Abstract
The paper presents the first proof of planktonic cyanoprokaryote genus Cuspidothrix as an anatoxin A (ATX) producer in Bulgarian wetlands. The results from polymerase chain reaction (PCR) obtained from two summer sampling campaigns in 26 selected lakes and reservoirs demonstrated presence of the anaC gene, responsible for ATX production in 21 strains of the genus. They were found in three waterbodies sampled in 2018 (coastal lake Vaya, coastal reservoir Poroy, inland reservoir Sinyata Reka) and in four waterbodies sampled in 2019 (inland reservoirs Duvanli, Koprinka, Plachidol 2, Sinyata Reka). The detected genetic diversity generally corresponds to the observations conducted by conventional light microscopy, by which we distinguished three species of Cuspidothrix (Cuspidothrix issatschenkoi, Cuspidothrix elenkinii and Cuspidothrix tropicalis, the latter considered alien in the country). Eleven strains showed high similarity to two sequences of C. issatschenkoi available from the National Centre for Biotechnology Information (NCBI). Ten other strains assembled in a group, which—in lack of available from NCBI genetic sequences—were presumed related to C. tropicalis and C. elenkinii after comparison with the results from light microscopy. Cuspidothrix strains found in Bulgarian waterbodies showed high genetic similarity to those isolated and sequenced from Asia (Japan, China) and Northern Europe (Norway, Finland).
Keywords:
Cyanobacteria; Cyanoprokaryota; cyanotoxins; ecosystem health; human health; phytoplankton; very fast death factor Key Contribution:
The paper presents the first genetic proof of occurrence of anatoxin A-producing algae in Bulgarian wetlands and relates all 21 obtained toxigenic strains with the cyanoprokaryotic genus Cuspidothrix. Comparison of the results obtained by PCR and light microscopy allowed us to hypothesize that besides the well-known C. issatschenkoi two more species of the genus (i.e., C. elenkinii and C. tropicalis) are potentially toxigenic in the country.
1. Introduction
Anatoxin A (ATX) and its analogues, anatoxins (ATXs), are alkaloid neurotoxins produced by more than 40 species of oxygenic photosynthetic prokaryotes from the phylum Cyanoprokaryota (blue–green algae)/Cyanobacteria) [1,2,3]. These toxigenic species occur mostly in freshwater, forming algal mats on the bottom of the waterbodies, or are floating in the water column. They were primarily referred to the following filamentous genera: Anabaena, Aphanizomenon, Blennothrix, Chrysosporum, Cuspidothrix, Cylindrospermum, Dolichospermum, Kamptonema, Lyngbya, Microcoleus, Microseira, Moorea, Nostoc, Oscillatoria, Planktothrix, Phormidium, Raphidiopsis (Syn. Cylindrospermopsis), Sphaerospermopsis and Tychonema [1,2,3,4,5,6,7,8,9,10,11], but two coccal genera, colonial Microcystis and Woronichinia, were also proved to be ATX producers [1,12,13]. The ability of cyanoprokaryotes to produce ATX is generic- and species-dependent, but one and the same species may comprise strains that are atoxic, while others can produce toxic metabolites [1,3]. Moreover, the strain specificity may contain a quantity of toxic compounds that can vary up to four orders of magnitude in different strains, thus explaining why high toxin concentrations could be achieved in nature even with low abundance from their producers (for details see [2]). In addition, almost all conducted studies indicated that presence of genes, responsible for the biochemical synthesis of certain toxins, is not sufficient for real toxin production and release in natural waters because gene expression is influenced by a variety of factors (e.g., [1]). An additional complication comes from the fact that the same species and even the same strain may contain multiple toxin operons and can produce diverse toxins from one “target” group (e.g., the neurotoxins ATX and saxitoxin) or even combinations of toxins from different groups (e.g., hepatotoxins and neurotoxins), depending on the conditions [12,13,14,15]. Therefore, assuming a given genus, species, or strain to be atoxic or toxic without proper investigation seems to be quite hazardous [13].
Physiological and toxicological research showed that ATX mimics the neurotransmitter acetylcholine, easily binds to its receptors of the boundary synapses located between nerve and muscle tissue, and stimulates them to release signals for muscle contractions in a persistent way [2,16]. Thus, ATX provokes neurotoxicoses in dogs, cattle and some wild animals, expressed in cramping, seizures and asphyxia with subsequent acute death [1,3,4,16,17,18,19,20,21,22,23,24]. The lethality can take 2–4 min in mice, and ATX was eloquently named “very fast death factor,” commonly abbreviated as VFDF [25,26,27,28]. Despite the fact that the effects for a long time were primarily related with fatal animal poisonings, ATX and its analogues (mainly homoanatoxin), may seriously impact human health through exposure to drinking water, recreational activities and seafood [2,3,29,30,31]. Consideration of the accumulated knowledge on their potential lasting implications led to the inclusion of ATXs in WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments [3]. There, the following provisional health-based reference values of “30 µg L−1 for acute or short-term exposure via drinking-water and 60 µg L−1 for recreational water exposure” for adults and of ca. “6 µg L−1 for short periods” for small children were mentioned [3] (p. 1).
Some studies have supposed that the importance of ATXs posed to human and environmental health is increasingly rising and highlighted the necessity of their consideration in risk assessment [32]. The detected worldwide spread of ATXs in surface waters has been enhanced by global warming and increasing eutrophication [10,22]. These factors are widely accepted as general driving forces for the production of diverse algal toxic compounds and for the troublesome ecological “success” of harmful algal blooms (for details see [2,13,33,34]). Despite the rising global importance of ATXs, there are regions and countries with little information on their occurrence and photosynthetic producers. One of these “white spots” on the global map is Bulgaria, a country on the Balkan Peninsula with about 9000 waterbodies, including important large drinking water reservoirs, small reservoirs used for irrigation, and recreation activities or as fisheries, but also different types of natural lakes, some of which are of global, regional, or national conservational importance [35].
During a 15-year period of investigations of toxins produced by Cyanoprokaryota (cyanotoxins) in Bulgaria, the occurrence of ATXs was reported only once [36]. They were found by application of HPLC in samples from the mountain reservoir Borovitsa, collected in July 2006 [37]. The present paper provides the first genetic evidence of the occurrence of potential ATX producers in the country. They were obtained after processing of phytoplankton samples collected during two sampling campaigns (summers of 2018 and 2019) from 26 selected according to their intensive use and importance for human health waterbodies in Central and Eastern Bulgaria. By polymerase chain reaction (PCR), 21 planktic strains related to the genus Cuspidothrix, which contained the specific ATX synthetase anaC gene, were identified in samples from the largest coastal lake, Vaya (2018), in the coastal reservoir Poroy (2018), in the large inland reservoir Koprinka (2019), as well as in the small inland reservoirs Duvanli (2019), Plachidol 2 (2019) and Sinyata Reka (2018, 2019). The comparison of morphological data, obtained by conventional light microscopy (LM) with the genetic sequences allowed us to extend the information on the distribution of species previously recorded in the country: Cuspidothrix issatschenkoi (Usachev) P. Rajaniemi, Komárek, R. Willame, P. Hrouzek, K. Kastovská, L. Hoffmann et K. Sivonen and C. elenkinii (Kisselev) P. Rajaniemi, J. Komárek, R. Willame, P. Hrouzek, K. Kastovská, L. Hoffmann et K. Sivonen. In addition, we provide data on the spread of the new (for Bulgaria) tropical species, C. tropicalis (Horecká et Komárek) Rajaniemi P., J. Komárek, L. Hoffmann, P. Hrouzek, K. Kastovská et K. Sivonen, and hypothesize its alien character.
2. Results
2.1. Results from Light Microscopy Observations
Conventional LM investigations of the phytoplankton samples collected in 2018 revealed the presence of 85 species of Cyanoprokaryota in all investigated waterbodies. Out of them, 58 cyanoprokaryotes were identified in the three waterbodies, in which strains with anaC genes were detected by PCR: 53 in Vaya, 8 in Poroy and 7 in Sinyata Reka. Among all determined cyanoprokaryotes, 39 species were from the following 10 genera, known as ATX producers: Anabaena, Aphanizomenon, Chrysosporum, Cuspidothrix, Dolichospermum, Microcystis, Oscillatoria, Phormidium, Planktothrix and Raphidiopsis. In accordance with the obtained PCR data, we report only results concerning findings on Cuspidothrix.
