Early Detrusor Application of Botulinum Toxin A Results in Reduced Bladder Hypertrophy and Fibrosis after Spinal Cord Injury in a Rodent Model
Abstract
:1. Introduction
2. Results
2.1. General Animal Health
2.2. Bladder Weight
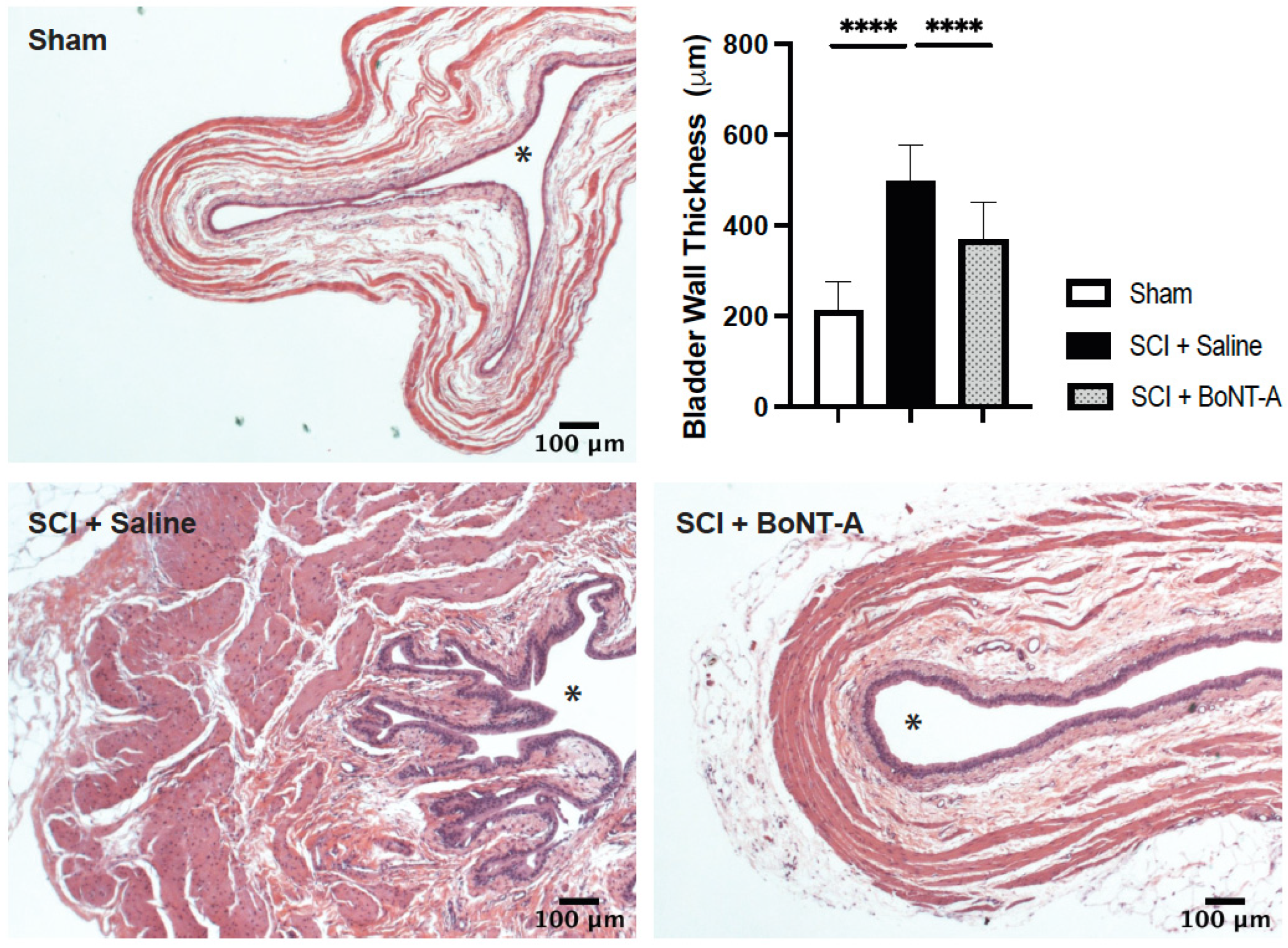
2.3. Histological Changes
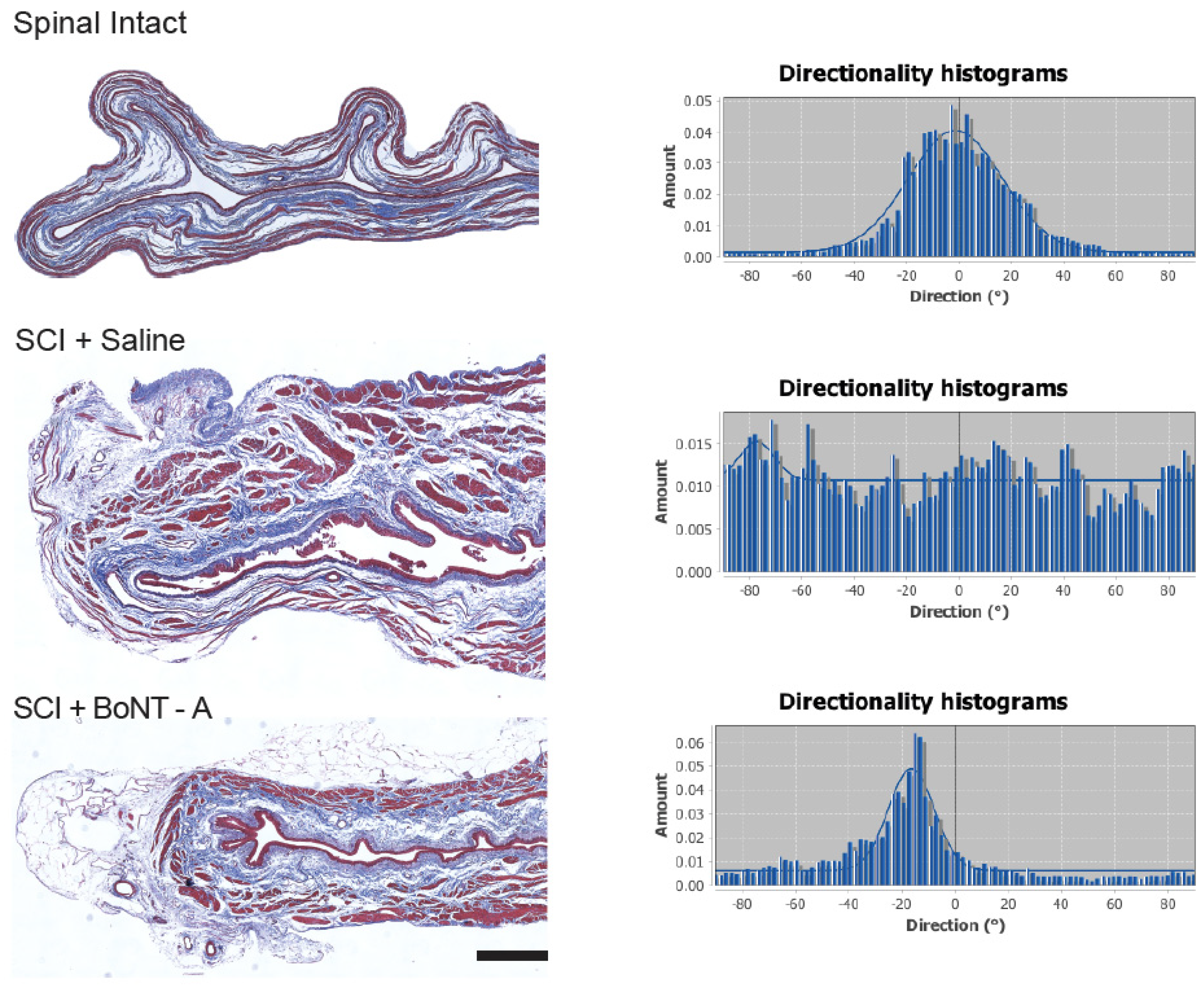
2.4. Collagen Directionality
2.5. Bladder Function after SCI
2.6. Spinal Cord Injury and Motor Function
3. Discussion
Translational Relevance and Study Limitations
4. Conclusions
5. Materials and Methods
5.1. Spinal Cord Injury Model
5.2. Intradetrusor Injection
5.3. Bladder Volumes
5.4. Locomotion after SCI
5.5. Histological Analysis
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshimura, N.; Chancellor, M.B. Neurophysiology of lower urinary tract function and dysfunction. Rev. Urol. 2003, 5, S3–S10. [Google Scholar] [PubMed]
- Allio, B.A.; Peterson, A.C. Urodynamic and physiologic patterns associated with the common causes of neurogenic bladder in adults. Transl. Androl. Urol. 2016, 5, 31–38. [Google Scholar] [PubMed]
- Harris, C.J.; Lemack, G.E. Neurourologic dysfunction: Evaluation, surveillance and therapy. Curr. Opin. Urol. 2016, 26, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.; Reitz, A.; Delcourt, A.; Capron, F.; Denys, P.; Chartier-Kastler, E. Histologic features in the urinary bladder wall affected from neurogenic overactivity—A comparison of inflammation, oedema and fibrosis with and without injection of botulinum toxin type A. Eur. Urol. 2006, 50, 1058–1064. [Google Scholar] [CrossRef]
- Janzen, J.; Vuong, P.N.; Bersch, U.; Michel, D.; Zaech, G.A. Bladder tissue biopsies in spinal cord injured patients: Histopathologic aspects of 61 cases. Neurourol. Urodyn. 1998, 17, 525–530. [Google Scholar] [CrossRef]
- Temeltas, G.; Tikiz, C.; Dagci, T.; Tuglu, I.; Yavasoglu, A. The effects of botulinum-A toxin on bladder function and histology in spinal cord injured rats: Is there any difference between early and late application? J. Urol. 2005, 174, 2393–2396. [Google Scholar] [CrossRef]
