Aflatoxins in Feed: Types, Metabolism, Health Consequences in Swine and Mitigation Strategies
Abstract
:1. Introduction

2. Types of Aflatoxins
| Aflatoxin Type | Molecular Formula | Molecular Weight (g /mol) | Melting Point (°C) | Fluorescence | |
|---|---|---|---|---|---|
| λ Excitation (nm) | λ Emission (nm) | ||||
| B1 [29] | C17H12O6 | 312 | 268–269 | 223 | 425 |
| B2 [29] | C17H14O6 | 314 | 286–289 | 265 | 425 |
| G1 [29] | C17H12O7 | 328 | 244–246 | 243 | 450 |
| G2 [29] | C17H14O7 | 330 | 237–240 | 265 | 450 |
| M1 [33] | C17H12O7 | 328 | 299 | 365 | 435 |
| M2 [34] | C17H14O7 | 330 | 293 | 360 | 450 |
| Aflatoxicol [32] | C17H14O6 | 314 | 225 | 325 | 425 |
| Aflatoxin Q1 [31] | C17H12O7 | 328 | 250 | 365 | 466 |
2.1. Aflatoxins B1 and B2
2.2. Aflatoxins G1 and G2
2.3. Aflatoxins M1 and M2
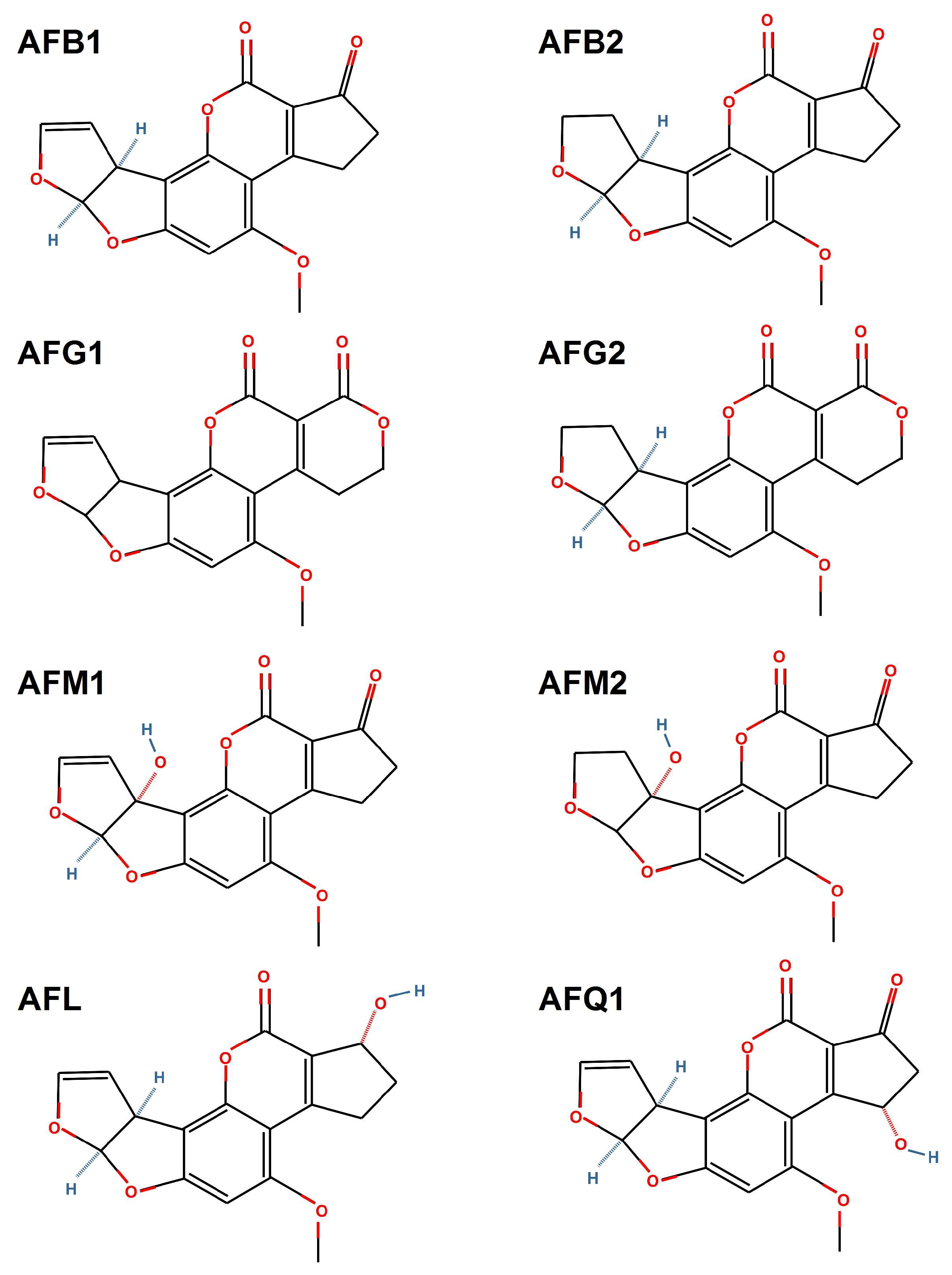
2.4. Aflatoxicol
2.5. Aflatoxin Q1
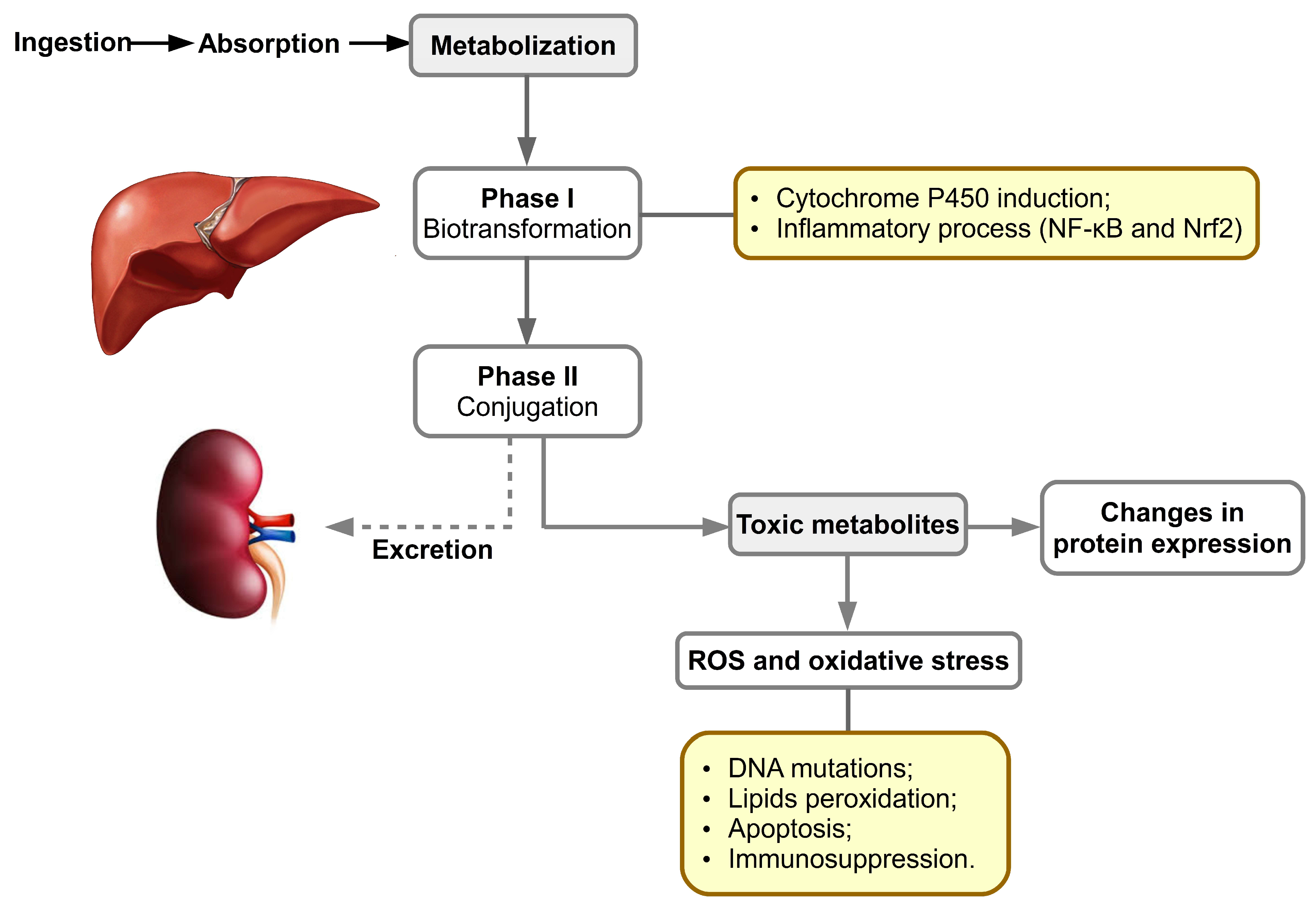
3. Aflatoxins’ Metabolism: Biochemical, Molecular and Cell Signaling Aspects
4. Aflatoxin’s Toxicity in Swine
5. Methods to Reduce Aflatoxins’ Toxicity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyo-Manzanares, N.; Huertas-Pérez, J.F.; García-Campaña, A.M.; Gámiz-Gracia, L. Aflatoxins in animal feeds: A straightforward and cost-effective analytical method. Food Control 2015, 54, 74–78. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilkumar, T.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Gräfenhan, T. Near-Infrared (NIR) hyperspectral imaging: Theory and applications to detect fungal infection and mycotoxin contamination in food products. Indian J. Entomol. 