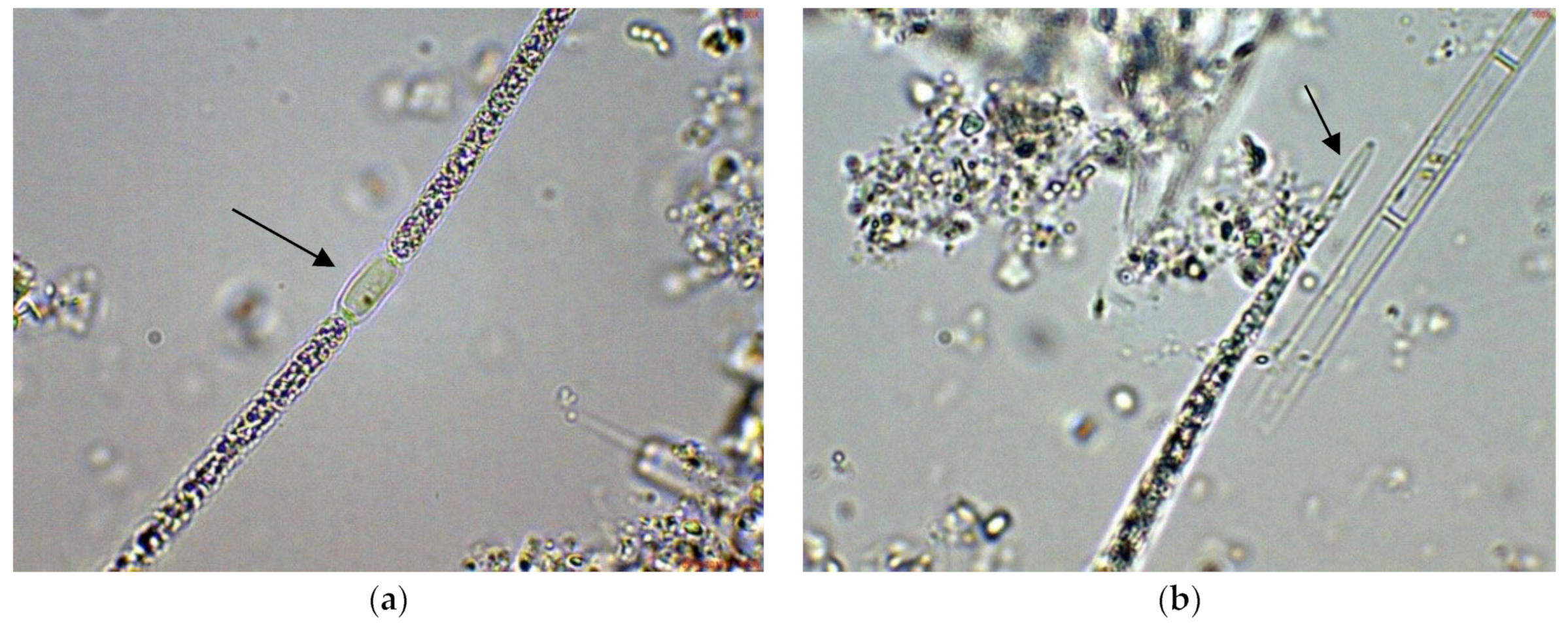
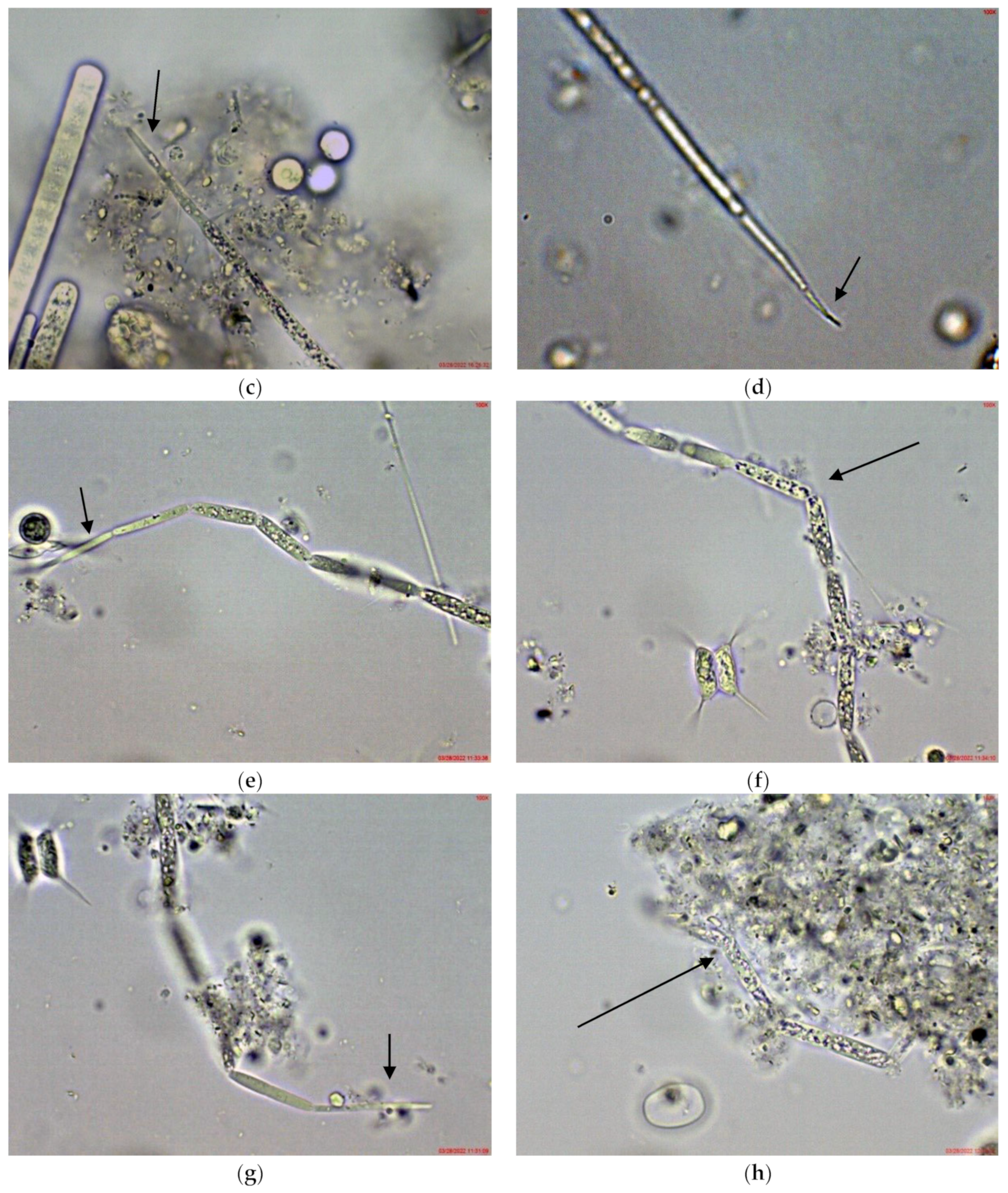
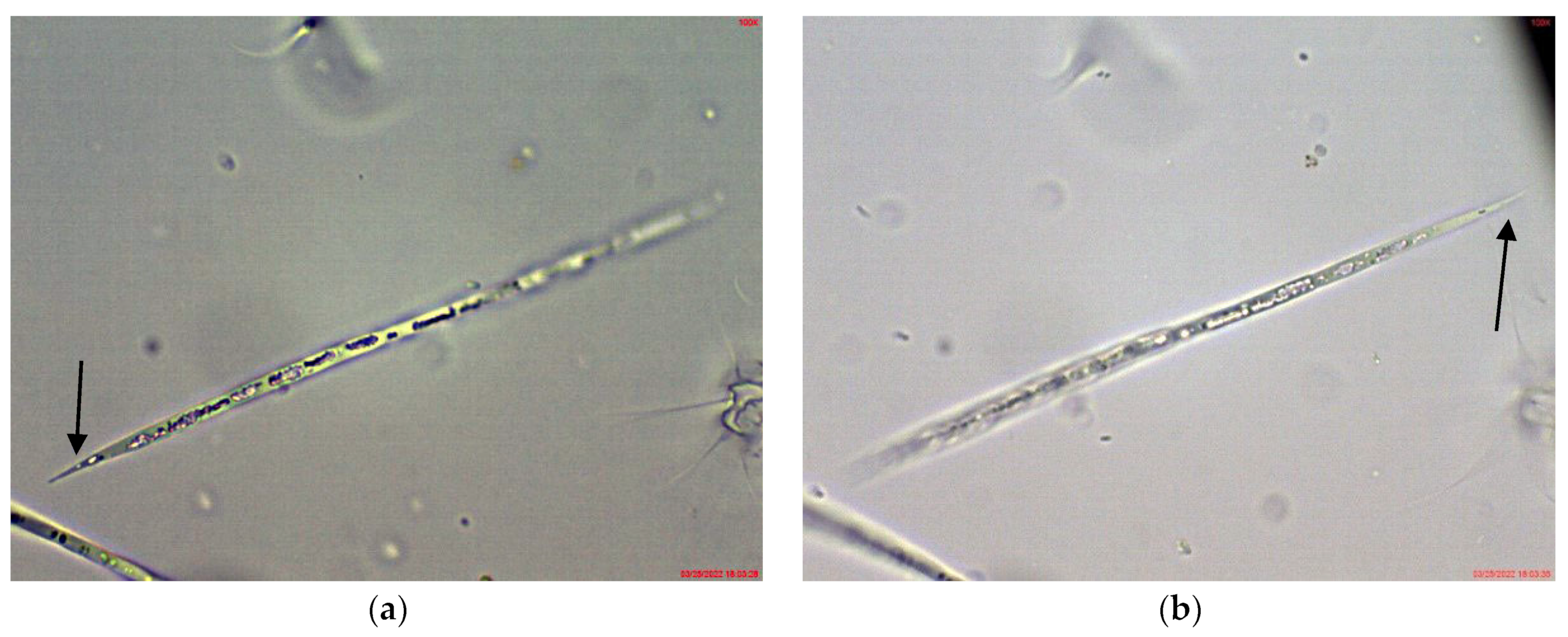
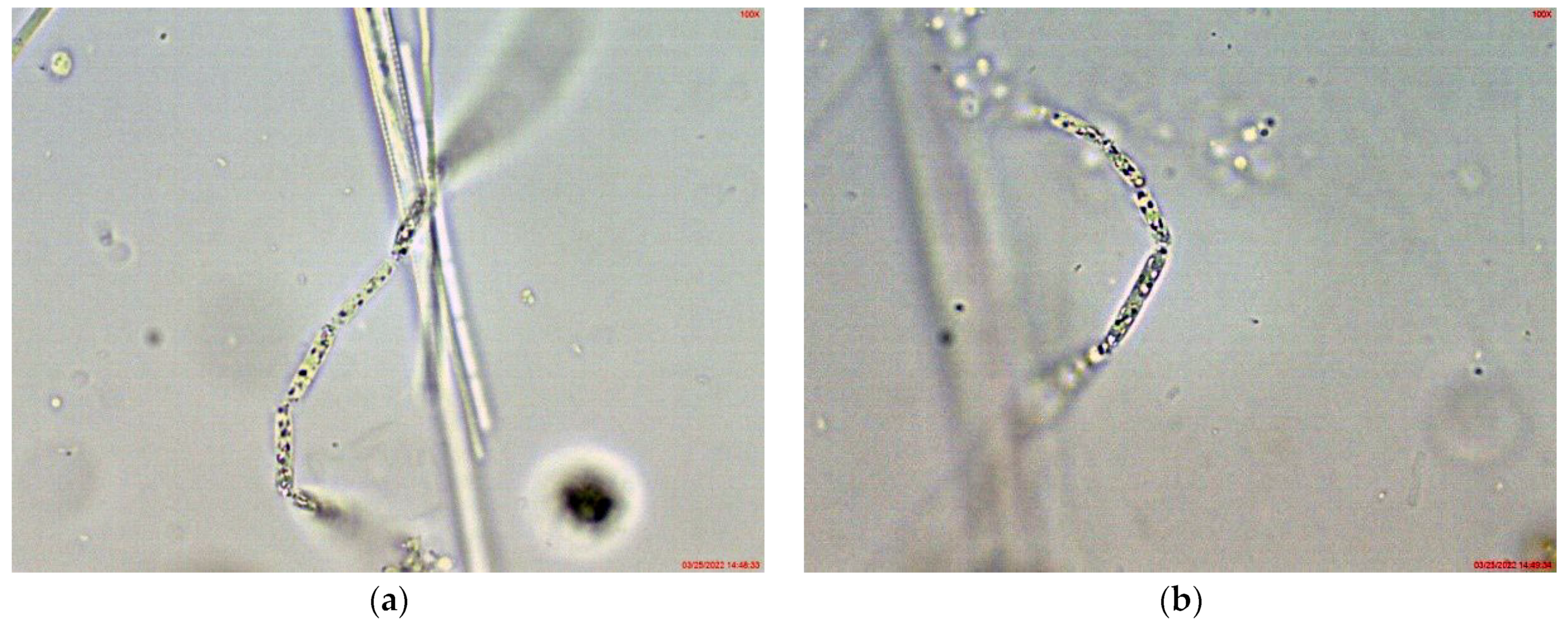
In the large, coastal Lake Vaya, specimens of Cuspidothrix were found in all studied sites, but in different quantities. In both sites 1 and 2, in samples collected from the western part of the lake, C. issatschenkoi was the most abundant, observed in different stages of development, often with heterocytes (Figure 1a) and non-matured akinetes. The morphological variation concerned mainly the length of apical cells, while the width of trichomes varied slightly from 2 to 3 (4) µm (Figure 1b–d). Some trichomes were young, non-heterocytous and sterile (i.e., without akinetes). Therefore, they could be only tentatively determined to species level, mainly on the basis of apical cells peculiar to each species. In addition, many trichomes were seen as vegetatively reproducing by disintegration in the typical for the genus “zig-zag” way (Figure 1e–g). Some fragments of disintegrating trichomes were also found in the samples (Figure 1h).
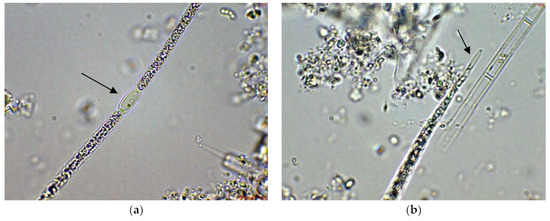
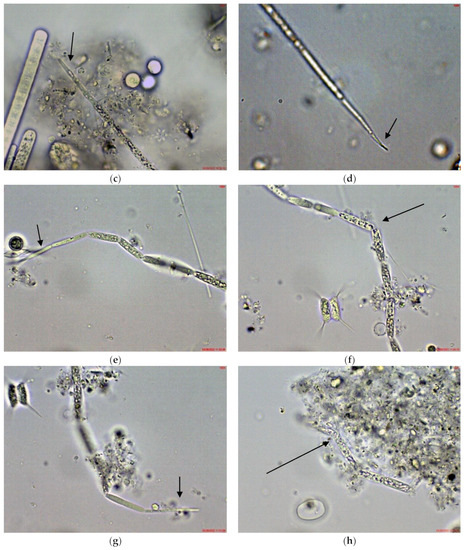
Figure 1.
