Inhibition of Essential Oils on Growth of Aspergillus flavus and Aflatoxin B1 Production in Broth and Poultry Feed
Abstract
:1. Introduction
2. Results
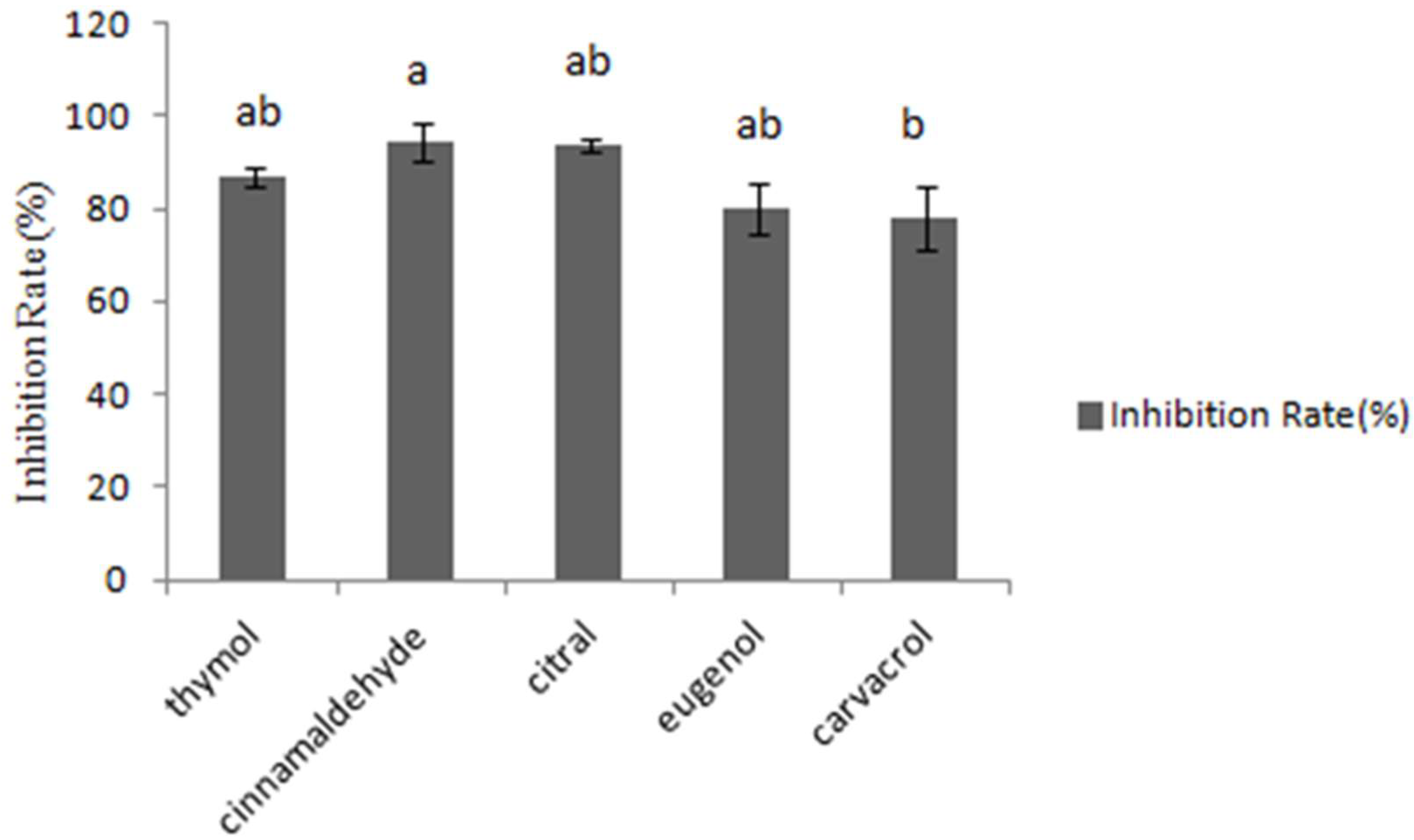
2.1. The Inhibition of Different Concentration of Essential Oils against A. flavus
2.2. MIC Tests and Synergy Effects of the Best Effect of Essential Oils on Fungal Growth in Broth by Using Checkerboard
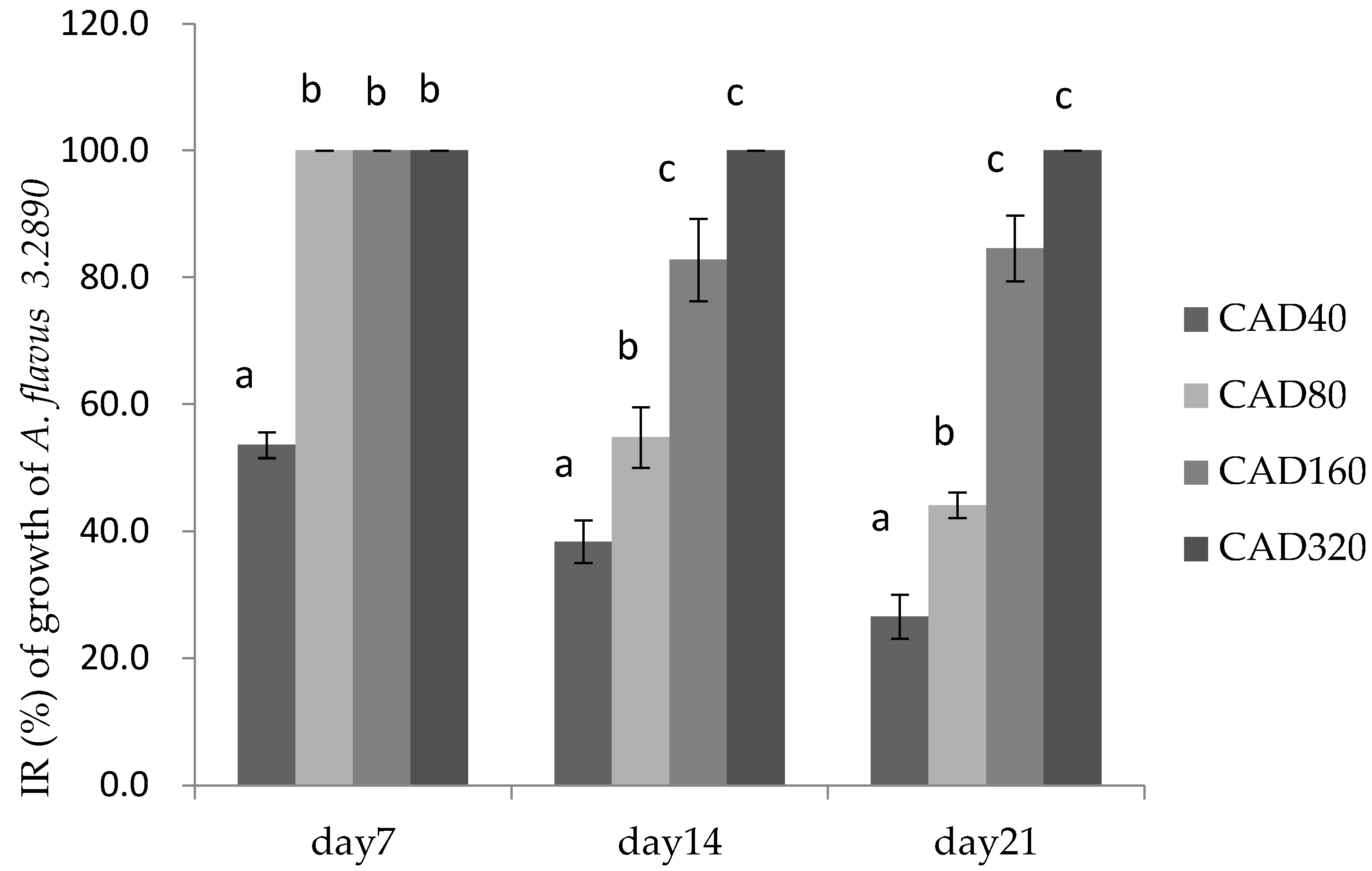
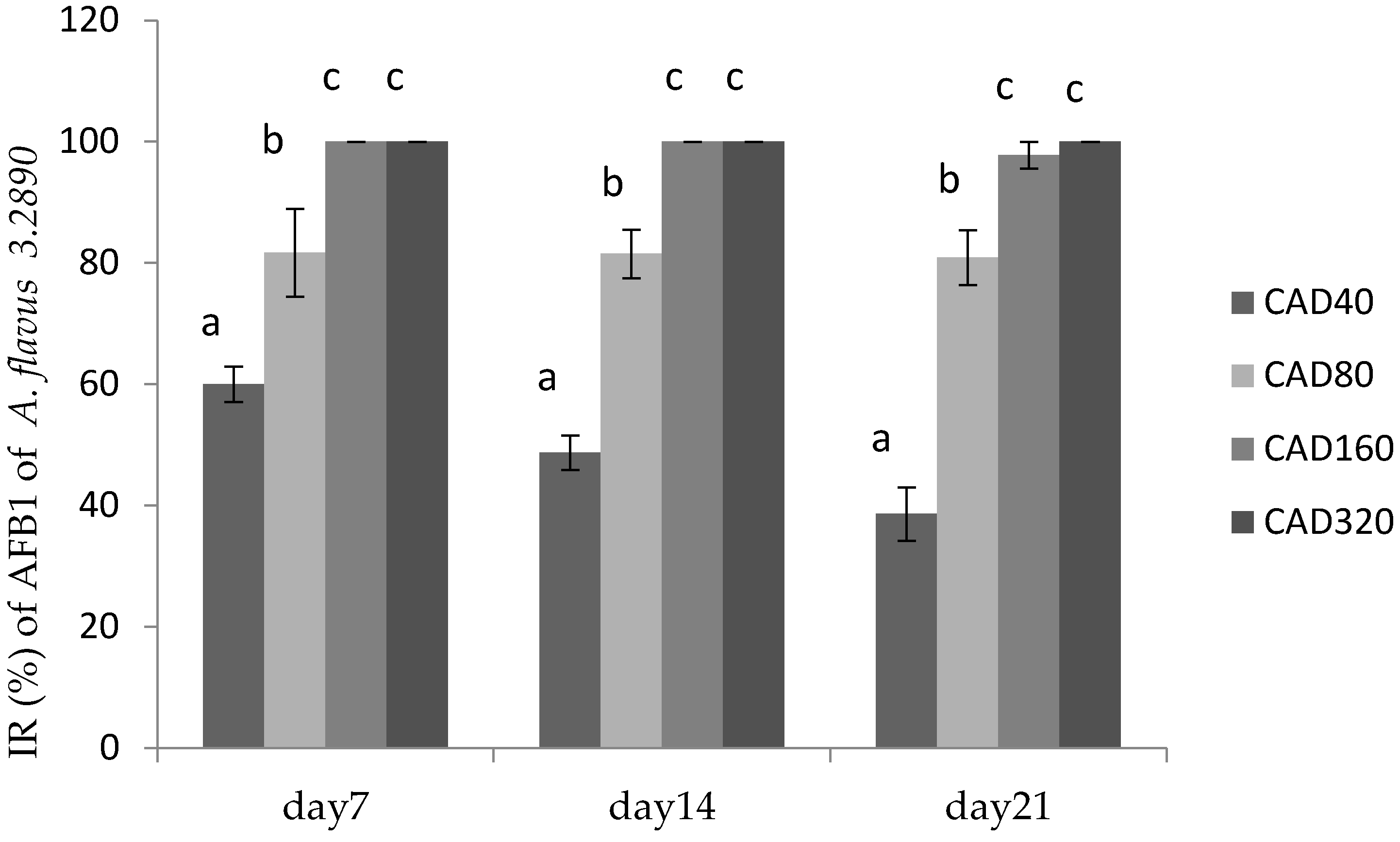
2.3. Effects of Essential Oils on Fungal Growth and AFB1 Production in Poultry Feed
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Microbial Cultures
5.2. Preparation of Essential Oils
5.3. Screening of Best Effect of Essential Oils on Mold Growth in Broth
5.4. MIC Tests and Synergy Effects of the Best Effect Essential Oils on Fungal Growth in Broth
5.5. Effects of the Best Effect Essential Oil on Fungal Growth and AFB1 Production in Poultry Feed
5.6. Determination of Mold Counts
5.7. Determination of AFB1 in Feed
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oguz, H.; Kececi, T.; Birdane, Y.O.; Onder, F. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Res. Vet. Sci. 2000, 69, 89–93. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B(1) in affecting broiler’s performance, immunity, and gastrointestinal tract: A review of history and contemporary issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef]