2016, 78, 91. [Google Scholar] [CrossRef]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological interactions between mycotoxins from ubiquitous fungi: Impact on hepatic and intestinal human epithelial cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pleadin, J.; Kovačević, D.; Perković, I. Impact of casing damaging on aflatoxin B 1 concentration during the ripening of dry-fermented meat sausages. J. Immunoass. Immunochem. 2015, 36, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Rheeder, J.P.; Marasas, W.F.O.; Vismer, H.F. Production of Fumonisin Analogs by. Society 2002, 68, 2101–2105. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; Kobbeman, K.; Montalbano, B.G.; Cotty, P.J. Aflatoxin-producing Aspergillus species from Thailand. Int. J. Food Microbiol. 2007, 114, 153–159. [Google Scholar] [CrossRef]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From simple to complex mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Fakruddin, M.; Chowdhury, A.; Hossain, M.N.; Ahmed, M.M. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. Springerplus 2015, 4, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wentzel, J.F.; Lombard, M.J.; Du Plessis, L.H.; Zandberg, L. Evaluation of the cytotoxic properties, gene expression profiles and secondary signalling responses of cultured cells exposed to fumonisin B1, deoxynivalenol and zearalenone mycotoxins. Arch. Toxicol. 2017, 91, 2265–2282. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Gagiu, V.; Mateescu, E.; Armeanu, I.; Dobre, A.A.; Smeu, I.; Cucu, M.E.; Oprea, O.A.; Iorga, E.; Belc, N. Post-harvest contamination with mycotoxins in the context of the geographic and agroclimatic conditions in Romania. Toxins 2018, 10, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.; Siri-Anusornsak, W.; Petchkongkaw, A.; Meneely, J.; Elliott, C. The Efficacy of Additives for the Mitigation of Aflatoxins in Animal Feed: A Systematic Review and Network Meta-Analysis. Toxins 2022, 14, 707. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Filazi, A.; Sireli, U.T. Chapter 7. Occurrence of aflatoxins in food. In Aflatoxins: Recent Advances and Future Prospects; Mehdi, R.-A., Ed.; InTech Open Access: London, UK, 2012; pp. 143–170. [Google Scholar]
- Feddern, V.; Dors, G.C.; Tavernari, F.; Mazzuco, H.; Cunha, J.A.; Krabbe, E.L.; Scheuermann, G.N. Aflatoxins: Importance on animal nutrition. Aflatoxins Recent Adv. Futur. Prospect. InTech Open Access Croat. 2013, 171–195. [Google Scholar] [CrossRef] [Green Version]
- Seetha, A.; Munthali, W.; Msere, H.W.; Swai, E.; Muzanila, Y.; Sichone, E.; Tsusaka, T.W.; Rathore, A.; Okori, P. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017, 33, 323–331. [Google Scholar] [CrossRef]
- Ismail, A.; Gonçalves, B.L.; de Neeff, D.V.; Ponzilacqua, B.; Coppa, C.F.S.C.; Hintzsche, H.; Sajid, M.; Cruz, A.G.; Corassin, C.H.; Oliveira, C.A.F. Aflatoxin in foodstuffs: Occurrence and recent advances in decontamination. Food Res. Int. 2018, 113, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Negash, D. A Review of Aflatoxin: Occurrence, Prevention, and Gaps in Both Food and Feed Safety. J. Appl. Microbiol. Res. 2018, 1, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Okoth, S. Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa; CTA Working Paper; 16/13; CTA: Wageningen, The Netherland, 2016; pp. 1–113. Available online: https://hdl.handle.net/10568/90118 (accessed on 23 September 2022).