Examples of Cuspidothrix from the large coastal lake Vaya (western part, sites 1 and 2; 2018): (a) Part of a trichome with a well-developed intercalar heterocyte (arrow); (b–d) Cuspidothrix issatschenkoi—apical parts of three trichomes with typical vegetative cylindrical cells, not constricted at cell walls and differently developed apical cells (arrows); (e–g) young trichome with typical terminal cells (arrows on (e,g)) in a process of disintegration (arrow on (f)); (h) fragment of Cuspidothrix trichome reproducing through disintegration (arrow).
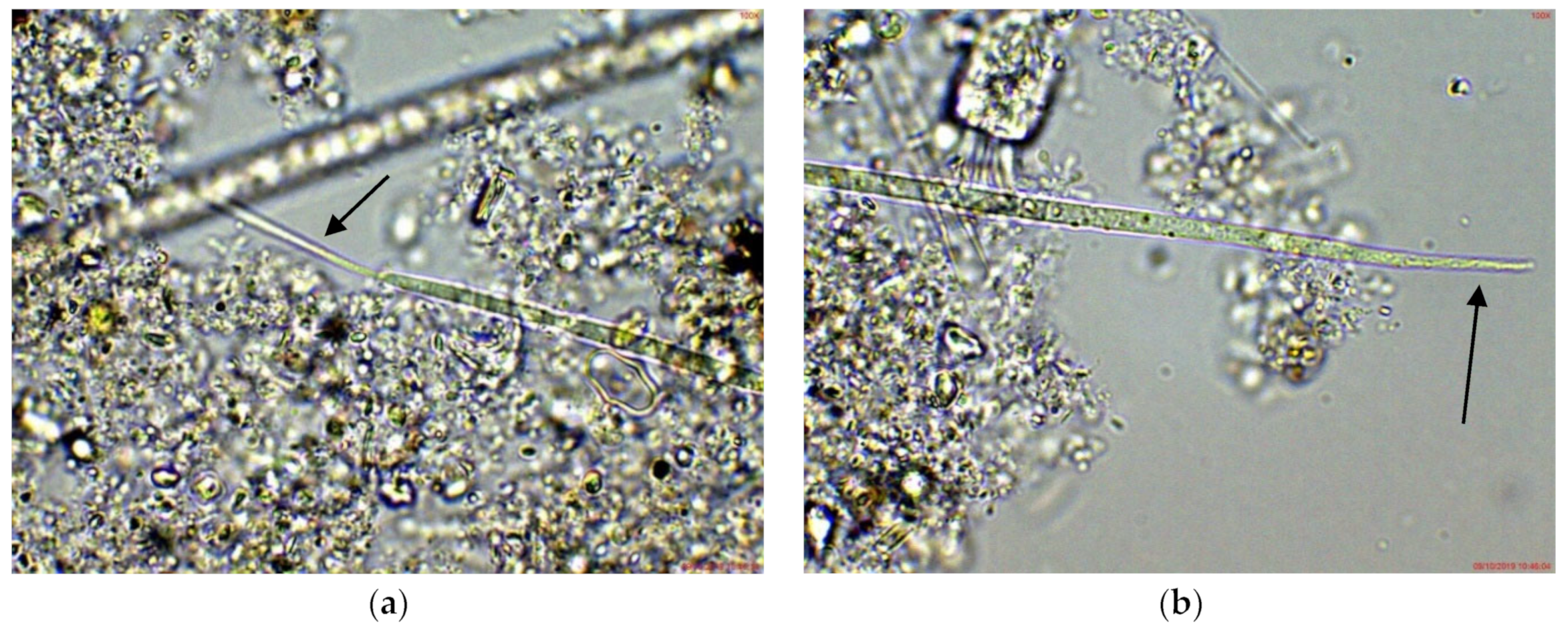
In the eastern part of the lake (site 3), Cuspidothrix was also observed, but mainly in young stages without heterocytes or as disintegrating fragments. However, the morphological diversity in this site was higher: according to the apical cells, most of the specimens resembled C. issatschenkoi, several looked similar to C. tropicalis, and a few had the peculiar thin, “thread-like” cells of C. elenkinii in at least at one of the poles (Figure 2), thus being sub-symmetric in the terminology of Komárek [38].
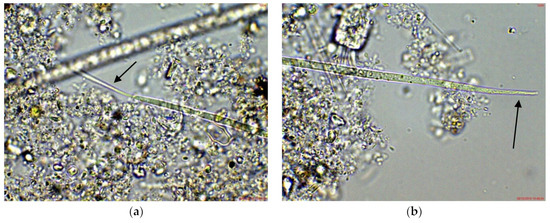
Figure 2.
Example of Cuspidothrix from the large coastal lake—Vaya (eastern part, site 3; 2018): (a,b) both poles (arrows) of a young, sub-symmetric trichome of C. elenkinii.
In the small coastal reservoir Poroy, situated at a distance of about 30 km from Lake Vaya, Cuspidothrix was relatively rare, but was also found in different stages, mainly as young, undeveloped trichomes without heterocytes. Some of them were without aerotopes (groups of gas vesicles [38]) in the cells. Very young symmetric trichomes with pointed elongated ends looked quite similar to some young trichomes of the phylogenetically related genus Raphidiopsis [38]. Its species R. raciborskii (Wołoszyńska) Aguilera A., Berrendero Gómez E., Kaštovský J., Echenique R.O. et Salerno G.L. in the same period was abundant in Poroy [39]. According to the shape of the apical cells, some better developed individuals looked like Cuspidothrix issatschenkoi and some were close to C. tropicalis (Figure 3). However, in the absence of akinetes their identification was only tentative. All observed fragments of disintegrating trichomes strongly resembled the “zig-zag” ones recorded by us in Vaya, where C. issatschenkoi was abundant.
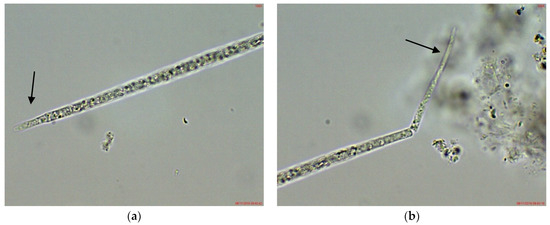
Figure 3.
Example of Cuspidothrix from the small coastal reservoir Poroy (2018): (a,b) Both poles (arrows) of a young, sub-symmetric trichome of C. tropicalis.
In the small inland reservoir Sinyata Reka, Microcystis wesenbergii (Komárek) Komárek ex Komárek dominated [40] and all other cyanoprokaryotes were quite rare. After obtaining the PCR results and additional processing of more than 10 slides from the concentrated sample, we managed to observe only several young trichomes without akinetes, similar to Cuspidothrix. Some of them had better-developed apical cells, which resembled C. tropicalis, and a few had very elongated apical cells, documented earlier for C. issatschenkoi (e.g., for its strain ORE27S2—Figure 9e in [41]) and recorded by us in some trichomes from Vaya (Figure 1c).
LM investigations of the summer phytoplankton samples collected in 2019 revealed a total of 128 species of Cyanoprokaryota. Out of them, 35 species occurred in the four reservoirs, from which strains containing ATX synthetase genes were recorded: 23 in Duvanli, 4 in Koprinka, 16 in Plachidol 2 and 6 in Sinyata Reka. Among all determined cyanoprokaryotes, 60 species belonged to the following 12 genera known as ATX-producers: Anabaena, Aphanizomenon, Chrysosporum, Cuspidothrix, Dolichospermum, Microcystis, Oscillatoria, Phormidium, Planktothrix, Raphidiopsis, Sphaerospermopsis and Woronichinia. As for 2018, below are discussed only results concerning findings of Cuspidothrix.
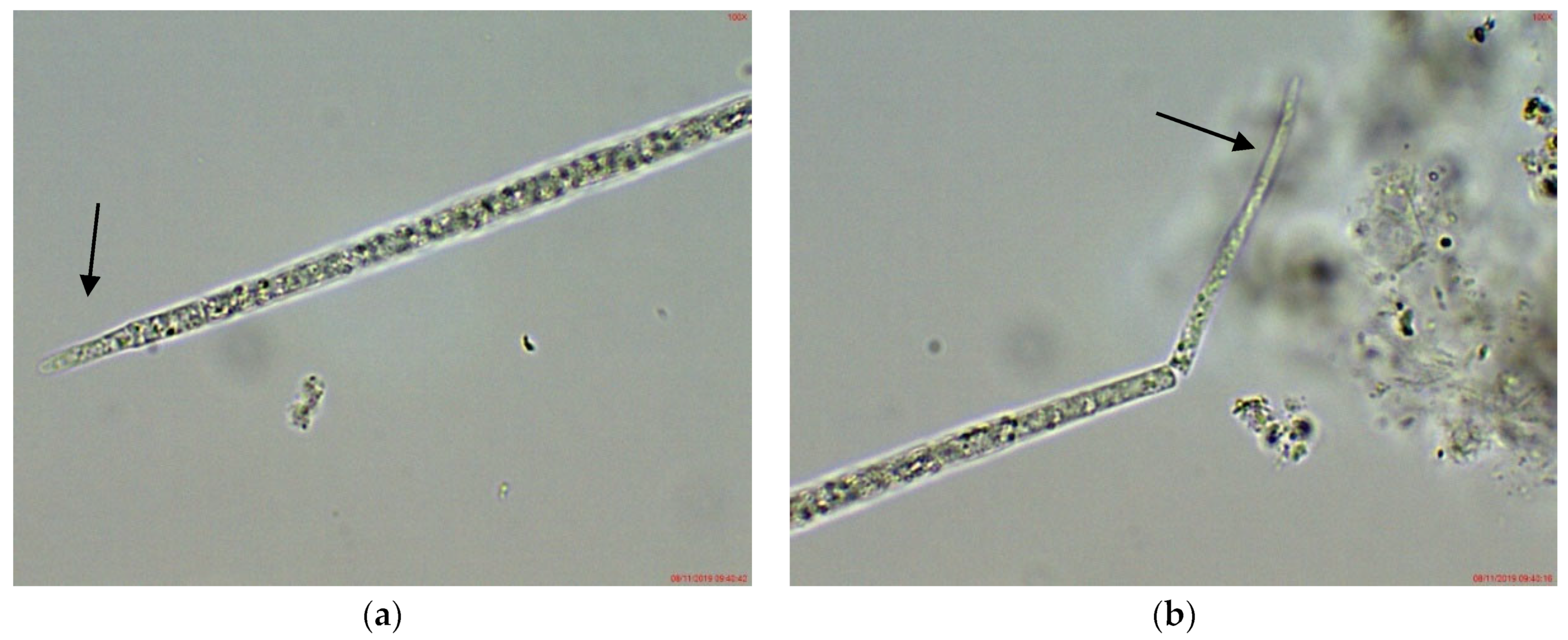
In the phytoplankton of the small inland reservoir Plachidol 2, dominated by Anabaena sp. ster., young symmetric trichomes with elongated, pointed apical cells and well developed aerotopes were rarely observed (Figure 4). They strongly resembled the young non-heterocytous trichomes of the genus Raphidiopsis, and of its species R. mediterranea Skuja, in particular. Due to lack of heterocytes and akinetes in all of them, their species identification by LM was impossible.
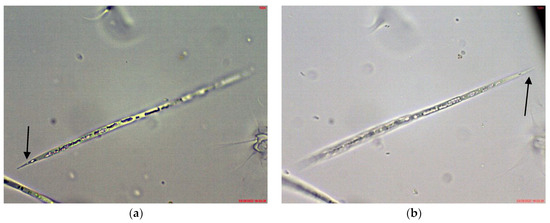
Figure 4.