- Oguz, H.; Kurtoglu, F.; Kurtoglu, V.; Birdane, Y.O. Evaluation of biochemical characters of broiler chickens during dietary aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2002, 73, 101–103. [Google Scholar] [CrossRef]
- Tessari, E.N.; Oliveira, C.A.; Cardoso, A.L.; Ledoux, D.R. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Brit. Poult. Sci. 2006, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.L. The pathology of mycotoxins. J. Pathol. 1988, 154, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.S. Mycotoxins: Food contamination, mechanism, carcinogenic potential and preventive measures. Mutat. Res. 1991, 259, 291–306. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods--a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Kohiyama, C.Y.; Yamamoto Ribeiro, M.M.; Mossini, S.A.; Bando, E. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015, 173, 1006–1010. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Dubey, N.K. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant based antimicrobial. Food Chem. Toxicol. 2010, 48, 539–543. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Sánchez, C.B.R. Vapor-Phase Activities of Cinnamon, Thyme, and Oregano Essential Oils and Key Constituents against Foodbore Microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Sivaranjani, R.; Rona, V. Mechanism of antioxidant and antifungal properties of Pimenta dioica (L.) leaf essential oil on Aspergillus flavus. J. Food Sci. Technol. 2021, 58, 2497–2506. [Google Scholar]
