September 2022).
- Hygiena HelicaTM Aflatoxin ELISA’s. Available online: https://www.hygiena.com/food-safety-solutions/mycotoxins/aflatoxin/ (accessed on 5 September 2022).
- Hygiena HelicaTM Deoxynivalenol (DON) ELISA’s. Available online: https://www.hygiena.com/food-safety-solutions/mycotoxins/deoxynivalenol-3/ (accessed on 5 September 2022).
- Hygiena HelicaTM Fumonisin ELISAs. Available online: https://www.hygiena.com/food-safety-solutions/mycotoxins/fumonisin-2/ (accessed on 5 September 2022).
- Hygiena HelicaTM Ochratoxin ELISAs. Available online: https://www.hygiena.com/food-safety-solutions/mycotoxins/ochratoxin/ (accessed on 5 September 2022).
- Hygiena HelicaTM Zearalenone ELISA. Available online: https://www.hygiena.com/food-safety-solutions/mycotoxins/zearalenone-2/ (accessed on 5 September 2022).
- Romer Labs AgraQuant® Aflatoxin B1 ELISA Test. Available online: https://www.romerlabs.com/shop/inter_en/agraquant-r-aflatoxin-b1-elisa-test/ (accessed on 5 September 2022).
- Romer Labs AgraQuant® Deoxynivalenol Plus ELISA Test. Available online: https://www.romerlabs.com/shop/inter_en/agraquant-r-deoxynivalenol-elisa-test/ (accessed on 5 September 2022).
- Romer Labs AgraQuant® Fumonisin ELISA Test. Available online: https://www.romerlabs.com/shop/inter_en/agraquant-r-fumonisin-elisa-test/ (accessed on 5 September 2022).
- Romer Labs AgraQuant® Ochratoxin ELISA Test. Available online: https://www.romerlabs.com/shop/inter_en/agraquant-r-ochratoxin-elisa-test/ (accessed on 5 September 2022).
- Romer Labs AgraQuant® Zearalenone ELISA Test. Available online: https://www.romerlabs.com/shop/inter_en/agraquant-r-zearalenone-elisa-test/ (accessed on 5 September 2022).
- Waters VICAM Afla-V ONE. Available online: https://www.vicam.com/products/afla-v-one (accessed on 5 September 2022).
- Waters VICAM DON-V AQUA. Available online: https://www.vicam.com/products/don-v-aqua (accessed on 5 September 2022).
- Waters VICAM Fumo-V. Available online: https://www.vicam.com/products/fumo-v (accessed on 5 September 2022).
- Waters VICAM Ochra-V AQUA. Available online: https://www.vicam.com/products/ochra-v-aqua (accessed on 5 September 2022).
- Waters VICAM Zearala-V AQUA. Available online: https://www.vicam.com/products/zearala-v-aqua (accessed on 5 September 2022).
- Ksenija, N. Mycotoxins-Climate Impact and Steps to Prevention Based on Prediction. Acta Veter. 2018, 68, 1–15. [Google Scholar] [CrossRef]