Example of Cuspidothrix from the small inland reservoir of Plachidol 2 (2019): (a,b) Both poles (arrows) of a young, symmetric, pointed trichome, comprised of cells containing aerotopes.
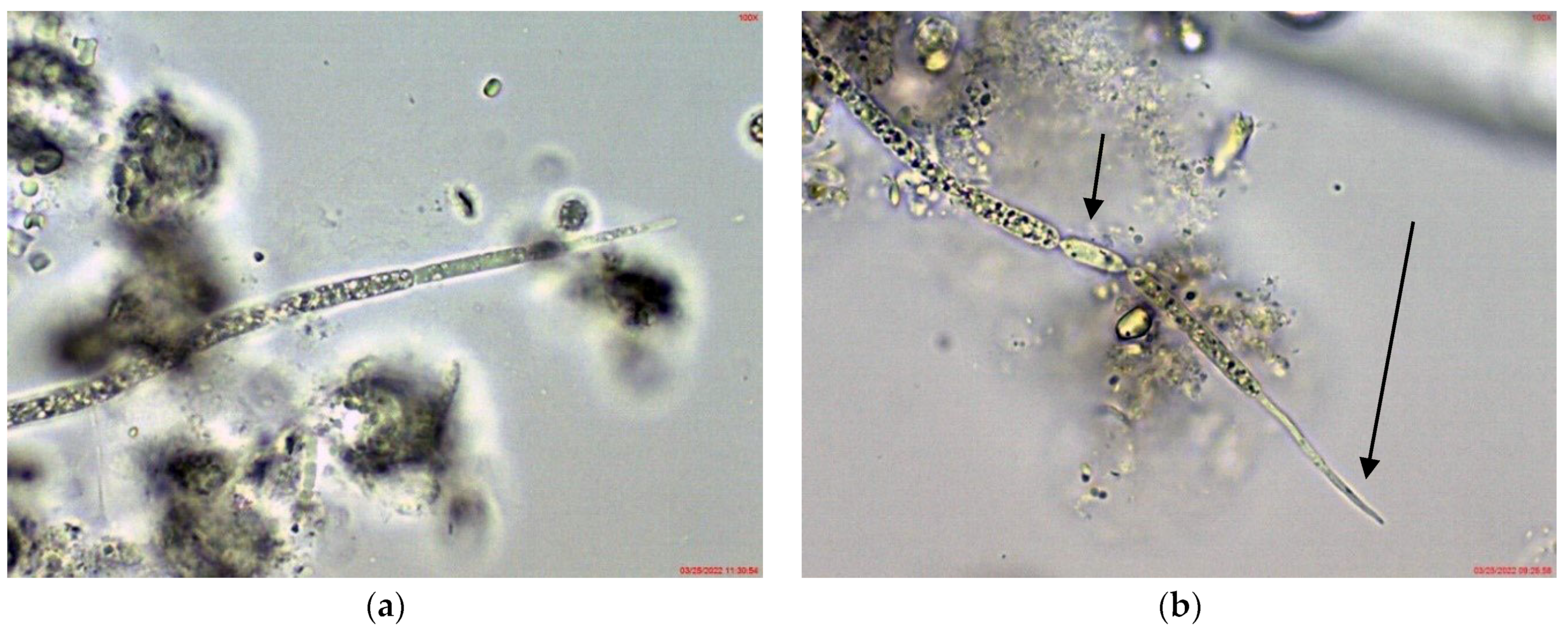
Cuspidothrix was extremely rarely observed in the microscopic slides, processed from the small inland reservoir Duvanli, the phytoplankton of which at that time was polydominated by Microcystis sp. div., Pseudanabaena limnetica (Lemmermann) Komárek and Romeria simplex (Hindák) Hindák [42]. There, we found only several disintegrating trichomes, with typical “zig-zag” outfit of Cuspidothrix (Figure 5). Although in this stage reliable species identification was impossible, due to the identical morphology with the disintegrating trichomes found in the western part of Vaya (sites 1 and 2, 2018; Figure 1h) where C. issatchenkoi was abundant, we supposed that specimens from Duvanli may also belong to this species.
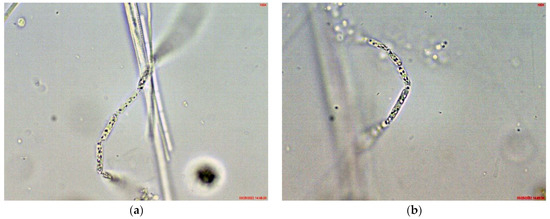
Figure 5.
Examples of Cuspidothrix from the small inland reservoir of Duvanli (2019): (a,b) Fragments of disintegrating sterile trichomes.
Cuspidothrix was rarely found in the phytoplankton of the small inland reservoir Sinyata Reka, in which at that time Sphaerospermopsis torques-reginae (Komárek) Werner, Laughinghouse IV, Fiore et Sant’Anna dominated and Microcystis wesenbergii was relatively abundant [43]. The several observed specimens were without akinetes and rarely had heterocytes, but according to the typical apical cells resembled C. tropicalis (Figure 6a).
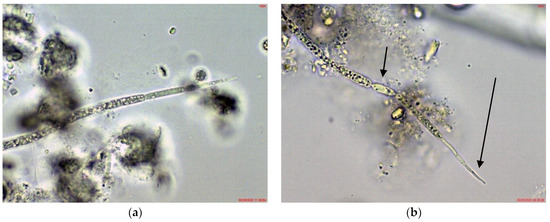
Figure 6.
Example of Cuspidothrix from the small inland reservoir of Sinyata Reka and large inland reservoir Koprinka (2019): (a) apical part of the trichome of Cuspidothrix tropicalis from Sinyata Reka; (b) apical part of the trichome of Cuspidothrix elenkinii with well-developed apical cell (long arrow) and typical intercalar heterocyte (short arrow) from Koprinka.
Cuspidothrix was a rare phytoplankter in the large inland reservoir Koprinka, where cryptophytes and diatoms dominated [42]. There, all observed trichomes of this genus had well-developed heterocytes and the peculiar “thread-like” apical cells typical of C. elenkinii (Figure 6b).
2.2. Results from Genetic-Molecular Studies
Considering that ATX is constructed through the polyketide synthase pathway (PKS) by a cluster of at least eight ana genes [6,44], for the identification of different ATX producers in the environmental phytoplankton samples, we applied a general primer pair, designed for amplification of the specific anaC genes [5]. Resulting PCR signals were recorded in seven of the samples. They were most intensive in the samples from Lake Vaya and Sinyata Reka, collected in 2018, and from Koprinka, collected in 2019, whereas weak signals were registered in the samples from Poroy, collected in 2018, and from Duvanli, Plachidol 2 and Sinyata Reka, collected in 2019. By processing of these materials, 21 sequences representing 21 strains were identified. They were compared with genotypes available from the National Centre for Biotechnology Information (NCBI; Bethesda, MD USA) [45]. The phylogenetic analysis and the first constructed phylogenetic tree showed that all 21 strains sequenced during this study were clearly separated from the outgroup, composed of three “foreign” strains, observed by BLAST [46] analysis: Oscillatoria sp. PCC 10601 (JF803652; orig. Finland) and Cylindrospermum stagnale PCC7417 (CP003642.1; orig. USA), clearly distant from Anabaena sp. WA102 (CP011456.1; orig. USA: Anderson Lake). Because of the low homology (<88%), this outgroup is not shown on the part of the phylogenetic tree in Figure 7.
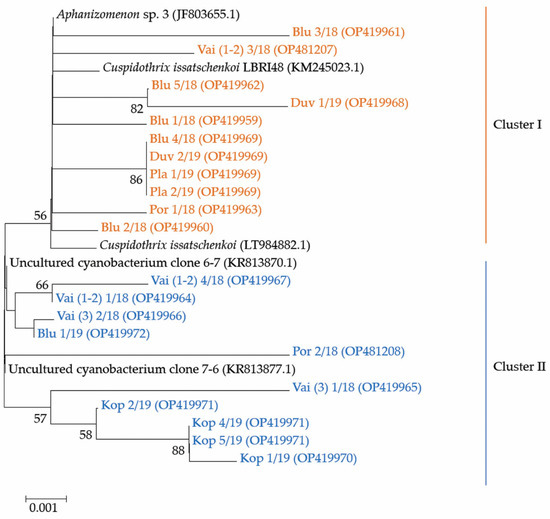
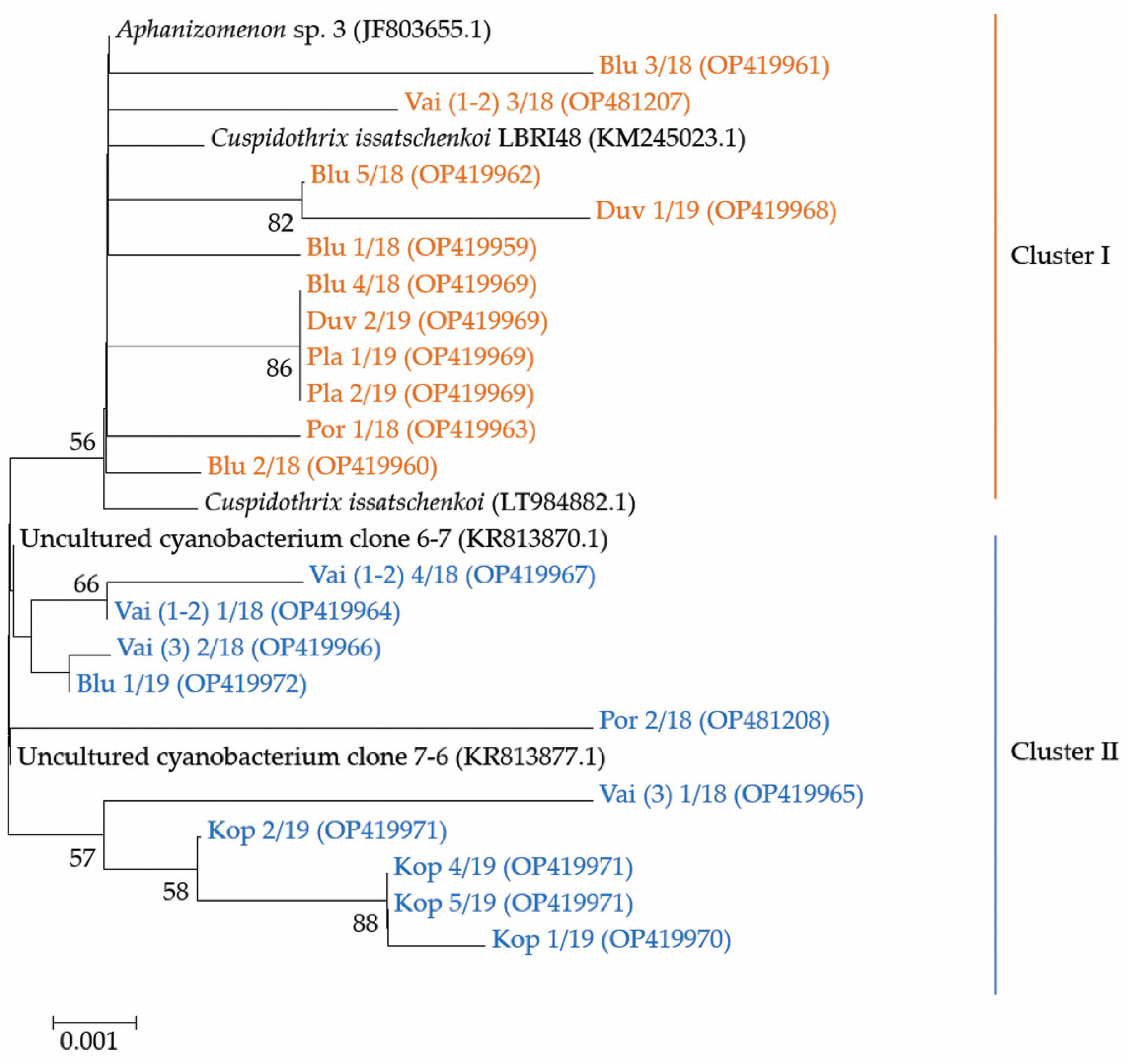
Figure 7.