- Shukla, R.; Kumar, A.; Singh, P.; Dubey, N.K. Efficacy of Lippia alba (Mill.) N.E. Brown essential oil and its monoterpene aldehyde constituents against fungi isolated from some edible legume seeds and aflatoxin B1 production. Int. J. Food Microbiol. 2009, 135, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.L.E. Antifungal action and antiaflatoxigenic properties of some essential oil constituents. Lett. Appl. Microbiol. 1994, 19, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Malo, A.; Maris Alzamora, S.; Palou, E. Aspergillus flavus growth in the presence of chemical preservatives and naturally occurring antimicrobial compounds. Int. J. Food Microbiol. 2005, 99, 119–128. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, L.; Lu, Z.; Liu, Y. In vitro anti-aflatoxigenic effect and mode of action of cinnamaldehyde against aflatoxin B1. Int. Biodeterior. Biodegrad. 2015, 104, 419–425. [Google Scholar] [CrossRef]
- Nogueira, J.H.; Goncalez, E.; Galleti, S.R.; Facanali, R. Ageratum conyzoides essential oil as aflatoxin suppressor of Aspergillus flavus. Int. J. Food Microbial. 2010, 137, 55–60. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Kembro, J.M.; Ballesteros, M.L.; Caliva, J.M.; Marin, R.H.; Labaque, M.C. Dynamics of thymol dietary supplementation in quail (Cotrrnix japonica): Linking bioavailability, effects on egg yolk total fatty acids and performance traits. PLoS ONE 2019, 4, 623–629. [Google Scholar]
- Abo Ghanima, M.M.; Elsadek, M.F.; Taha, A.E.; Abd El-Hack, M.E.; Alagawany, M.; Ahmed, B.M.; Elshafie, M.M.; El-Sabrout, K. Effect of Housing System and Rosemary and Cinnamon Essential Oils on Layers Performance, Egg Qualiyt, Haematological Traits, Blood Chemistry, Immunity, and Antioxidant. Animals 2020, 10, 245. [Google Scholar] [CrossRef]