- Zong, C.; Jiang, F.; Wang, X.; Li, P.; Xu, L.; Yang, H. Imaging Sensor Array Coupled with Dual-Signal Amplification Strategy for Ultrasensitive Chemiluminescence Immunoassay of Multiple Mycotoxins. Biosens. Bioelectron. 2021, 177, 112998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, S.; Jin, Y.; Yang, C.; He, L.; Wang, J.; Chen, Y. Multiplex SERS-Based Lateral Flow Immunosensor for the Detection of Major Mycotoxins in Maize Utilizing Dual Raman Labels and Triple Test Lines. J. Hazard Mater. 2020, 393, 122348. [Google Scholar] [CrossRef] [PubMed]
- Pum, J.K.W. Evaluating Sample Stability in the Clinical Laboratory with the Help of Linear and Non-Linear Regression Analysis. Clin. Chem. Lab. Med. 2020, 58, 188–196. [Google Scholar] [CrossRef]
- McDaniel, G. New CLSI Guideline for Evaluation of Stability of In Vitro Diagnostic Reagents. Lab. Med. 2010, 41, 18–19. [Google Scholar] [CrossRef][Green Version]
- Bakir, A.; Mustafa, M.; Alacam, S.; Sig, A.K.; Bekiroglu, N.; Yavuz, M.T. Assessment of Index Value Performance of Enzyme Immunoassay Test in Predicting the Diagnosis of Human Brucellosis. J. Infect Dev. Ctries 2022, 16, 807–812. [Google Scholar] [CrossRef]
- Luo, J.; Brakel, A.; Krizsan, A.; Ludwig, T.; Mötzing, M.; Volke, D.; Lakowa, N.; Grünewald, T.; Lehmann, C.; Wolf, J.; et al. Sensitive and Specific Serological ELISA for the Detection of SARS-CoV-2 Infections. Virol. J. 2022, 19, 50. [Google Scholar] [CrossRef]
- Ma, L.; Wang, W.; Li, L.; Chen, Y.; Chen, B.; Shao, M.; Cheng, Y.; Zhou, R. Comparison of Different Assays for the Detection of Anticyclic Citrullinated Peptide Antibodies in Patients with Rheumatoid Arthritis. Front. Immunol. 2022, 13, 940713. [Google Scholar] [CrossRef] [PubMed]
- D’Agnello, P.; Vita, V.; Franchino, C.; Urbano, L.; Curiale, A.; Debegnach, F.; Iammarino, M.; Marchesani, G.; Chiaravalle, A.E.; Pace, R.D. ELISA and UPLC/FLD as Screening and Confirmatory Techniques for T-2/HT-2 Mycotoxin Determination in Cereals. Appl. Sci. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Ghassabian, S.; Rethwan, N.S.A.; Griffiths, L.; Smith, M.T. Fully Validated LC–MS/MS Method for Quantification of Homocysteine Concentrations in Samples of Human Serum: A New Approach. J. Chromatogr. B 2014, 972, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.R.; Schultz, G.A.; Eckstein, J.A.; Ackermann, B.L. Surrogate Matrix and Surrogate Analyte Approaches for Definitive Quantitation of Endogenous Biomolecules. Bioanalysis 2012, 4, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Leaney, A.E.; Horner, C.; Grace, P.B.; Mawson, D.H. Selection of a Surrogate Matrix for the Quantification of an Endogenous Analyte in Dried Whole Blood. Bioanalysis 2020, 12, 409–418. [Google Scholar] [CrossRef]