Neighbor-joining phylogenetic tree obtained after processing of phytoplankton samples from the summer phytoplankton of 6 Bulgarian waterbodies. The tree is constructed using 21 newly obtained nucleotides sequences (OP419959-OP419972, OP481207, OP481208) and closest sequences retrieved after BLAST [46] search in NCBI database [45] with indication of their original name, year of sampling and the relevant accession number in NCBI [45]. For the identical sequences (IS) only one accession number is provided: (i) The IS Duv 2/19, Blu4/18, Pla1/19, Pla 2/19 are with accession number OP419969; (ii) The IS Kop 4/19 and Kop 5/19, as well as the shorter sequence Kop 2/19 have accession number OP419971. Bootstrap values are shown at branch points (percentage of 1000 resamplings). Legend: Blu—Reservoir Sinyata Reka (=Blue River); Duv—Reservoir Duvanli; Kop—Reservoir Koprinka; Pla—Reservoir Plachidol 2; Por—Reservoir Poroy; Vai—Lake Vaya. For more details see the text of the paper.
The phylogenetic analysis of the 21 sequences obtained by us revealed their grouping in two clusters (Figure 7). The first cluster contained eleven strains: nine were from the inland reservoirs Duvanli, Plachidol 2, and Sinyata Reka, and two strains were from the coastal reservoir Poroy and Lake Vaya. It is worthy to note that all strains, sequenced from Plachidol 2 and Duvanli, were only in this cluster, which comprised strains that showed high homology (>99%) with the available sequences of the identified species Cuspidothrix issatschenkoi. Seven of the eleven strains in this cluster were affiliated with C. issatschenkoi (LT984882.1) isolated from Norway, three were more similar to C. issatschenkoi LBRI48 (KM245023.1) from Lake Biwa (Japan), and only one strain (i.e., Blu 3/18, OP419961) could be tentatively related to the genus Aphanizomenon (JF803655.1) from Lake Sääskjärvi (Finland).
The second cluster contained 10 strains, sequenced from samples collected from the inland reservoirs Koprinka and Sinyata Reka, as well as from the coastal reservoir Poroy and Lake Vaya (Figure 7). These strains showed relatively high homology with two uncultured cyanobacterial strains, viz. clone 6-7 (KR813870.1) and clone 7-6 (KR813877.1) isolated from Dainchi Lake (China). Four of the strains were closer to clone 6-7 (KR813870.1) and six were affiliated with clone 7-6 (KR813877.1). Here, we would like to underline that all four sequences obtained from Koprinka were grouped only in this second cluster near clone 7-6 (KR813877.1).
3. Discussion
According to microscopic analysis by conventional LM of the phytoplankton samples from all 26 investigated Bulgarian lakes and reservoirs, 129 species of Cyanoprokaryota were determined. Out of them, 62 species (39 in 2018 and 60 in 2019), according to the literature, could be suspected as ATX producers. However, by application of genetic-molecular studies, we proved the presence of potential ATX producers only in seven samples, collected from three waterbodies in 2018 (Vaya, Poroy and Sinyata Reka) and from four waterbodies in 2019 (Duvanli, Koprinka, Plachidol 2, and Sinyata Reka). Only in one of them (Sinyata Reka) were ATX producers found in both sampling years, but with variance in toxigenic strains.
The differences in the occurrence of ATX producers in 2018 and 2019 could be explained by: (1) the dissimilarity in the environmental conditions during both campaigns. For example, the sampling in August 2019 was preceded by strong rains atypical for the country, which started in May and caused dilution of waters with decreased nutrient content and less pronounced algal blooms. By contrast, before the sampling campaign in June 2018, the weather was extremely hot and dry, which enhanced algal blooms (for details see [43]); (2) the shallow character of most Bulgarian waterbodies, in which frequent disturbances lead to a great algal diversity and rapid changes of dominants (e.g., [35,43]).
Regarding genetic diversity of the 21 toxigenic strains, identified in this study, and their coexistence, it is possible to state that highest diversity (five strains obtained in 2018) was detected in Lake Vaya, which is an important from conservational point of view waterfowl site on the bird migration route Via Pontica [35,47]. Similar diversity was recorded in the small inland strongly eutrophicated reserve Siniyata Reka, from which five strains were isolated in 2018 (but only one in 2019). According to the number of co-existing strains, the other waterbodies could be arranged in the following order: Koprinka (four strains), followed by Duvanli, Plachidol 2 and Poroy, with two strains isolated from each of them.
The results obtained by the applied PCR method revealed that algae containing the anaC gene belonged to the genera Cuspidothrix and Aphanizomenon. However, in 2005, based on genetic data, the genus Cuspidothrix was derived from the genus Aphanizomenon (with C. issatschenkoi formerly being known as A. issatschenkoi Kisselev—for details see [38,41]). Therefore, with a high level of probability it was supposed that the single sequence of unidentified species of Aphanizomenon, which appeared from BLAST [46] analysis nearby the sequences of C. issatschenkoi, in fact belonged to Cuspidothrix. Thus, we presumed that all potentially toxic cyanoprokaryote strains genetically identified in our study represented only the genus Cuspidothrix, three different species of which (i.e., C. issatschenkoi, C. elenkinii and C. tropicalis) were distinguished by LM in the relevant investigated samples.
Except in these samples, Cuspidothrix was discovered by LM also in the June 2018 phytoplankton of coastal reservoir Mandra (which together with Lake Vaya comprises a part of the large group of so-called Burgas lakes [35]), where it was rarely represented by single trichomes of C. issatschenkoi, and in the coastal lake Durankulak, where C. tropicalis occurred, forming <5% of the total biomass (for details see [39]). The lack of PCR signal for anaC genes in all samples from these two waterbodies, allows to suppose the atoxic character of their strains, or presence of other ana genes, which could not be covered by the used set of primers.
In general, the diazotrophic genus Cuspidothrix, which commonly bears specific heterocytes that contain nitrogenase (which is considered to be the responsible enzyme for the assimilation of gaseous atmospheric nitrogen (nitrogen fixation) [38]), was not commonly found in the studied waterbodies. Its rare occurrence and unequal distribution coincided with the relatively high content of TN detected during both sampling campaigns and is in accordance with the well-known decrease of the amount and diversity of heterocytous algae in rich in nutrient-eutrophicated waters (e.g., [48,49,50,51]). Since the facultative lack of heterocytes in environments rich in nitrogen has been documented [38] and toxigenic abilities of such non-heterocytous strains have been reported regarding ATX production [1,52,53,54], it is worthy to recall that in this study we found mainly non-heterocytous trichomes of Cuspidothrix. The only exception was the large reservoir Koprinka, in which TN was in low concentrations, and all observed specimens had well developed heterocytes (Figure 6b). Then, the finding of strains with toxigenic anaC gene in 16% of the investigated samples can be taken as an alert for potentially larger spread of ATXs in Bulgarian waterbodies, and especially in eutrophicated ones. There, the availability of nutrients, and the high nitrogen content in particular, may promote the production of ATXs, which contain nitrogen in their molecules [2,10,55,56].
Comparison of results achieved after application of LM and PCR methods, showed their general conformity. All genetic and morphological data pointed on the heterogeneity of Cuspidothrix in Bulgarian waterbodies, which once more demonstrates the great genetic diversity of cyanoprokaryote strains, highlighted in our previous works [39,40,43,57]. Considering biogeographical data on the origin of NCBI [45] strains affiliated with our material (Japan, China, Norway and Finland) the results from this study are in accordance with earlier conclusions on genetic similarity of European and Asian strains of the genus Cuspidothrix (e.g., [58]).
During the LM work, we found problems to distinguish reliably between young, non-heterocytous trichomes of Cuspidothrix and Raphidiopsis (preliminary R. mediterranea, but also R. raciborskii) especially in the reservoirs Poroy and Plachidol 2. Similar confusion between both genera was reported earlier and some subsequent genetic studies confirmed that strains, looking like R. mediterranea and its variety grandis, in particular, belonged to Cuspidothrix (e.g., [53,54,56,59,60]). On the other hand, we have to consider the long-lasting discussions on misidentifications and the validity of the genus Raphidiopsis (for details see [61,62]) and the fact that R. mediterranea is still a taxonomically accepted unit [63], which in the opinion of Moustaka-Gouni et al. [64] is clearly morphologically and genetically distinguishable from C. issatschenkoi. Therefore, we would like to highlight the need for further comparative LM and molecular-genetic studies of all species of Raphidiopsis and its phylogenetic relations with Cuspidothrix. At present, taking into account the obtained data on the spread of anaC genes in the sampled waterbodies, we accepted that at least a part of observed by LM trichomes in the reservoirs Poroy and Plachidol 2 belonged to Cuspidothrix. Moreover, on the constructed phylogenetic tree all four toxigenic strains from Poroy and Plachidol 2 were affiliated to C. issatschenkoi (Figure 7). It is the best studied and the most widely distributed species of the genus Cuspidothrix (e.g., [38,63]).
Beside these four strains, seven other strains sequenced from Duvanli, Sinyata Reka and Vaya, also fitted to C. issatschenkoi. This species was recorded by LM in the same waterbodies and its morphological variation, observed by LM, generally corresponded to the detected genetic diversity of strains. Here we would like to note particularly the case of disintegrating trichomes, found in Duvanli, Poroy and Vaya, which on the basis of their morphological similarity in LM, were presumed as belonging to C. issatschenkoi. In accordance with this conclusion, the strains sequenced from these three waterbodies, grouped in the same subcluster of the phylogenetic tree, showing high similarity to C. issatschenkoi (Figure 7). We would like to mention also that frequent disintegration of trichomes was recorded during the LM study of this species from Nakdong River of South Korea [60].
Ten strains obtained during this study from Koprinka, Poroy, Sinyata Reka and Vaya, grouped tightly with uncultured cyanobacterium clone 6-7 (KR813870.1) and uncultured cyanobacterium clone 7-6 (KR813877.1). In the same waterbodies, by LM we distinguished C. elenkinii and C. tropicalis, for which, according to our best knowledge, no sequences are available from NCBI [45].
At present, taking into account all abovementioned considerations from comparison of our LM data and clear groups of Cuspidothrix strains identified by PCR method, and following the recent taxonomy of Cuspidothrix with five accepted species names [63], we concluded that at least three species, i.e., C. issatschenkoi, C. elenkinii and C. tropicalis, were potential ATX producers in the studied Bulgarian lakes and reservoirs. In respect to their distribution in Bulgaria, we would like to note that:
- C. issatschenkoi has already been recorded by LM from “standing and running waters in Sofia region,” in the lakes Vaya, Durankulak and Sreburna, as well as in the reservoirs Burzina, Mandra and Poroy [39,65,66,67,68,69]);
- C. elenkinii has been reported from the reservoirs Aheloy, Antimovo, Boyka, Devets, Dubnika, Koprinka, Ovchi Kladenets, Poroy, Rabisha, Rasovo, Telish, Vulchovets, and Yastrebino [70], but was not found in a subsequent study of the phytoplankton dynamics of the reservoir Koprinka [71];
- Our first finding of C. tropicalis in Bulgaria in June 2018 (in Vaya, Durankulak and Poroy) was already documented by its inclusion in the list of potential producers of another important cyanotoxin—cylindrospermopsin [39]. However, there we had not published more detailed LM and the supporting genetic data, which we provide in this paper, extending information on its occurrence by data from samplings conducted in 2019. In addition, here, based on our long expertise in phytoplankton studies in Bulgarian waterbodies, we would like to underline the presumed alien character of this tropical species. This statement is strongly supported by the fact that it was observed mainly in coastal waterbodies along the Black Sea, which provide resting and nesting sites on the important bird migration route Via Pontica, used by waterfowl and other species flying back from tropical Africa [47].
