Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens
Abstract
:1. Introduction
2. Results
2.1. Growth Performance and Organ Indexes
2.2. Oxidative Status of the Intestine and Liver
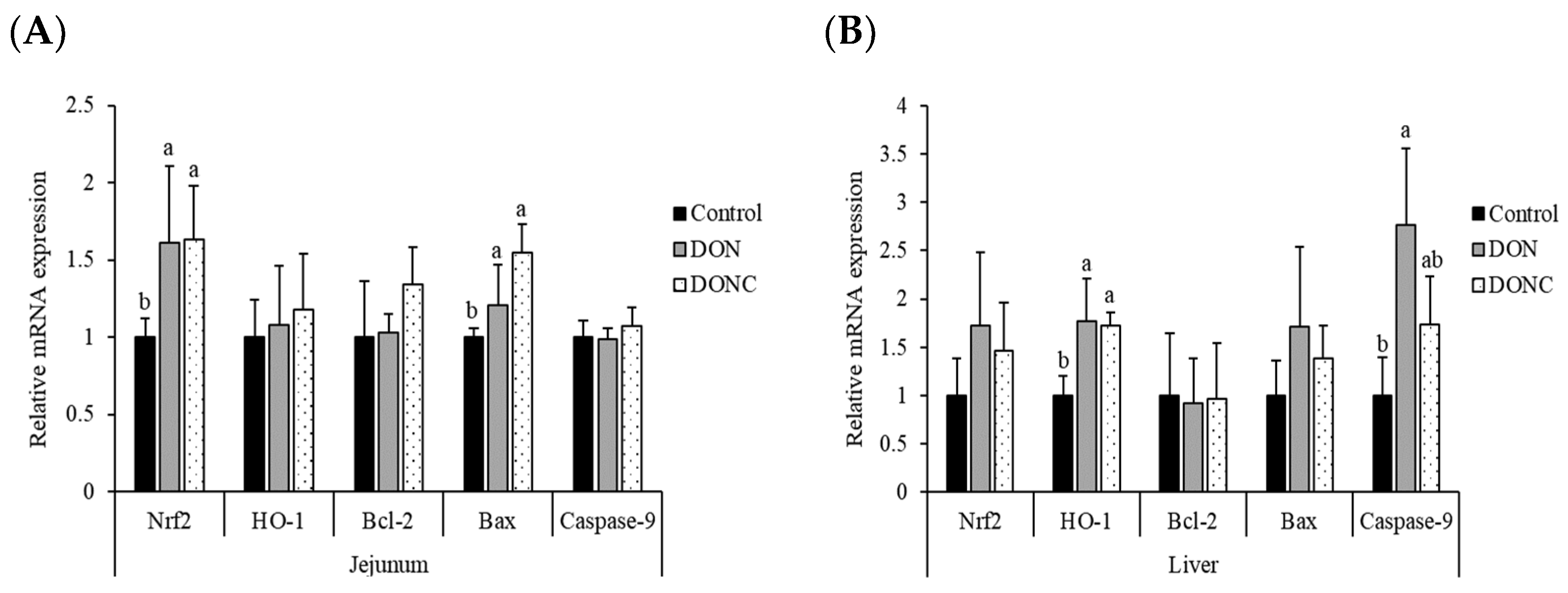
2.3. Relative mRNA Expression of Antioxidation- and Apoptosis-Related Genes
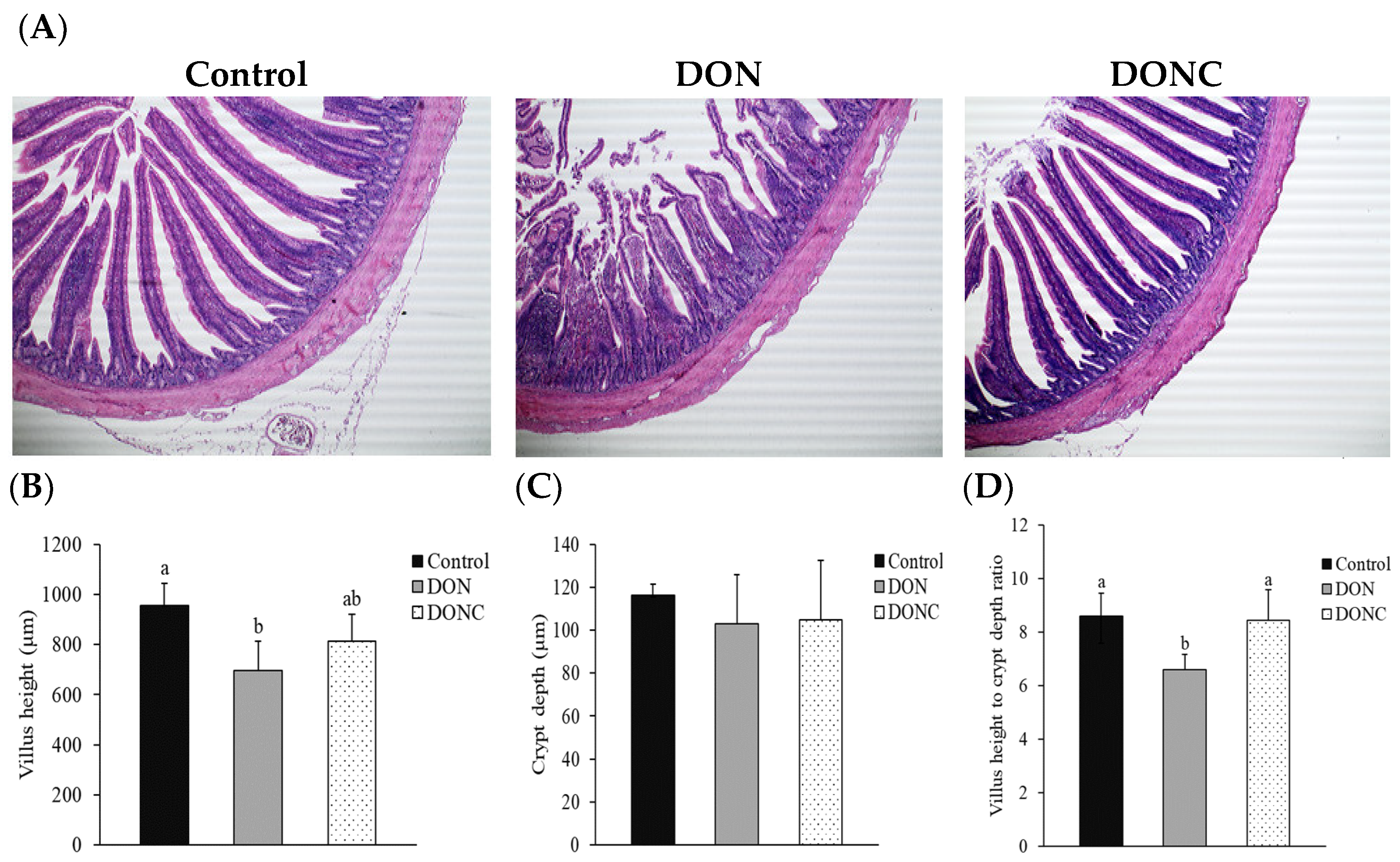
2.4. Intestinal Morphological Structure
2.5. Gut Microbiota
2.5.1. Diversity of Gut Microbiota
2.5.2. Gut Microbial Composition
2.5.3. Bacterial Richness among Groups
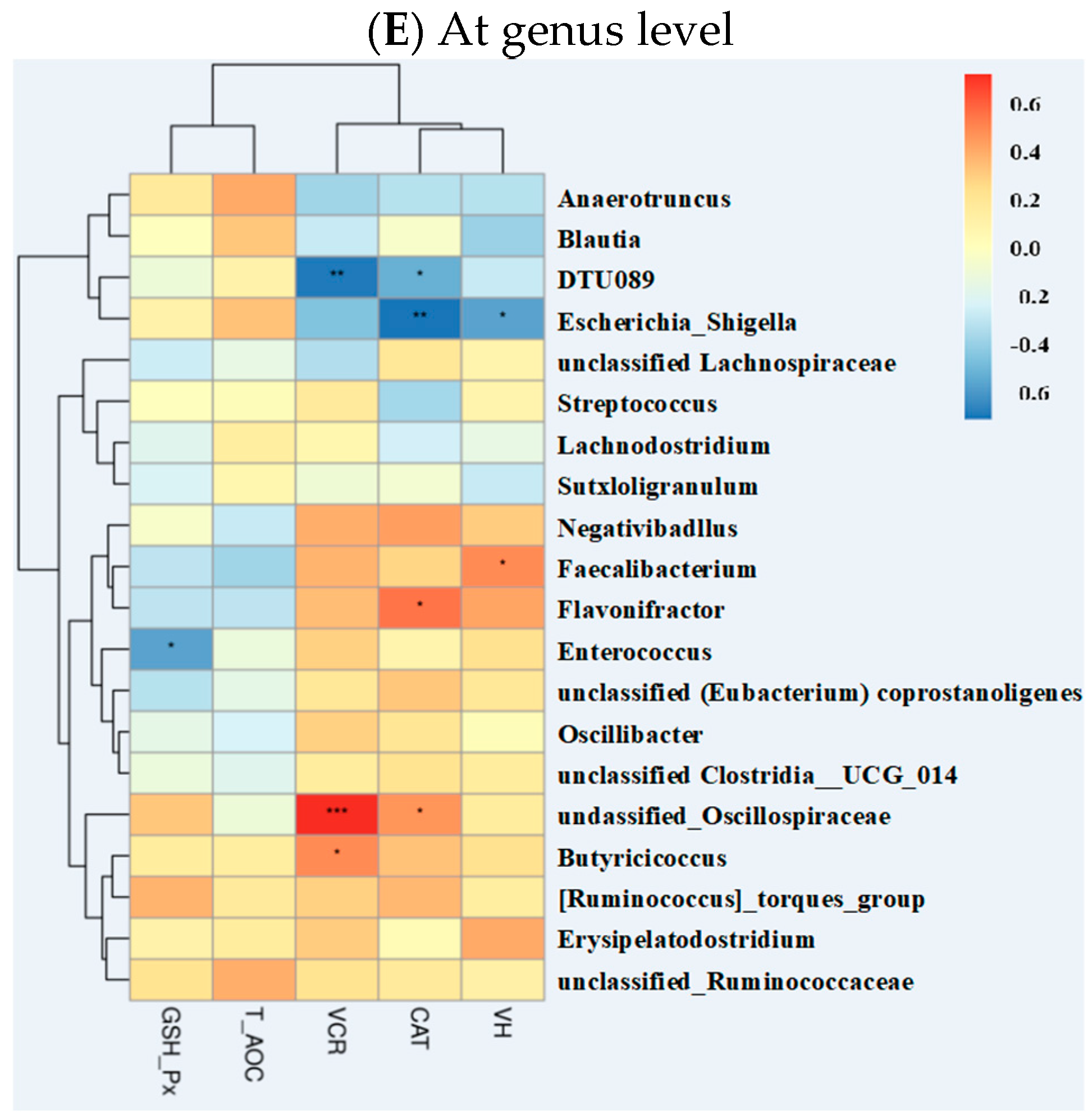
2.5.4. Correlations of Gut Microbiota with Other Intestinal Parameters
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Experimental Design
5.2. Fabrication of DON-Enriching Rices
5.3. Determination of Growth Performance and Organ Indexes
5.4. Measurement of Oxidative Status
5.5. Measurement of Gene Expression
5.6. Analysis of Intestinal Morphological Structure
5.7. High-Throughput Sequencing of Gut Microbiota
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M.A. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.Y.; Zhao, L.H.; Fan, Y.; Jia, Y.X.; Sun, L.; Ma, S.S.; Ji, C.; Ma, Q.G.; Zhang, J.Y. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechno. 2014, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Guo, Y.P.; Zhao, L.H.; Fan, Y.; Ji, C.; Zhang, J.Y.; Ma, Q.G. Protective effects of Devosia sp ANSB714 on growth performance, immunity function, antioxidant capacity and tissue residues in growing-finishing pigs fed with deoxynivalenol contaminated diets. Food Chem. Toxicol. 2018, 121, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Li, X.Y.; Ji, C.; Rong, X.P.; Liu, S.J.; Zhang, J.Y.; Ma, Q.G. Protective effect of Devosia sp ANSB714 on growth performance, serum chemistry, immunity function and residues in kidneys of mice exposed to deoxynivalenol. Food Chem. Toxicol. 2016, 92, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Tech. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Paraskeuas, V.; Griela, E.; Bouziotis, D.; Fegeros, K.; Antonissen, G.; Mountzouris, K.C. Effects of deoxynivalenol and fumonisins on broiler gut cytoprotective capacity. Toxins 2021, 13, 729. [Google Scholar] [CrossRef]