- Hackler, R.H.; Hall, M.K.; Zampieri, T.A. Bladder Hypocompliance in the Spinal Cord Injury Population. J. Urol. 1989, 141, 1390–1393. [Google Scholar] [CrossRef]
- Apostolidis, A.; Gonzales, G.E.; Fowler, C.J. Effect of Intravesical Resiniferatoxin (RTX) on Lower Urinary Tract Symptoms, Urodynamic Parameters, and Quality of Life of Patients with Urodynamic Increased Bladder Sensation. Eur. Urol. 2006, 50, 1299–1305. [Google Scholar] [CrossRef]
- Khera, M.; Somogyi, G.T.; Salas, N.A.; Kiss, S.; Boone, T.B.; Smith, C.P. In vivo effects of botulinum toxin A on visceral sensory function in chronic spinal cord-injured rats. Urology 2005, 66, 208–212. [Google Scholar] [CrossRef]
- Krhut, J.; Zvara, P. Intravesical instillation of botulinum toxin A: An in vivo murine study and pilot clinical trial. Int. Urol. Nephrol. 2010, 43, 337–343. [Google Scholar] [CrossRef]
- Zinck, N.; Rafuse, V.; Downie, J. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp. Neurol. 2007, 204, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Bywater, M.; Tornic, J.; Mehnert, U.; Kessler, Y.M. Detrusor Acontractility after Acute Spinal Cord Injury-Myth or Reality? J. Urol. 2018, 199, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Behr-Roussel, D.; Oger, S.; Pignol, B.; Pham, E.; Le Maux, A.; Chabrier, P.-E.; Caisey, S.; Compagnie, S.; Picaut, P.; Bernabé, J.; et al. Minimal Effective Dose of Dysport and Botox in a Rat Model of Neurogenic Detrusor Overactivity. Eur. Urol. 2012, 61, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Huynh Le Maux, A.; Pignol, B.; Behr-Roussel, D.; Blachon, J.-L.; Chabrier, P.-E.; Compagnie, S.; Picaut, P.; Bernabé, J.; Giuliano, F.; Denys, P. Does Reduction of Number of Intradetrusor Injection Sites of aboBoNTA (Dysport(R)) Impact Efficacy and Safety in a Rat Model of Neurogenic Detrusor Overactivity? Toxins 2015, 7, 5462–5471. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Wognum, S.; Joyce, E.M.; Freytes, D.O.; Sacks, M.S.; Badylak, S.F. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials 2008, 29, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Deveaud, C.M.; Macarak, E.J.; Kucich, U.; Ewalt, D.H.; Abrams, W.R.; Howard, P.S. Molecular analysis of collagens in bladder fibrosis. J. Urol. 1998, 160, 1518–1527. [Google Scholar] [CrossRef]
- Fouad, K.; Pearse, D.D.; Tetzlaff, W.; Vavrek, R. Transplantation and repair: Combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord 2009, 47, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Shimizu, T.; Takai, S.; Shimizu, N.; Kanai, A.J.; Tyagi, P.; Kakizaki, H.; Yoshimura, N. Post-injury bladder management strategy influences lower urinary tract dysfunction in the mouse model of spinal cord injury. Neurourol. Urodyn. 2016, 36, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Weld, K.J.; Graney, M.J.; Dmochowski, R.R. Differences in bladder compliance with time and associations of bladder management with compliance in spinal cord injured patients. J. Urol. 2000, 163, 1228–1233. [Google Scholar] [CrossRef]
- Kadekawa, K.; Yoshizawa, T.; Wada, N.; Shimizu, T.; Majima, T.; Tyagi, P.; de Groat, W.C.; Sugaya, K.; Yoshimura, N. Effects of liposome-based local suppression of nerve growth factor in the bladder on autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury. Exp. Neurol. 2017, 291, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.C.; Tooke, K.; Malley, S.E.; Soulas, A.; Weiss, T.; Ganesh, N.; Saidi, N.; Daugherty, S.; Saragovi, U.; Ikeda, Y.; et al. Role of proNGF/p75 signaling in bladder dysfunction after spinal cord injury. J. Clin. Investig. 2018, 128, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Ergin, G.; Kibar, Y.; Ebiloğlu, T.; Irkılata, H.; Kopru, B.; Kaya, E.; Uyanık, M.; Tapan, S.; Dayanc, M.M. The role of urinary nerve growth factor for the diagnosis and assessment of the biofeedback success in children with dysfunctional voiding. J. Pediatr. Urol. 2015, 12, 118.e1–118.e6. [Google Scholar] [CrossRef] [PubMed]