In conclusion, the present work provides the first genetically proved data on the occurrence and distribution of the potentially toxic strains of cyanoprokaryote genus Cuspidothrix in Bulgaria, demonstrating presence of ATX synthetase gene anaC in 21 strains, spread in five reservoirs and one natural lake of the country. Considering that all cyanotoxins are of rising global concern [72,73], pose serious threat to the vulnerable Bulgarian waterbodies [35,74] influencing both environmental and human health strongly related with national security [75], further exploration is motivated to clarify the current spread and toxigenicity of cyanoprokaryotes in the whole country. Therefore, future enlargement of the monitoring of the waterbodies in the country combined with chemical detection of ATX and ATXs is strongly needed. The results may provide further insights to the biogeography of globally important ATX producers, but it has to be stressed that such comprehensive information is crucially important for achieving reliable risk assessment of Bulgarian waterbodies and for taking of adequate measures in a timely manner.
4. Materials and Methods
4.1. Studied Sites and Field Sampling
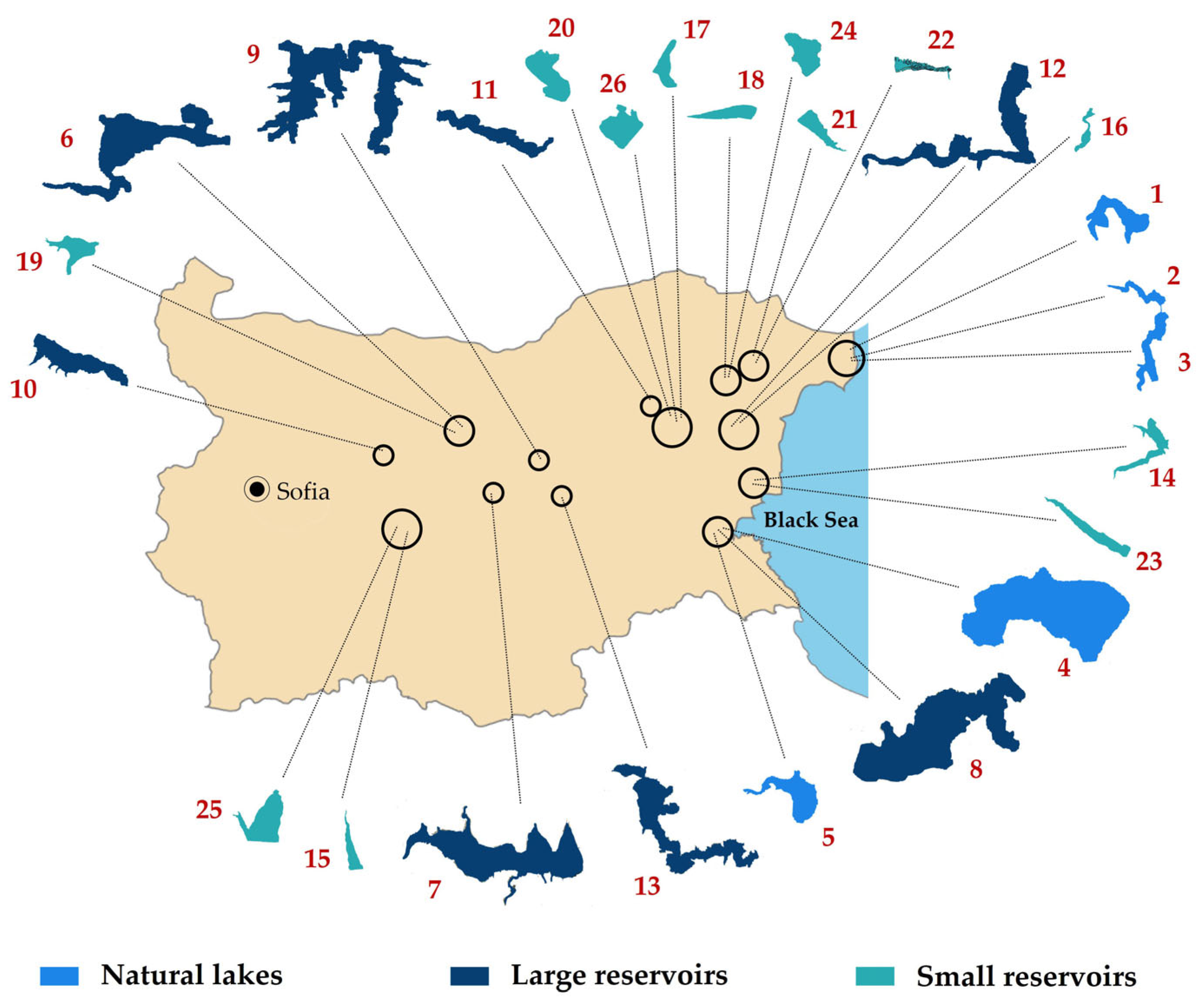
The study was based on 44 processed surface phytoplankton samples from 26 selected waterbodies in Central and Eastern Bulgaria. Sampling campaigns were conducted in June 2018 (17 sites of nine waterbodies) and in August 2019 (27 sites of 25 waterbodies)—Table 1, Figure 8. Detailed hydro-morphological data and descriptions with notes on their use and conservational status are available from the Inventory of Bulgarian wetlands [35] and, therefore, the identification number from inventory’s database (IBWXXXX) is provided in Table 1.

Table 1.
Sampling sites in Bulgarian waterbodies and their environmental parameters during both sampling campaigns of June 2018 and August 2019. First are enlisted the natural lakes, followed by the large reservoirs, and small reservoirs, all organized in alphabetical order. Legend: WBN—name of the water body; IBW—number in the Inventory of Bulgarian Wetlands [35]; E—Eastern part of the waterbody, W—western part of the waterbody (the nearest site from the former year is additionally labelled with one of these letters); Res—reservoir; L—lake; Site—number or name of given site, only in case of more than one sampled sites; Alt—altitude above the sea level (m); WT—water temperature (°C), SD—Secchi disk Secchi disk transparency (m); TTB—total transparent to bottom; CN—conductivity (S m−1); TDS—total dissolved solids (µg L−1); DO—oxygen concentration (mg L−1); TP—total phosphorus (mg L−1); TN—total nitrogen (mg L−1).
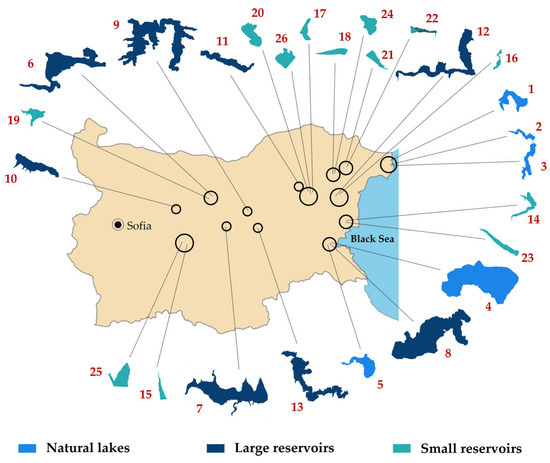
Figure 8.
Map of Bulgaria with indication of the locations and shapes of all sampled 26 waterbodies. Numbers follow those in Table 1. For more details see the text of the paper.
Due to the specific aim to search for algal blooms, in both years sampling was preceded by sending of a drone with a camera (DJI Mavic Pro, Model M1P GL200A; SZ DJI Technology, Shenzhen, Guangdong, China, in 2018, and DJI Mavic 2 Enterprise Dual; Shenzhen, Guangdong, China, in 2019). The observations of the aquatories of the visited wetlands aimed to discover and document the visible accumulations of cyanoprokaryotes (blooming areas or spots) distinguished by changes in the water color [34]. Subsequently, the chosen sites were reached by inflatable boats. In cases when algal accumulations were not visible, samples were collected at sites, visited in our previous studies. Geographical and physical parameters, such as altitude, coordinates, water temperature, water pH, water hardness (TDS), oxygen content (DO), and conductivity, were measured in situ with Aquameter AM-200 and Aquaprobe AP-2000 from Aquaread water monitoring instruments, 2012 Aquaread Ltd. (Broadstairs, UK), whereas the transparency was estimated according to Secchi disk data (Table 1). The main nutrients, total nitrogen (TN) and total phosphorus (TP) were measured ex situ using an Aqualytic AL410 photometer from Aqualytic® (Dortmund, Germany) at the first possibility after collection (Table 1).
At each site, phytoplankton samples were collected for genetic and molecular studies (viz. PCR) and for taxonomic identification by LM. All samples were taken from the surface layer (0–20 cm) in a volume of 0.5 or 1 L, depending on the water color intensity, with increasing volume at brighter color of the water. The samples for algal determination and enumeration were fixed with 2–4% formalin at the site and transported to the lab, where they were concentrated by sedimentation, lasting minimum 48 h and further processed by LM. The samples for PCR were filtered ex situ at the first possibility after collection, and the obtained filters, kept in sterile plastic tubes, were transported to the lab in a dry ice.
4.2. Microscopic (LM) Processing of the Phytoplankton Samples and Species Identification
The LM work was carried in the lab using Motic BA and Motic B1 microscopes (Wetzlar, Germany), with a Moticam 2000 and Moticam 2.0 mp camera, both supplied by Motic Images 2 and 3 Plus software program, respectively.
The identification of algae was done on non-permanent slides under magnification 100× with application of immersion oil and was based on standard European taxonomic literature consulted with recent data in AlgaeBase [63] and relevant papers ([41,61,76], etc.). Regarding Cyanoprokaryota, the three volumes of the Freshwater Middle European Flora were used as main determination source [38,77,78]. It has to be noted that LM work was conducted twice: first, in 2018–2019 after each of the sampling campaigns, when from each site at least two slides were processed, and then, in 2021–2022, it was repeated on more slides with special attention to the sites, in which toxin producers had been detected genetically.
For morphological identification of Cuspidothrix species in particular, the following diagnostic features were followed: (1) shape and size of vegetative cells; (2) presence/absence of constrictions at cell walls; (3) presence or absence of mucilage sheath; (4) shape and size of apical cells; (5) shape, size and position of heterocytes; (6) shape, size and position of the specific resting and reproductive cells—akinetes [38]. In the text, the specific term “trichome” was used to name the organization of cells in a thread-like unit without a mucilage sheath, since “filament” in cyanoprokaryote studies is commonly applied in cases when trichome is surrounded by such a sheath (e.g., [38]).
The potential ATX and ATXs producers among the planktic cyanoprokaryotes were evaluated according to the data provided by [1,3,79]. The term “alien” was used for non-native, allochthonous species [80,81,82,83,84].
4.3. Molecular and Genetic Studies
The frequent spread of eight ATX synthetase genes (ana genes) in both sediment and water samples was shown by Legrand et al. [85], who were the first to assess the molecular tools for detection of ATXs in waters. Sequencing of the ana gene clusters of different strains of Cuspidothrix issatschenkoi demonstrated that their arrangement patterns were identical, but differed from those of Oscillatoria and Anabaena, except for the conserved section with anaB-anaG genes [57]. Therefore, anaC was amplified using a general primer set, originally designed for multi-generic detection of different ATX- and ATXs-producing genera [5]. The primer sequences used were F-ATGGTCAGAGGTTTTACAAG and R-CGACTCTTAATCATGCGATC [5].