- Wu, Q.H.; Wang, X.; Yang, W.; Niissler, A.K.; Xiong, L.Y.; Kuca, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- You, L.; Zhao, Y.Y.; Kuca, K.; Wang, X.; Oleksak, P.; Chrienova, Z.; Nepovimova, E.; Jacevic, V.; Wu, Q.H.; Wu, W.D. Hypoxia, oxidative stress, and immune evasion: A trinity of the trichothecenes T-2 toxin and deoxynivalenol (DON). Arch. Toxicol. 2021, 95, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Nemoto, T.; Kawakami, S.; Yamashita, F.; Hashida, M. Efficient protection by cationized catalase against H2O2 injury in primary cultured alveolar epithelial cells. J. Control. Release 2007, 121, 74–80. [Google Scholar] [CrossRef]
- Switala, J.; Loewen, P.C. Diversity of properties among catalases. Arch. Biochem. Biophys. 2002, 401, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Red. Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Guo, G.L.; Zhou, T.T.; Ren, F.Y.; Sun, J.Z.; Deng, D.; Huang, X.G.; Wassie, T.; Qazi, I.H.; Wu, X. Effect of maternal catalase supplementation on reproductive performance, antioxidant activity and mineral transport in sows and piglets. Animals 2022, 12, 828. [Google Scholar] [CrossRef]
- Tang, M.H.; Fang, R.J.; Xue, J.J.; Yang, K.L.; Lu, Y. Effects of catalase on growth performance, antioxidant capacity, intestinal morphology, and microbial composition in yellow broilers. Front. Vet. Sci. 2022, 9, 802051. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.L.; Jiang, X.M.; Chen, L.; Hong, L.; Zhuo, Y.; Lin, Y.; Fang, Z.Z.; Che, L.Q.; Feng, B.; et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2020, 98, 1–10. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, F.C.; Yang, W.R.; Jiang, S.Z.; Li, Y. Supplementation with exogenous catalase from Penicillium notatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol. Spectr. 2021, 9, e0065421. [Google Scholar] [CrossRef]
- Sun, X.J.; Piao, L.G.; Jin, H.F.; Nogoy, K.M.C.; Zhang, J.F.; Sun, B.; Jin, Y.; Lee, D.H.; Choi, S.; Li, X.Z. Dietary glucose oxidase and/or catalase supplementation alleviates intestinal oxidative stress induced by diquat in weaned piglets. Anim. Sci. J. 2021, 92, e13634. [Google Scholar] [CrossRef]
- Bindari, Y.R.; Gerber, P.F. Centennial review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult. Sci. 2022, 101, 101612. [Google Scholar] [CrossRef]