- MdQuadri, S.H.; Niranjan, M.; Chaluvaraju, K.; Shantaram, U.; Enamul, H. An Overview on Chemistry, Toxicity, Analysis and Control of Aflatoxins. Int. J. Chem. Life Sci. 2017, 2, 1071–1078. [Google Scholar]
- Sailaja, O.; Krishnaven, G.; Manoranjani, M. Identification and High-performance Liquid Chromatography Quantification of Aflatoxins in Red Chili. Asian J. Pharm. 2017, 2017, 933–937. [Google Scholar]
- Akeberegn, D.; Alemneh, T.; Zewudie, D. Effects of Aflatoxin Contamination in Milk: A Review. MRJMBS 2018, 6, 118–128. [Google Scholar]
- Sedova, I.; Kiseleva, M.; Tutelyan, V. Mycotoxins in Tea: Occurrence, Methods of Determination and Risk Evaluation. Toxins 2018, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Vijaya Kumar, V. Aflatoxins: Properties, Toxicity and Detoxification. Nutr. Food Sci. Int. J. 2018, 6, 555696. [Google Scholar] [CrossRef]
- Bbosa, G.S.; Kitya, D.; Odda, J.; Ogwal-Okeng, J. Aflatoxins metabolism. Health 2013, 5, 14–34. [Google Scholar] [CrossRef] [Green Version]
- Franco, C.M.; Fente, C.A.; Vázquez, B.I.; Cepeda, A.; Mahuzier, G.; Prognon, P. Interaction between cyclodextrins and aflatoxins Q1, M1 and P1. Fluorescence and chromatographic studies. J. Chromatogr. A 1998, 815, 21–29. [Google Scholar] [CrossRef]
- Carvajal, M.; Rojo, F.; Méndez, I.; Bolños, A. Aflatoxin B1and its interconverting metabolite aflatoxicol in milk: The situation in Mexico. Food Addit. Contam. 2003, 20, 1077–1086. [Google Scholar] [CrossRef]
- Behfar, A.; Khorasgani, Z.N.; Alemzadeh, Z.; Goudarzi, M.; Ebrahimi, R.; Tarhani, N. Determination of Aflatoxin M1 Levels in Produced Pasteurized Milk in Ahvaz City by Using HPLC. Jundishapur J. Nat. Pharm. Prod. 2012, 7, 80–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Lee, K.G. Analysis of aflatoxin M1 and M2 in commercial dairy products using high-performance liquid chromatography with a fluorescence detector. Food Control. 2015, 50, 467–471. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Asker, A.A. Aflatoxins in rabbit production: Hazards and control. Trop. Subtrop. Agroecosystems 2008, 8, 1–28. [Google Scholar]
- Rawal, S.; Kim, J.E.; Coulombe, R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.H.E.-D. (Ed.) Mycotoxins-Induced Oxidative Stress and Disease. In Mycotoxin and Food Safety in Developing Countries; InTech Open Access: London, UK, 2013; pp. 63–92. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, A.T.; Hirooka, E.Y.; e Silva, P.L.A.; Bracarense, A.P.F.R.L.; Da Costa, K.K.M.; Akagi, C.Y.; Kawamura, O.; Da Costa, M.C.; Itano, E.N. Impact of a single oral acute dose of aflatoxin b1on liver function/cytokines and the lymphoproliferative response in C57BL/6 mice. Toxins 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.B.; Abdel Kader, M.M.; Wick, E.L.; Wogan, G.N. Aflatoxin B2: Chemical identity and biological activity. Science 1963, 142, 1191–1192. [Google Scholar] [CrossRef]
- Yabe, K.; Ando, Y.; Hamasaki, T. Biosynthetic Relationship among aflatoxins B1, B2, G1 and G2. Appl. Environ. Microbiol. 1988, 54, 2101–2106. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Corey, E.J. Short, enantioselective total synthesis of aflatoxin B2 using an asymmetric [3+2]-cycloaddition step. J. Am. Chem. Soc. 2005, 127, 11958–11959. [Google Scholar] [CrossRef]