- Kürekci, C.; Özsoy, B.; Hassan, E.; Özkan, H.; Gundoğdu, A.; Özsoy, Ş.Y.; Yakan, A. Effect of essential oil supplementation to diet on meat quality, fatty acid composition, performance parameters and intestinal microbiota of Japanese quails. J.Anim. Physiol. Anim. Nutr. 2021, 105, 927–937. [Google Scholar] [CrossRef]
- Alessandro, S.; Nunziatina, R.; Antonino, M.; Carmine, S.; Antonio, N.; Carmine, S.; Antonio, N.; Cinzia, C.; Cinzia, L.R.; Massimiliano, L. Effect of essential oil supplementation to diet on meat quality, fatty acid composition, performance parameters and intestinal microbiota of Japanese quails. Animals 2020, 10, 640. [Google Scholar]
- Gorran, A.; Farzaneh, M.; Shivazad, M.; Rezaeian, M. Aflatoxin B1-reduction of Aspergillus flavus by three medicinal plants (Lamiaceae). Food Control 2013, 31, 218–223. [Google Scholar] [CrossRef]
- Karaca, N.; Sener, G.; Demirci, B.; Demirci, F. Synergistic antibacterial combination of Lavandula latifolia Medik. essential oil with camphor. Z. Naturforsch. C J. Biosci. 2021, 76, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.B.; Chen, C.H.; Kollanoor-Johny, A.; Darre, M.J. Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans-cinnamaldehyde. Poult. Sci. 2015, 94, 2183–2190. [Google Scholar] [CrossRef] [PubMed]
| Essential Oils | MICs |
|---|---|
| Cinnamaldehyde | 80 ppm |
| Citral | 80 ppm |