- Steers, W.D.; Tuttle, J.B. Mechanisms of Disease: The role of nerve growth factor in the pathophysiology of bladder disorders. Nat. Clin. Pract. Urol. 2006, 3, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Breyer, B.N.; Fandel, T.M.; Alwaal, A.; Osterberg, E.C.; Shindel, A.W.; Lin, G.; Tanagho, E.A.; Lue, T.F. Comparison of spinal cord contusion and transection: Functional and histological changes in the rat urinary bladder. Br. J. Urol. 2016, 119, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Khaing, Z.Z.; Cates, L.N.; Hyde, J.; DeWees, D.M.; Hammond, R.; Bruce, M.; Hofstetter, C.P. Contrast-Enhanced Ultrasound for Assessment of Local Hemodynamic Changes Following a Rodent Contusion Spinal Cord Injury. Mil. Med. 2020, 185, 470–475. [Google Scholar] [CrossRef] [PubMed]
- David, B.T.; Steward, O. Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp. Neurol. 2010, 226, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bushnell, J.Y.; Cates, L.N.; Hyde, J.E.; Hofstetter, C.P.; Yang, C.C.; Khaing, Z.Z. Early Detrusor Application of Botulinum Toxin A Results in Reduced Bladder Hypertrophy and Fibrosis after Spinal Cord Injury in a Rodent Model. Toxins 2022, 14, 777. https://doi.org/10.3390/toxins14110777
Bushnell JY, Cates LN, Hyde JE, Hofstetter CP, Yang CC, Khaing ZZ. Early Detrusor Application of Botulinum Toxin A Results in Reduced Bladder Hypertrophy and Fibrosis after Spinal Cord Injury in a Rodent Model. Toxins. 2022; 14(11):777. https://doi.org/10.3390/toxins14110777
Chicago/Turabian StyleBushnell, Juliana Y., Lindsay N. Cates, Jeffrey E. Hyde, Christoph P. Hofstetter, Claire C. Yang, and Zin Z. Khaing. 2022. "Early Detrusor Application of Botulinum Toxin A Results in Reduced Bladder Hypertrophy and Fibrosis after Spinal Cord Injury in a Rodent Model" Toxins 14, no. 11: 777. https://doi.org/10.3390/toxins14110777