Standard reactions (25 μL) were performed with 12.5 μL MyTaqHS Mix (Bioline, London, UK), which included 10 pmol (1 μL) primers straight and inverted. The reaction mixtures were incubated in a QB-96 Thermal Cycler using the following program: denaturation at 95 °C for 3 min, 35 cycles of denaturation (10 s at 95 °C), annealing at 52 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The obtained anaC PCR products were purified and cloned using GeneJET™ Thermo Scientific and Clone JET PCR purification kits for cloning (Thermo Fisher Scientific, Waltham, MA, USA). Then the recombinant clones were sent to Macrogen Europe (Amsterdam, The Netherlands), where they were Sanger sequenced with the same pJET primers. Depending on the obtained PCR product intensity, different number of clones per sample were sent to Macrogen Europe (Amsterdam, The Netherlands) for sequencing: four or five anaC clones per sample were send in cases of intensive PCR signal, while at a weak PCR amplification, only one or two clones were used for sequencing. Obtained data were manually edited and initially analyzed using Vector NTI 11.5 (Thermo Fisher Scientific, Waltham, MA, USA) software package. Afterwards, a phylogenetic tree was constructed using Mega 6.0. program [86] and the Neighbor-Joining method with 1000 bootstrap values. The obtained anaC sequences were deposited in the NCBI GenBank database [45] under the accession numbers OP419959—OP419972, OP481207, and OP481208.
Author Contributions
Conceptualization, MSG and KS; methodology, M.S.-G., B.U., G.G., K.S. and M.R.; investigation—Light micrsocopy: M.S.-G., B.U. and G.G.; Polymerase Chain Reaction: K.S. and M.R.; resources, B.U. and M.S.-G.; writing—original draft preparation, M.S.-G.; writing—review and editing, M.S.-G., B.U., G.G., K.S. and M.R.; visualization, M.S.-G., B.U. and K.S.; supervision, G.G.; project administration, B.U.; funding acquisition, B.U., M.S.-G. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Scientific Fund of the Bulgarian Ministry of Education and Science (NSF-BMES), grants DN13/9 from 15 December 2017 (for all sites sampled in 2018), KP-06-OPR06/2/18.12.2018, and KP-06-OPR03/18/19.12.2018 (for sampling in 2019). The APC was funded by NSF-BMES grant DN13/9 from 15 December 2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results can be found in the NCBI GenBank database (https://www.ncbi.nlm.nih.gov, accessed on 26 September 2022) under the accession numbers OP419959-OP419972, OP481207, and OP481208.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Meriluoto, J.; Spoof, L.; Codd, J. (Eds.) Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons, Ltd.: Chichester, UK, 2017. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cyanobacterial Toxins: Anatoxin-a and Analogues. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Wood, S.A.; Puddick, J.; Fleming, R.; Heussner, A.H. Detection of anatoxin-producing Phormidium in a New Zealand farm pond and an associated dog death. New Zealand J. Bot. 2017, 55, 36–46. [Google Scholar] [CrossRef]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.M.; Mueller, R.S.; Shepardson, J.W.; Landry, Z.C.; Morré, J.T.; Maier, C.S.; Hardy, F.G.; Dreher, T.W. Structural and functional analysis of the finished genome of the recently isolated toxic Anabaena sp. WA102. BMC Genom. 2016, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef]
- Ballot, A.; Fastner, J.; Lentz, M.; Wiedner, C. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 2010, 56, 964–971. [Google Scholar] [CrossRef]
- Shams, S.; Capelli, C.; Cerasino, L.; Ballot, A.; Dietrich, D.R.; Sivonen, K.; Salmaso, N. Anatoxin-a producing Tychonema (cyanobacteria) in European waterbodies. Water Res. 2015, 69, 68–79. [Google Scholar] [CrossRef]
- Tao, S.; Wang, S.; Song, L.; Gan, N. Understanding the differences in the growth and toxin production of anatoxin-producing Cuspidothrix issatschenkoi cultured with Inorganic and organic N sources from a new perspective: Carbon/Nitrogen metabolic blance. Toxins 2020, 12, 724. [Google Scholar] [CrossRef]
- Bouma-Gregson, K.; Olm, M.R.; Probst, A.J.; Anantharaman, K.; Power, M.E.; Banfield, J.F. Impacts of microbial assemblage and environmental conditions on the distribution of anatoxin-a producing cyanobacteria within a river network. ISME J. 2019, 13, 1618–1634. [Google Scholar] [CrossRef]
- Park, H.D.; Watanabe, M.F.; Harda, K.; Nagai, H.; Suzuki, M.; Watanabe, M.; Hayashi, H. Hepatotoxin (microcystin) and neurotoxin (anatoxin-a) contained in natural blooms and strains of cyanobacteria from Japanese freshwaters. Nat. Toxins 1993, 1, 353–360. [Google Scholar] [CrossRef]
- Paerl, H.W.; Barnard, M.A. Mitigating the global expansion of harmful cyanobacterial blooms: Moving targets in a human- and climatically-altered world. Harmful Algae 2020, 96, 101845. [Google Scholar] [CrossRef] [PubMed]
- Al-Lay, J.K.; Poon, G.K.; Codd, G.A. Isolation and purification of peptide and alkaloid toxins from Anabaena flos-aquae using high performance thin-layer chromatography. J. Microbiol. Methods 1998, 7, 251–258. [Google Scholar] [CrossRef]
- Harada, K.-I.; Ogawa, K.; Kimura, Y.; Murata, H.; Suzuki, M.; Thorn, P.M.; Evans, W.R.; Carmichael, W.W. Microcystins from Anabaena flos-aquae NRC 525- 17. Chem. Res. Toxicol. 1991, 4, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management; E & FN Spon: London, UK, 1999. [Google Scholar]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.F.; Guette, K.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of anatoxin-a poisoning in dogs from North America. J. Vet. Diagn. Invest. 2008, 20, 80–92. [Google Scholar] [CrossRef]
- Edwards, C.; Beattie, K.A.; Scrimgeour, C.M.; Codd, G.A. Identifcation of anatoxin-a in benthic cyanobacteria (blue-green algae) and in associated dog poisonings at Loch Insh, Scotland. Toxicon 1992, 30, 1165–1175. [Google Scholar] [CrossRef]
- Fastner, J.; Beulker, C.; Geiser, B.; Hoffmann, A.; Kröger, R.; Teske, K.; Hoppe, J.; Mundhenk, L.; Neurath, H.; Sagebiel, D.; et al. Fatal neurotoxicosis in dogs associated with tychoplanktic, anatoxin-a producing Tychonema sp. in mesotrophic Lake Tegel, Berlin. Toxins 2018, 10, 60. [Google Scholar] [CrossRef]
- Wood, S.A.; Heath, M.; McGregor, G.; Holland, P.T.; Munday, R.; Ryan, K. Identification of a benthic microcystin producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon 2010, 55, 897–903. [Google Scholar] [CrossRef]
- John, N.; Baker, L.; Ansell, B.R.E.; Newham, S.; Crosbie, N.D.; Jex, A.R. First report of anatoxin-a producing cyanobacteria in Australia illustrates need to regularly up-date monitoring strategies in a shifting global distribution. Sci. Rep. 2019, 9, 10894. [Google Scholar] [CrossRef]
- Bauer, F.; Fastner, J.; Bartha-Dima, B.; Breuer, W.; Falkenau, A.; Mayer, C.; Raeder, U. Mass occurrence of anatoxin-a- and dihydroanatoxin-a-producing Tychonema sp. in mesotrophic reservoir Mandichosee (River Lech, Germany) as a cause of neurotoxicosis in dogs. Toxins 2020, 12, 726. [Google Scholar] [CrossRef]
- Blahova, L.; Sehnal, L.; Lepsova-Skacelova, O.; Szmucova, V.; Babica, P.; Hilscherova, K.; Teikari, J.; Sivonen, K.; Blaha, L. Occurrence of cylindrospermopsin, anatoxin-a and their homologs in the southern Czech Republic—Taxonomical, analytical, and molecular approaches. Harmful Algae 2021, 108, 102101. [Google Scholar] [CrossRef] [PubMed]
- Gorham, P.R.; McLachlan, J.; Hammer, U.T.; Kim, W.K. Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. SIL Proc. 1964, 15, 796–804. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Biggs, D.F.; Gorham, P.R. Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science 1975, 187, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Gorham, P.R.; Biggs, D.F. Two laboratory case studies on the oral toxicity to calves of the freshwater cyanophyte (blue-green alga) Anabaena flos-aquae NRC-44-1. Can. Vet. J. 1977, 18, 71–75. [Google Scholar]
- Devlin, J.; Edwards, O.; Gorham, P.; Hunter, N.; Pike, R.; Stavric, B. Anatoxin-a, a toxic alkaloid from Anabaena flos-aquae NRC-44h. Can. J. Chem. 1977, 55, 1367–1371. [Google Scholar] [CrossRef]
- Botana, L.M.; James, K.; Crowley, J.; Duphard, J.; Lehane, M.; Furey, A. Anatoxin-a and analogues: Discovery, distribution, and toxicology. In Phycotoxins: Chemistry and Biochemistry, Botana, L., ed; Blackwell Publishing: Oxford, UK, 2007; pp. 141–158. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. “Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Adamski, M.; Zimolag, E.; Kaminski, A.; Drukała, J.; Bialczyk, J. Effects of cylindrospermopsin, its decomposition products, and anatoxin-a on human keratinocytes. Sci. Total Environ. 2020, 765, 142670. [Google Scholar] [CrossRef]
- Cerasino, L.; Salmaso, N. Co-occurrence of anatoxin-a and microcystins in Lake Garda and other deep subalpine lakes: Co-occurrence of anatoxin-a and microcystins in Lake Garda. Adv. Oceanogr. Limnol. 2020, 11, 11–21. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Descy, J.-P.; Gärtner, G.; Draganova, P.H.; Borisova, C.I.; Pavlova, V.; Mitreva, M. Pilot application of drone-observations and pigment marker detection by HPLC in the studies of CyanoHABs in Bulgarian inland waters. Mar. Freshw. Res. 2019, 71, 606–616. [Google Scholar] [CrossRef]
- Michev, T.; Stoyneva, M. (Eds.) Inventory of Bulgarian Wetlands and Their Biodiversity; Elsi-M: Sofia, Bulgaria, 2007. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Descy, J.-P.; Latli, A.; Uzunov, B.; Pavlova, V.; Bratanova, Z.; Babica, P.; Maršálek, B.; Meriluoto, J.; Spoof, L. Assessment of cyanoprokaryote blooms and of cyanotoxins in Bulgaria in a 15-years period (2000-2015). Adv. Oceanogr. Limnol. 2017, 8, 131–152. [Google Scholar] [CrossRef][Green Version]
- Teneva, I.; Mladenov, R.; Belkinova, D.; Dimitrova-Dyulgerova, I.; Dzhambazov, B. Phytoplankon community of the drinking after supply reservoir Borovitsa (South Bulgaria) with an emphasis on cyanotoxins and water quality. Cent. Eur. J. Biol. 2010, 5, 231–239. [Google Scholar] [CrossRef]
- Komárek, J. Cyanoprokaryota. 3rd Part: Heterocytous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier, Spektrum Akad. Verl.: Heidelberg, Germany, 2014. [Google Scholar]
- Stefanova, K.; Radkova, M.; Uzunov, B.; Gärtner, G.; Stoyneva-Gärtner, M.P. Pilot search for cylindrospermopsin-producers in nine shallow Bulgarian waterbodies reveals nontoxic strains of Raphidiopsis raciborskii, R. mediterranea and Chrysosporum bergii. Biotechnol. Biotechnol. Equip. 2020, 34, 384–394. [Google Scholar] [CrossRef]
- Radkova, M.; Stefanova, K.; Uzunov, B.; Gärtner, G.; Stoyneva-Gärtner, M. Morphological and molecular identification of microcystin-producing cyanobacteria in nine shallow Bulgarian water bodies. Toxins 2020, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Rajaniemi, P.; Komárek, J.; Hoffmann, L.; Hrouzek, P.; Kastocská, K.; Sivonen, K. Taxonomic consequences from the combined molecular and phenotype evaluation of selected Anabaena and Aphanizomenon strains. Algol. Stud. 2005, 117, 371–391. [Google Scholar] [CrossRef]
- Uzunov, B.; Stefanova, K.; Radkova, M.; Descy, J.-P.; Gärtner, G.; Stoyneva-Gärtner, M. Microcystis species and their toxigenic strains in phytoplankton of ten Bulgarian wetlands (August 2019). Botanica 2021, 27, 77–94. Available online: 09_uzunov_et_al__botanica27_12021_60d3db833f272.pdf(gamtc.lt) (accessed on 7 November 2022). [CrossRef]
- Stoyneva-Gärtner, M.; Stefanova, K.; Descy, J.-P.; Uzunov, B.; Radkova, M.; Pavlova, V.; Mitreva, M.; Gärtner, G. Microcystis aeruginosa and M. wesenbergii were the primary planktonic microcystin producers in several Bulgarian waterbodies (August 2019). Appl. Sci. 2021, 11, 357. [Google Scholar] [CrossRef]
- Méjean, A.; Paci, G.; Gautier, V.; Ploux, O. Biosynthesis of anatoxin-a and analogues (anatoxins) in cyanobacteria. Toxicon 2014, 91, 15–22. [Google Scholar] [CrossRef]
- NCBI: National Centre for Biotechnology Information (NCBI). Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 12 May 2021).