- Wong, J.J.; Hsieh, D.P.H. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc. Natl. Acad. Sci. USA 1976, 73, 2241–2244. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Galvano, F.; Galofaro, V.; Galvano, G. Occurrence and Stability of Aflatoxin M 1 in Milk and Milk Products: A Worldwide Review. J. Food Prot. 1996, 59, 1079–1090. [Google Scholar] [CrossRef]
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlberg, S.; Grace, D.; Kiarie, G.; Kirino, Y.; Lindahl, J. A risk assessment of Aflatoxin M1 exposure in low and mid-income dairy consumers in Kenya. Toxins 2018, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Puga-Torres, B.; Salazar, D.; Cachiguango, M.; Cisneros, G.; Gómez-Bravo, C. Determination of aflatoxin M1 in raw milk from different provinces of Ecuador. Toxins 2020, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Righetti, L.; Rolli, E.; Dellafiora, L.; Galaverna, G.; Suman, M.; Bruni, R.; Dall’Asta, C. Thinking Out of the Box: On the Ability of Zea mays L. to Biotrasform Aflatoxin B1 Into Its Modified Forms. Front. Plant Sci. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Salhab, A.S.; Edwards, G.S. Comparative in Vitro Metabolism of Aflatoxicol by Liver Preparations from Animals and Humans. Cancer Res. 1976, 37, 1016–1021. [Google Scholar] [CrossRef] [Green Version]
- Schoenhard, G.L.; Hendricks, J.D.; Nixon, J.E.; Lee, D.J.; Wales, J.H.; Sinnhuber, R.O.; Pawlowski, N.E. Aflatoxicol-induced hepatocellular carcinoma in Rainbow Trout (Salmogairdneri) and the synergistic effects of cyclopropenoid fatty acids. Cancer Res. 1981, 41, 1011–1014. [Google Scholar]
- Nakazato, M.; Morozumi, S.; Saito, K.; Fujinuma, K.; Nishima, T.; Kasai, N. Interconversion of aflatoxin B1 and aflatoxicol by several fungi. Appl. Environ. Microbiol. 1990, 56, 1465–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabulut, S.; Paytakov, G.; Leszczynski, J. Reduction of aflatoxin B1 to aflatoxicol: A comprehensive DFT study provides clues to its toxicity. J. Sci. Food Agric. 2014, 94, 3134–3140. [Google Scholar] [CrossRef]
- Yourtee, D.M.; Bean, T.A.; Kirk-Yourtee, C.L. Human aflatoxin B1metabolism: An investigation of the importance of aflatoxin Q1as a metabolite of hepatic post-mitochondrial fraction. Toxicol. Lett. 1987, 38, 213–224. [Google Scholar] [CrossRef]
- Hendricks, J.D.; Sinnhuber, R.O.; Nixon, J.E.; Wales, J.H.; Masri, M.S.; Hsieh, D.P. Carcinogenic response of rainbow trout (Salmo gairdneri) to aflatoxin Q1 and synergistic effect of cyclopropenoid fatty acids. J. Natl. Cancer Inst. 1980, 64, 523–528. [Google Scholar] [PubMed]
- Fan, T.S.L.; Zhang, G.S.; Chu, F.S. Production and characterization of antibody against aflatoxin Q1. Appl. Environ. Microbiol. 1986, 47, 526–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonissen, G.; Devreese, M.; De Baere, S.; Martel, A.; Van Immerseel, F.; Croubels, S. Impact of Fusarium mycotoxins on hepatic and intestinal mRNA expression of cytochrome P450 enzymes and drug transporters, and on the pharmacokinetics of oral enrofloxacin in broiler chickens. Food Chem. Toxicol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Carvajal-Moreno, M. Metabolic Changes of Aflatoxin B1 to become an Active Carcinogen and the Control of this Toxin. Immunome Res. 2015, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Mulero, J.; Martínez, G.; Oliva, J.; Cermeño, S.; Cayuela, J.M.; Zafrilla, P.; Martínez-Cachá, A.; Barba, A. Phenolic compounds and antioxidant activity of red wine made from grapes treated with different fungicides. Food Chem. 2015, 180, 25–31. [Google Scholar] [CrossRef]