- Qiu, Y.Q.; Yang, J.; Wang, L.; Yang, X.F.; Gao, K.G.; Zhu, C.; Jiang, Z.Y. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.C.; Zhang, B.Y.; Zhang, L.; Wu, K.T.; Yang, A.; Li, C.; Wang, Y.N.; Zhang, J.C.; Qi, D.S. Potential link between gut microbiota and deoxynivalenol-induced feed refusal in weaned piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef]
- Qing, H.R.; Huang, S.M.; Zhan, K.; Zhao, L.H.; Zhang, J.Y.; Ji, C.; Ma, Q.G. Combined toxicity evaluation of ochratoxin A and aflatoxin B1 on kidney and liver injury, immune inflammation, and gut microbiota alteration through pair-feeding pullet model. Front. Immunol. 2022, 13, 920147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.L.; Zhang, L.J.; Zhan, X.Y.; Liu, Z.G.; Zhuo, Y.; Lin, Y.; Fang, Z.Z.; Che, L.Q.; Feng, B.; et al. Effects of a diet supplemented with exogenous catalase from Penicillium notatum on intestinal development and microbiota in weaned piglets. Microorganisms 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dersjant-Li, Y.M.; Verstegen, M.W.A.; Gerrits, W.J.J. The impact of low concentrations of aflatoxin, deoxynivalenol or fumonisin in diets on growing pigs and poultry. Nutr. Res. Rev. 2003, 16, 223–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, T.; Daneshyar, M.; Allymehr, M.; Jalali, A.S.; Behroozyar, H.K.; Tukmechi, A. The impact of deoxynivalenol contaminated diet on performance, immune response, intestine morphology and jejunal gene expression in broiler chicken. Toxicon 2021, 199, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Hess, M.; Twarużek, M.; Grajewski, J.; Kosicki, R.; Böhm, J.; Zentek, J. The impact of the Fusarium mycotoxin deoxynivalenol on the health and performance of broiler chickens. Int. J. Mol. Sci. 2011, 12, 7996–8012. [Google Scholar] [CrossRef]
- Chen, S.S.; Li, Y.H.; Lin, M.F. Chronic exposure to the Fusarium mycotoxin deoxynivalenol: Impact on performance, immune organ, and intestinal integrity of slow-growing chickens. Toxins 2017, 9, 334–354. [Google Scholar] [CrossRef] [Green Version]
- Awad, W.A.; Bohm, J.; Razzazi-Fazeli, E.; Ghareeb, K.; Zentek, J. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 2006, 85, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.R.; Liu, Y.L.; Duan, Y.L.; Wang, F.Y.; Guo, F.S.; Yan, F.; Yang, X.J.; Yang, X. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum JM113 and consequentially altered gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Cheng, Y.F.; Wen, C.; Wang, W.B.; Kang, Y.R.; Wang, A.Q.; Zhou, Y.M. The protective effects of modified palygorskite on the broilers fed a purified zearalenone-contaminated diet. Poult. Sci. 2019, 98, 3802–3810. [Google Scholar] [CrossRef]