- BLAST: Basic Local Alignment Search Tool (BLAST). Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 12 May 2021).
- Michev, T.M.; Profirov, L.A.; Karaivanov, N.P.; Michev, B.T. Migration of soaring birds over Bulgaria. Acta Zool. Bulg. 2012, 64, 33–41. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Stoyneva-Gärtner, M.P.; Morana, C.; Borges, A.V.; Okello, W.; Bouillon, S.; Deirmendjian, L.; Lambert, T.; Roland, F.; Nankabirwa, A.; Nabafu, E.; et al. Diversity and ecology of phytoplankton in Lake Edward (East Africa): Present status and long-term changes. JGLR 2020, 46, 741–751. [Google Scholar] [CrossRef]
- Stoyneva, M.P. Development of the phytoplankton of the shallow Srebarna lake (North-Eastern Bulgaria) across the trophic gradient. Hydrobiologia 1998, 369, 259–367. [Google Scholar] [CrossRef]
- Deirmendjian, L.; Descy, J.-P.; Morana, C.; Okello, W.; Stoyneva-Gärtner, M.P.; Bouillon, S.; Borges, A.V. Limnological changes in Lake Victoria since the mid-20th century. Freshw. Biol. 2021, 66, 1630–1648. [Google Scholar] [CrossRef]
- Namikoshi, M.; Murakami, T.; Watanabe, M.F.; Oda, T.; Yamada, J.; Tsujimura, S.; Nagai, H.; Oishi, S. Simultaneous production of homoanatoxin-a, anatoxin-a, and a new non-toxic 4-hydroxyhomoanatoxin-a by the cyanobacterium Raphidiopsis mediterranea Skuja. Toxicon 2003, 42, 533–538. [Google Scholar] [CrossRef]
- Watanabe, M.F.; Tsujimura, S.; Oishi, S.; Niki, T.; Namikoshi, M. Isolation and identification of homoanatoxina from a toxic strain of the cyanobacterium Raphidiopsis mediterranea Skuja isolated from Lake Biwa, Japan. Phycologia 2003, 42, 364–369. [Google Scholar] [CrossRef]
- Hodoki, Y.; Ohbayashi, K.; Kobayashi, Y.; Takasu, H.; Okuda, N.; Nakano, S. Anatoxin-a-producing Raphidiopsis mediterranea Skuja var. grandis Hill is one ecotype of non-heterocytous Cuspidothrix issatschenkoi (Usačev) Rajaniemi et al. in Japanese lakes. Harmful Algae 2013, 21–22, 44–53. [Google Scholar] [CrossRef]
- Gagnon, A.; Pick, F.R. Effect of nitrogen on cellular production and release of the neurotoxin anatoxin-a in a nitrogen-fixing cyanobacterium. Front. Microbiol. 2012, 3, 211. [Google Scholar] [CrossRef]
- Wood, S.A.; Rasmussen, J.P.; Holland, P.T.; Campbell, R.; Crowe, A.L.M. First report of the cyanotoxin anatoxin-a from Aphanizomenon issatschenkoi (cyanobacteria). J. Phycol. 2007, 43, 356–365. [Google Scholar] [CrossRef]
- Uzunov, B.; Stefanova, K.; Radkova, M.; Descy, J.-P.; Gärtner, G.; Stoyneva-Gärtner, M. First report on Microcystis as a potential microviridin producer in Bulgarian waterbodies. Toxins 2021, 13, 448. [Google Scholar] [CrossRef]
- Ballot, A.; Scherer, P.I.; Wood, S.A. Variability in the anatoxin gene clusters of Cuspidothrix issatschenkoi from Germany, New Zealand, China and Japan. PLoS ONE 2018, 13, e0200774. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, G.; Pan, Q.; Yang, Y.; Li, R. Identification of genes for anatoxin-a biosynthesis in Cuspidothrix issatschenkoi. Harmful Algae 2015, 46, 43–48. [Google Scholar] [CrossRef]
- Ryu, H.S.; Shin, R.Y.; Lee, J.H. Morphology and taxonomy of the Aphanizomenon spp. (Cyanophyceae) and related species in the Nakdong River, South Korea. J. Ecol. Environ. 2017, 41, 6. [Google Scholar] [CrossRef]
- Aguilera, A.; Berrendero Gómez, E.; Kaštovský, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Kormas, K.A.; Vardaka, E.; Katsiapi, M.; Gkelis, S. Raphidiopsis mediterranea Skuja represents non-heterocytous lifecycle stages of Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba Raju in Lake Kastoria (Greece), its type locality: Evidence by morphological and phylogenetic analysis. Harmful Algae 2009, 8, 864–872. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. Available online: http://www.algaebase.org/ (accessed on 26 July 2022).
- Moustaka-Gouni, M.; Kormas, K.A.; Polykarpou, P.; Gkelis, S.; Bobori, D.C.; Vardaka, E. Polyphasic evaluation of Aphanizomenon issatschenkoi and Raphidiopsis mediterranea in a Mediterranean lake. J. Plankton. Res. 2010, 32, 927–936. [Google Scholar] [CrossRef][Green Version]
- Vodenicharov, D.; Draganov, S.; Temniskova, D. Flora of Bulgaria. In Algae; Narodna Prosveta: Sofia, Bulgaria, 1971. [Google Scholar]
- Stoyneva, M. 1998a. Algae. In Biodiversity of the Srebarna Biosphere Reserve. Checklist and Bibliography; Michev, T.M., Georgiev, B.B., Petrova, A.V., Stoyneva, M.P., Eds.; Co-publ. Context & Pensoft: Sofia, Bulgaria, 1998; pp. 10–37. [Google Scholar]
- Stoyneva, M.P. Algological studies of Bulgarian coastal wetlands. I. Species composition of the phytoplankton of Durankulak and Shabla-Ezeretz lakes. Ann. Univ. Sof. 2000, 91, 27–48. [Google Scholar]
- Stoyneva, M.P. Contribution to the studies of aero- and hydrobiontic prokaryotic and eukaryotic algae of Bulgaria. Ph.D. Thesis, Sofia University “St Kliment Ohridski”, Faculty of Biology, Sofia, Bulgaria, 2014. [Google Scholar]
- Dimitrova, R.; Nenova, E.; Uzunov, B.; Shishiniova, M.; Stoyneva, M. Phytoplankton composition of Vaya Lake (2004–2006). Bulg. J. Agric. Sci. Suppl. 2014, 20, 165–172. [Google Scholar]
- Stoyanov, P.; Teneva, I.; Mladenov, R.; Belkinova, D. Diversity and ecology of the phytoplankton of filamentous blue-green algae (Cyanoprokaryota, Nostocales) in Bulgarian standing waters. Ecol. Balc. 2013, 5, 1–6. [Google Scholar]
- Dochin, K.; Ivanova, A.; Iliev, I. The phytoplankton of Koprinka Reservoir (Central Bulgaria): Species composition and dynamics. J. BioSci. Biotechnol. 2017, 6, 73–82. [Google Scholar]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hardy, F.J. Integrative Monitoring of Marine and Freshwater Harmful Algae in Washington State for Public Health Protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Descy, J.-P.; Stoyneva-Gärtner, M.P.; Uzunov, B.A.; Dimitrova, P.H.; Pavlova, V.T.S.; Gärtner, G. Studies on cyanoprokaryotes of the water bodies along the Bulgarian Black Sea Coast (1890–2017): A review, with special reference to new, rare and harmful taxa. Acta Zool. Bulgar. Suppl. 2018, 11, 43–52. [Google Scholar]
- Stoyneva-Gärtner, M.; Uzunov, B.; Dimitrova, P.; Pavlova, V. Algal toxins—New risk factors for national security in Bulgaria. In Proceedings of the Actual Problems of the Security, Veliko Turnovo, Bulgaria, 26–27 October 2017; Electronic publication, Publishing house complex of NVU “Vasil Levski”: Veliko Turnovo, Bulgaria, 2017; pp. 435–445, ISBN 2367-7473. [Google Scholar]
- Komárek, J.; Komarková, J. Diversity of Aphanizomenon-like cyanobacteria. Czech. Phycol. 2006, 6, 1–32. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Teil: Chroococcales. In Süßwasserflora von Mitteleuropa. Bd. 19/1; Ettl, H., Gärtner, G., Heynig, G., Mollenhauer, D., Eds.; Gustav Fischer: Jena, Germany; Stuttgart, Germany; Lübeck, Germany; Ulm, Germany, 1999. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Teil: Oscillatoriales. In Süßwasserflora von Mitteleuropa. Bd. 19/2; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Elsevier, Spektrum Akad. Verl.: Heidelberg, Germany; München, Germany, 2005. [Google Scholar]
- Catherine, Q.; Wood, S.; Echenigue-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Walther, G.-R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Woźniak, E.; Najberck, K. Towards clarifying the presence of alien algae in inland waters—Can we predict places of their occurrence? Biologia 2013, 68, 838–844. [Google Scholar] [CrossRef]
- Kaštovský, J.; Hauer, T.; Mareš, J.; Krautová, M.; Bešta, T.; Komárek, J.; Desortová, B.; Heteša, J.; Hindáková, A.; Houk, V.; et al. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biol. Invasions 2010, 12, 3599–3625. [Google Scholar] [CrossRef]
- Stoyneva, M.P. Allochtonous planctonic algae recorded in Bulgaria during the last 25 years and their possible dispersal agents. Hydrobiologia 2016, 764, 53–64. [Google Scholar] [CrossRef]
- Savadova, K.; Mazur-Marzec, H.; Karosiene, J.; Kasperoviciene, J.; Vitonyte, I.; Torunska-Sitarz, A.; Koreiviene, J. Effect of increased temperature on native and alien nuisance cyanobacteria from temperate lakes: An experimental approach. Toxins 2018, 10, 445. [Google Scholar] [CrossRef]
- Legrand, B.; Lesobre, J.; Colombet, J.; Latour, D.; Sabart, M. Molecular tools to detect anatoxin-a genes in aquatic ecosystems: Toward a new nested PCR-based method. Harmful Algae 2016, 58, 16–22. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).