- Jarolim, K.; Del Favero, G.; Pahlke, G.; Dostal, V.; Zimmermann, K.; Heiss, E.; Ellmer, D.; Stark, T.D.; Hofmann, T.; Marko, D. Activation of the Nrf2-ARE pathway by the Alternaria alternata mycotoxins altertoxin I and II. Arch. Toxicol. 2017, 91, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Burkina, V.; Rasmussen, M.K.; Oliinychenko, Y.; Zamaratskaia, G. Porcine cytochrome 2A19 and 2E1. Basic Clin. Pharmacol. Toxicol. 2019, 124, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nannelli, A.; Chirulli, V.; Longo, V.; Gervasi, P.G. Expression and induction by rifampicin of CAR- and PXR-regulated CYP2B and CYP3A in liver, kidney and airways of pig. Toxicology 2008, 252, 105–112. [Google Scholar] [CrossRef]
- Yao, M.; Dai, M.; Liu, Z.; Huang, L.; Chen, D.; Wang, Y.; Peng, D.; Wang, X.; Liu, Z.; Yuan, Z. Comparison of the substrate kinetics of pig CYP3A29 with pig liver microsomes and human CYP3A4. Biosci. Rep. 2011, 31, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, E.; Gervasi, P.G.; La Marca, M.; Beffy, P.; Longo, V. Expression and inducibility by phenobarbital of CYP2C33, CYP2C42, CYP2C49, CYP2B22, and CYP3As in porcine liver, kidney, small intestine, and nasal tissues. Xenobiotica 2010, 40, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Nuffer, J.H.; Muñiz-Papandrea, V.A.; Colón, W.; Siegel, R.W.; Dordick, J.S. Cytochrome c on silica nanoparticles: Influence of nanoparticle size on protein structure, stability, and activity. Small 2009, 5, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Morozumi, T. Cloning of six full-length cDNAs encoding pig cytochrome P450 enzymes and gene expression of these enzymes in the liver and kidney. J. Health Sci. 2004, 50, 518–529. [Google Scholar] [CrossRef]
- Achour, B.; Barber, J.; Rostami-Hodjegan, A. Correction to “Cytochrome P450 pig liver pie: Determination of individual cytochrome P450 isoform contents in microsomes from two pig livers using liquid chromatography in conjunction with mass spectroscopy” (Drug Metabolism and Disposition (2011) 39, (2130-2134)). Drug Metab. Dispos. 2012, 40, 227. [Google Scholar] [CrossRef]
- Lehman-McKeeman, L.D.; Ruepp, S.U. Biochemical and Molecular Basis of Toxicity, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128098424. [Google Scholar]
- Gallagher, E.P.; Kunze, K.L.; Stapleton, P.L.; Eaton, D.L. The kinetics of aflatoxin B1oxidation by human cDNA-expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicol. Appl. Pharmacol. 1996, 141, 595–606. [Google Scholar] [CrossRef]
- Devreese, M.; De Backer, P.; Croubels, S. Overview of the most important mycotoxins for the pig and poultry husbandry Overzicht van de meest belangrijke mycotoxines voor de varkens-en pluimveehouderij. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 171–180. [Google Scholar] [CrossRef]
- Dhama, K.; Singh, K.P. Aflatoxins- Hazard to Livestock and Poultry Production: A Review. Vet. Immunol. Immunopathol. 2007, 9, 1–15. [Google Scholar]
- Diaz, G.J.; Murcia, H.W.; Cepeda, S.M.; Boermans, H.J. The role of selected cytochrome P450 enzymes on the bioactivation of aflatoxin B1 by duck liver microsomes. Avian Pathol. 2010, 39, 279–285. [Google Scholar] [CrossRef]
- Pauletto, M.; Tolosi, R.; Giantin, M.; Guerra, G.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Insights into Aflatoxin B1 Toxicity in Cattle: An in vitro whole-transcriptomic approach. Toxins 2020, 12, 429. [Google Scholar] [CrossRef]