- Reddy, K.E.; Song, J.; Lee, H.J.; Kim, M.; Kim, D.W.; Jung, H.J.; Kim, B.; Lee, Y.; Yu, D.; Kim, D.W.; et al. Effects of high levels of deoxynivalenol and zearalenone on growth performance, and hematological and immunological parameters in pigs. Toxins 2018, 10, 114. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sugiyama, H.; Makino, H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 2005, 68, 1018–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A.; Bracarense, A.P.F.R.L. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon 2020, 185, 203–212. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Magesh, S.; Chen, Y.; Hu, L.Q. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Chen, L.K.; Peng, Z.; Wang, D.; Song, Y.D.; Wang, H.Y.; Yao, P.; Yan, H.; Nüssler, A.K.; Liu, L.G.; et al. Embryotoxicity caused by DON-induced oxidative stress mediated by Nrf2/HO-1 pathway. Toxins 2017, 9, 188. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liang, S.S.; Guo, F.S.; Ren, Z.Z.; Yang, X.J.; Long, F.Y. Gut microbiota mediates the protective role of Lactobacillus plantarum in ameliorating deoxynivalenol-induced apoptosis and intestinal inflammation of broiler chickens. Poult. Sci. 2020, 99, 2395–2406. [Google Scholar] [CrossRef]
- Li, J.B.; Bai, Y.S.; Ma, K.D.; Ren, Z.S.; Li, J.P.; Zhang, J.; Shan, A.S. Dihydroartemisinin alleviates deoxynivalenol induced liver apoptosis and inflammation in piglets. Ecotoxicol. Environ. Saf. 2022, 241, 113811. [Google Scholar] [CrossRef]
- Davey, L.; Halperin, S.A.; Lee, S.F. Thiol-disulfide exchange in gram-positive Firmicutes. Trends Microbiol. 2016, 24, 902–915. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, A.P.; Culpepper, T.; Saldivar, B.; Anton, S.; Stoll, S.; Handberg, E.M.; Xu, K.; Pepine, C.; Triplett, E.W.; Aggarwal, M. A six-day, lifestyle-based immersion program mitigates cardiovascular risk factors and induces shifts in gut microbiota, specifically Lachnospiraceae, Ruminococcaceae, Faecalibacterium prausnitzii: A pilot study. Nutrients 2021, 13, 3459. [Google Scholar] [CrossRef]