- Arce-López, B.; Lizarraga, E.; Irigoyen, Á.; González-Peñas, E. Presence of 19 Mycotoxins in Human Plasma in a Region of Northern Spain. Toxins 2020, 12, 750. [Google Scholar] [CrossRef]
- Facorro, R.; Llompart, M.; Dagnac, T. Combined (d)SPE-QuEChERS Extraction of Mycotoxins in Mixed Feed Rations and Analysis by High Performance Liquid Chromatography-High-Resolution Mass Spectrometry. Toxins 2020, 12, 206. [Google Scholar] [CrossRef]
- Tangni, E.K.; Motte, J.-C.; Callebaut, A.; Pussemier, L. Cross-Reactivity of Antibodies in Some Commercial Deoxynivalenol Test Kits against Some Fusariotoxins. J. Agr. Food Chem. 2010, 58, 12625–12633. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Zherdev, A.V.; Zvereva, E.A.; Eremin, S.A.; Dzantiev, B.B. Changing Cross-Reactivity for Different Immunoassays Using the Same Antibodies: Theoretical Description and Experimental Confirmation. Appl. Sci. 2021, 11, 6581. [Google Scholar] [CrossRef]
- Lee, K.R.; Dipaolo, B.; Ji, X. Calibration and LOD/LOQ Estimation of a Chemiluminescent Hybridization Assay for Residual DNA in Recombinant Protein Drugs Expressed in E. Coli Using a Four-Parameter Logistic Model. Drug Dev. Ind. Pharm. 2000, 26, 661–669. [Google Scholar] [CrossRef]
- Cumberland, W.N.; Fong, Y.; Yu, X.; Defawe, O.; Frahm, N.; Rosa, S.D. Nonlinear Calibration Model Choice between the Four and Five-Parameter Logistic Models. J. Biopharm. Stat. 2014, 25, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Warrens, M.J.; Jurman, G. The Coefficient of Determination R-Squared Is More Informative than SMAPE, MAE, MAPE, MSE and RMSE in Regression Analysis Evaluation. Peer Comput. Sci. 2021, 7, e623. [Google Scholar] [CrossRef] [PubMed]
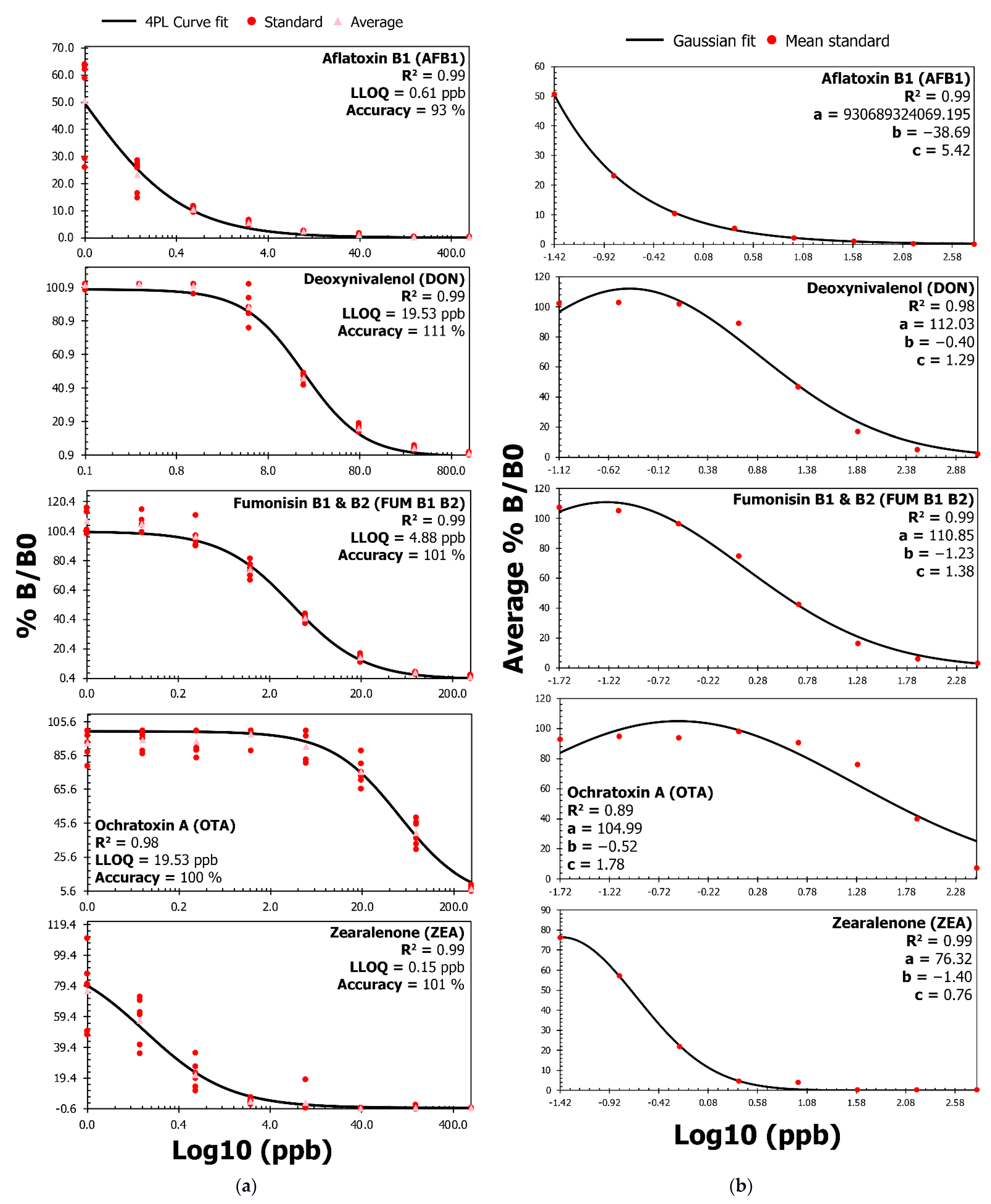
| Mycotoxin | Spike (ppb) | Mean Recovery (%) | Mean Repeatability (CV%) | Mean Matrix Effect (%) | Mean Matrix Factor |
|---|---|---|---|---|---|
| AFB1 | 0.61 | 96 | 0.05 | −72.11 | 0.27 |
| 2.44 | 97 | 4 | −65.91 | 0.33 | |
| 9.77 | 96 | 4 | −49.71 | 0.48 | |
| 39.06 | 101 | 2 | −40.50 | 0.60 | |
| DON | 19.53 | 98 | 4 | −9.28 | 0.88 |
| 78.13 | 94 | 5 | −0.78 | 0.93 | |
| 312.50 | 94 | 7 | 6.19 | 1.00 | |
| 1250 | 91 | 17 | 8.75 | 0.99 | |
| FUM B1/B2 | 4.88 | 73 | 0.1 | 5.47 | 0.77 |
| 19.53 | 81 | 11 | −0.44 | 0.80 | |
| 78.13 | 79 | 6 | 3.12 | 0.81 | |
| 312.50 | 91 | 2 | −3.76 | 0.88 | |
| OTA | 19.53 | 81 | 0.4 | −9.51 | 0.74 |
| 78.13 | 86 | 21 | −10.16 | 0.76 | |
| 312.50 | 79 | 3 | −3.69 | 0.76 | |