| Cinnamaldehyde | 0 | 1/4 × MIC | 1/2 × MIC | 1 × MIC | 2 × MIC | |
|---|---|---|---|---|---|---|
| Citral | ||||||
| 0 | 0.439 ± 0.012 | 0.428 ± 0.022 | 0.427 ± 0.038 | 0.377 ± 0.034 | 0.376 ± 0.027 | |
| 1/4 × MIC | 0.419 ± 0.009 | 0.401 ± 0.028 | 0.369 ± 0.029 | 0.371 ± 0.011 | 0.371 ± 0.051 | |
| 1/2 × MIC | 0.418 ± 0.025 | 0.383 ± 0.003 | 0.425 ± 0.082 | 0.372 ± 0.028 | 0.374 ± 0.044 | |
| 1 × MIC | 0.419 ± 0.018 | 0.392 ± 0.008 | 0.372 ± 0.022 | 0.368 ± 0.036 | 0.375 ± 0.051 | |
| 2 × MIC | 0.406 ± 0.003 | 0.392 ± 0.053 | 0.366 ± 0.004 | 0.377 ± 0.017 | 0.376 ± 0.032 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Fu, G.-W.; Wang, J.-Q. Inhibition of Essential Oils on Growth of Aspergillus flavus and Aflatoxin B1 Production in Broth and Poultry Feed. Toxins 2022, 14, 655. https://doi.org/10.3390/toxins14100655
Han B, Fu G-W, Wang J-Q. Inhibition of Essential Oils on Growth of Aspergillus flavus and Aflatoxin B1 Production in Broth and Poultry Feed. Toxins. 2022; 14(10):655. https://doi.org/10.3390/toxins14100655
Chicago/Turabian StyleHan, Bing, Guang-Wu Fu, and Jin-Quan Wang. 2022. "Inhibition of Essential Oils on Growth of Aspergillus flavus and Aflatoxin B1 Production in Broth and Poultry Feed" Toxins 14, no. 10: 655. https://doi.org/10.3390/toxins14100655
APA StyleHan, B., Fu, G.-W., & Wang, J.-Q. (2022). Inhibition of Essential Oils on Growth of Aspergillus flavus and Aflatoxin B1 Production in Broth and Poultry Feed. Toxins, 14(10), 655. https://doi.org/10.3390/toxins14100655