- Khorraminezhad, L.; Leclercq, M.; O’Connor, S.; Julien, P.; Weisnagel, S.J.; Gagnon, C.; Droit, A.; Rudkowska, I. Dairy product intake modifies gut microbiota composition among hyperinsulinemic individuals. Eur. J. Nutr. 2021, 60, 159–167. [Google Scholar] [CrossRef]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, In Vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.H.; Gao, F.; Zhu, Q.F.; Li, C.; Jiang, Y.; Dai, S.F.; Zhou, G.H. Dietary sodium butyrate alleviates the oxidative stress induced by corticosterone exposure and improves meat quality in broiler chickens. Poult. Sci. 2011, 90, 2592–2599. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, C.; Xu, J.; Xu, H.M.; Liu, L.; Zhao, H.L.; Wang, J.Q.; Huang, W.Q.; Peng, W.; Chen, Y.; et al. Gut microbiota is a potential biomarker in inflammatory bowel disease. Front. Nutr. 2022, 8, 818902. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Chen, B.W.; Chen, B.W.; Yi, J.; Long, H.P.; Wen, H.Q.; Tian, F.M.; Liu, Y.F.; Xiao, L.; Li, L.S. Liuwei Dihuang Decoction alleviates cognitive dysfunction in mice with D-galactose-induced aging by regulating lipid metabolism and oxidative stress via the microbiota-gut-brain axis. Front. Neurosci. 2022, 16, 949298. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.X.; Zhang, Z.X.; Morales, M.; Wang, Y.N.; Khafipour, E.; Friel, J. Feeding practice influences gut microbiome composition in very low birth weight preterm infants and the association with oxidative stress: A prospective cohort study. Free Radic. Biol. Med. 2019, 142, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Zhang, B.; Li, L.; Li, X.B.; Zhang, J.Q.; Chen, G.Y. Fermented noni (Morinda citrifolia L.) fruit juice improved oxidative stress and insulin resistance under the synergistic effect of Nrf2/ARE pathway and gut flora in db/db mice and HepG2 cells. Food Funct. 2022, 13, 8254–8273. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, B.K.; Tang, L.; Zhou, Y.H.; Wang, Q.; Gong, L.; Ni, J.J.; Li, W.F. Probiotic Bacillus alleviates oxidative stress-induced liver injury by modulating gut-liver axis in a rat model. Antioxidants 2022, 11, 291. [Google Scholar] [CrossRef]