| 1250 | 92 | 6 | −7.43 | 0.85 | |
| ZEA | 0.15 | 85 | 4 | −39.22 | 0.52 |
| 0.61 | 95 | 3 | −29.88 | 0.66 | |
| 2.44 | 89 | 10 | −12.69 | 0.78 | |
| 9.77 | 106 | 18 | −10.12 | 0.94 |
| Parameters or Studies for Comparison | AFB1 (ppb) | DON (ppb) | FUM (ppb) | OTA (ppb) | ZEA (ppb) |
|---|---|---|---|---|---|
| Lowest EU guidance levels for food ° | 2 ◊ | 500 ◊ | 800 ◊ | 2 ◊ | 50 ◊ |
| LLOQ from the present study * | 0.61 | 19.53 | 4.88 | 19.53 | 0.15 |
| Cusabio (ELISA) § | 1.5 | 100 | 30 | 1.5 | 30 |
| Elabscience (ELISA) ⊗ | 0.6 | 150 | 20 | 5 | 6 |
| HelicaTM (ELISA) ⊕ | 4 | 500 | 100 | 1 | NI |
| AgraQuant® (ELISA) # | 2 | 250 | 250 | 2 | 25 |
| VICAM (LFIA) ^ | 2 | 250 | 200 | 2.5 | 100 |
| Wu et al. 2020 (LFIA) ∅ | 0.1 | NA | 4 | 0.2 | 0.8 |
| Xing et al. 2020 (LFIA) | 4 | 200 | 20 | NA | 40 |
| Charlermroj et al. 2021 (LFIA) ∅ | 5 | 10 | 0.5 | NA | 10 |
| Joshi et al. 2016 (SPR) ∅ | 3 | 26 | 10 | 13 | 16 |
| Wie et al. 2019 (SPR) ∅ | 0.9 | 5.3 | NA | 1.9 | 10.3 |
| Parameters or Studies for Comparison | Recovery (%) | ||||
|---|---|---|---|---|---|
| AFB1 | DON | FUM | OTA | ZEA | |
| Present study | 96–101 | 91–98 | 73–91 | 79–92 | 85–106 |
| Elabscience (ELISA) | 69–99 | 70–100 | 78–108 | 70–100 | 70–100 |
| Hygiena HelicaTM (ELISA) | 82–109 | 74–82 | 82–119 | 95–101 | 89–102 |
| Wu et al. 2020 (LFIA) | 85–112 | NA | 88–112 | 82–116 | 88–104 |
| Charlermroj et al. 2021 (LFIA) | 87–111 | 87–109 | 88–108 | NA | 89–123 |
| Wie et al. 2019 (SPR) | 92–104 | 88–104 | NA | 95–111 | 89–103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, K.; Villavicencio-Aguilar, F.; Solano-Rivera, F.; Gilbert, L. Analytical Validation of a Direct Competitive ELISA for Multiple Mycotoxin Detection in Human Serum. Toxins 2022, 14, 727. https://doi.org/10.3390/toxins14110727
Garg K, Villavicencio-Aguilar F, Solano-Rivera F, Gilbert L. Analytical Validation of a Direct Competitive ELISA for Multiple Mycotoxin Detection in Human Serum. Toxins. 2022; 14(11):727. https://doi.org/10.3390/toxins14110727
Chicago/Turabian StyleGarg, Kunal, Fausto Villavicencio-Aguilar, Flora Solano-Rivera, and Leona Gilbert. 2022. "Analytical Validation of a Direct Competitive ELISA for Multiple Mycotoxin Detection in Human Serum" Toxins 14, no. 11: 727. https://doi.org/10.3390/toxins14110727
APA StyleGarg, K., Villavicencio-Aguilar, F., Solano-Rivera, F., & Gilbert, L. (2022). Analytical Validation of a Direct Competitive ELISA for Multiple Mycotoxin Detection in Human Serum. Toxins, 14(11), 727. https://doi.org/10.3390/toxins14110727