- Wang, W.W.; Wang, Y.; Duan, Y.X.; Meng, Z.Q.; An, X.P.; Qi, J.W. Regulation of wheat bran feruloyl oligosaccharides in the intestinal antioxidative capacity of rats associated with the p38/JNK-Nrf2 signaling pathway and gut microbiota. J. Sci. Food. Agric. 2022, 102, 6992–7002. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, S.D.; Liu, X.W.; Wen, L.F.; Zou, J.X. Effects of dietary antimicrobial peptides on intestinal morphology, antioxidant status, immune responses, microbiota and pathogen disease resistance in grass carp Ctenopharyngodon idellus. Microb. Pathogenesis 2022, 165, 105386. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, P.H.; Hao, S.S.; Kim, S.W.; Du, T.R.; Hua, J.D.; Huang, R.H. Characteristics of gut microbiota in sows and their relationship with apparent nutrient digestibility. Int. J. Mol. Sci. 2019, 20, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2015, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.Y.; Zhang, S.; Wu, S.; Madsen, M.H.; Shi, S.R. Supplementing the early diet of broilers with soy protein concentrate can improve intestinal development and enhance short-chain fatty acid-producing microbes and short-chain fatty acids, especially butyric acid. J. Anim. Sci. Biotechnol. 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Treatments 2 | p-Value | |||
|---|---|---|---|---|
| Control | DON | DONC | ||
| IBW (g) | 33.60 ± 0.21 | 33.50 ± 0.13 | 33.51 ± 0.17 | 0.593 |
| FBW (g) | 319.47 ± 22.41 | 309.83 ± 15.06 | 313.39 ± 13.59 | 0.635 |
| ADG (g) | 15.97 ± 1.12 | 15.49 ± 0.75 | 15.67 ± 0.68 | 0.636 |
| ADFI (g) | 31.53 ± 1.56 | 29.54 ± 1.38 | 30.77 ± 1.49 | 0.093 |
| FCR | 1.98 ± 0.12 | 1.91 ± 0.15 | 1.96 ± 0.13 | 0.676 |
| Treatments 2 | p-Value | |||
|---|---|---|---|---|
| Control | DON | DONC | ||
| Spleen (g/kg) | 1.30 ± 0.33 | 1.52 ± 0.25 | 1.18 ± 0.45 | 0.419 |
| Liver (g/kg) | 23.66 ± 1.77 b | 28.56 ± 1.81 a | 25.16 ± 2.58 b | 0.016 |
| Bursa of Fabricius (g/kg) | 3.28 ± 0.96 | 3.21 ± 0.31 | 3.16 ± 0.41 | 0.957 |
| Kidney (g/kg) | 9.56 ± 0.71 | 8.46 ± 0.77 | 9.19 ± 0.95 | 0.078 |
| Treatments 2 | p-Value | ||||
|---|---|---|---|---|---|
| Control | DON | DONC | |||
| Jejunum | ROS | 62.12 ± 5.94 | 64.88 ± 3.94 | 64.83 ± 7.56 | 0.667 |
| O2− | 111.62 ± 48.46 | 115.78 ± 78.19 | 101.99 ± 35.02 | 0.924 | |
| OH | 6.51 ± 2.67 | 6.96 ± 1.80 | 5.46 ± 1.21 | 0.466 | |
| 8-OHdG | 27.49 ± 0.97 | 29.02 ± 2.59 | 28.09 ± 1.77 | 0.394 | |
| MDA | 7.49 ± 0.22 | 7.30 ± 0.40 | 7.34 ± 0.31 | 0.327 | |
| CAT | 545.59 ± 29.53 a | 464.41 ± 8.66 c | 497.50 ± 11.86 b | <0.001 | |
| GSH-Px | 114.79 ± 2.21 a | 111.18 ± 2.29 b | 117.75 ± 3.63 a | 0.008 | |
| T-AOC | 3.68 ± 0.31 b | 3.67 ± 0.23 b | 4.62 ± 0.65 a | 0.006 | |
| Liver | ROS | 27.56 ± 0.52 b | 29.75 ± 1.32 a | 28.52 ± 1.34 ab | 0.031 |
| O2− | 421.58 ± 35.68 | 445.83 ± 53.19 | 443.60 ± 73.66 | 0.753 | |
| OH | 33.26 ± 2.77 | 34.10 ± 6.16 | 33.16 ± 5.50 | 0.949 | |
| 8-OHdG | 27.56 ± 0.52 b | 29.75 ± 1.32 a | 28.52 ± 1.34 ab | 0.031 | |
| MDA | 7.24 ± 0.086 b | 7.76 ± 0.20 a | 7.00 ± 0.26 b | 0.012 | |
| CAT | 554.118 ± 19.88 | 565.15 ± 18.03 | 545.41 ± 30.67 | 0.764 | |
| GSH-Px | 118.92 ± 5.68 | 123.71 ± 5.04 | 121.78 ± 3.50 | 0.255 | |
| T-AOC | 5.74 ± 0.19 a | 5.17 ± 0.42 b | 5.27 ± 0.07 b | 0.017 | |
| Genes 1 | Primer Sequence 2 (5′-3′) | Accession No. |
|---|---|---|
| GAPDH | F: GAGGGTAGTGAAGGCTGCTG | NM_204305.1 |
| R: CATCAAAGGTGGAGGAATGG | ||
| Nrf2 | F: GATGTCACCCTGCCCTTAG | NM_205117.1 |
| R: CTGCCACCATGTTATTCC | ||
| HO-1 | F: GCTGAAGAAAATCGCCCAA | NM_205344.1 |
| R: ATCTCAAGGGCATTCATTCGG | ||
| Bax | F: CAACAGGAAGAACACGCTGA | XM_015290060.1 |
| R: TCAGTCTCGGCCCACTATCT | ||
| Bcl-2 | F: GACAACGGAGGATGGGATG | NM_205339.2 |
| R: CAGGCTCAGGATGGTCTTCA | ||
| Caspase-9 | F: TCGAGCTGGCTCTGACATAGACTG | XM_424580.5 |
| R: AGGATGACCACGAGGCAGCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhu, J.; Cao, Q.; Zhang, C.; Dong, Z.; Feng, D.; Ye, H.; Zuo, J. Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens. Toxins 2022, 14, 830. https://doi.org/10.3390/toxins14120830
Wang W, Zhu J, Cao Q, Zhang C, Dong Z, Feng D, Ye H, Zuo J. Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens. Toxins. 2022; 14(12):830. https://doi.org/10.3390/toxins14120830
Chicago/Turabian StyleWang, Weiwei, Jingqiang Zhu, Qingyun Cao, Changming Zhang, Zemin Dong, Dingyuan Feng, Hui Ye, and Jianjun Zuo. 2022. "Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens" Toxins 14, no. 12: 830. https://doi.org/10.3390/toxins14120830
APA StyleWang, W., Zhu, J., Cao, Q., Zhang, C., Dong, Z., Feng, D., Ye, H., & Zuo, J. (2022). Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens. Toxins, 14(12), 830. https://doi.org/10.3390/toxins14120830