- Iori, S.; Pauletto, M.; Bassan, I.; Bonsembiante, F.; Gelain, M.E.; Bardhi, A.; Barbarossa, A.; Zaghini, A.; Dacasto, M.; Giantin, M. Deepening the Whole Transcriptomics of Bovine Liver Cells Exposed to AFB1: A Spotlight on Toll-like Receptor 2. Toxins 2022, 14, 504. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kim, T.H.; Hong, M.W.; Park, T.S.; Lee, H.; Lee, S.J. Transcriptomic alterations induced by aflatoxin B1 and ochratoxin A in LMH cell line. Poult. Sci. 2020, 99, 5265–5274. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, Y.; Wu, T.; Srinivas, S.; Yang, W.; Fan, J.; Yang, C.; Wang, S. Aflatoxin B1 Negatively Regulates Wnt/β-Catenin Signaling Pathway through Activating miR-33a. PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, J.; Huang, K.; Luo, Y.; Zhang, B.; Xu, W. miR-34a screened by miRNA profiling negatively regulates Wnt/β-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caloni, F.; Cortinovis, C. Toxicological effects of aflatoxins in horses. Vet. J. 2011, 188, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Y.; Guo, Y.; Ma, Q.; Ji, C.; Zhao, L. Transcriptional profiling of aflatoxin b1-induced oxidative stress and inflammatory response in macrophages. Toxins 2021, 13, 401. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research progress in toxicological effects and mechanism of aflatoxin B1 toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef] [PubMed]
- Velayudhan, D.E.; Kim, I.H.; Nyachoti, C.M. Characterization of dietary energy in swine feed and feed ingredients: A review of recent research results. Asian-Australasian J. Anim. Sci. 2015, 28, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in Corn: Occurrence, Impacts, and Management, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-811971-6. [Google Scholar]
- Diekman, M.A.; Coffey, M.T.; Purkhiser, E.D.; Reeves, D.E.; Young, L.G. Mycotoxins and Swine Performance. Nutrition 1914, 6, 1–6. [Google Scholar]
- Guerre, P. Worldwide mycotoxins exposure in pig and poultry feed formulations. Toxins 2016, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Park, C.S.; Kim, B.G. Evaluation of a mycotoxin adsorbent in swine diets containing barley naturally contaminated with Fusarium mycotoxins. Rev. Colomb. Ciencias Pecu. 2016, 29, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Tiemann, U.; Dänicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanora, A.; Maes, D. The role of mycotoxins in pig reproduction: A review. Vet. Med. 2009, 54, 565–576. [Google Scholar] [CrossRef] [Green Version]
- Chaytor, A.C.; Hansen, J.A.; Van Heugten, E.; See, M.T.; Kim, S.W. Occurrence and decontamination of mycotoxins in swine feed. Asian-Australasian J. Anim. Sci. 2011, 24, 723–738. [Google Scholar] [CrossRef]
- Prodanov-Radulovic, J.; Dosen, R.; Stojanov, I.; Pusic, I.; Zivkov-Balos, M.; Ratajac, R. Influence of mycotoxin zearalenone on the swine reproductive failure. Zb. Matice Srp. Za Prir. Nauk. 2013, 124, 121–129. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Fan, Y.; Jia, Y.; Sun, L.; Ma, S.; Ji, C.; Ma, Q.; Zhang, J. Occurrence of mycotoxins in feed ingredients and complete feeds obtained from the Beijing region of China. J. Anim. Sci. Biotechnol. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, J.; Moyo, K.M.; Park, K.; Jeong, J.; Kim, H.; Ryu, Y.; Kim, J.; Kim, J.; Lee, S.; Go, G. Meat Quality Traits of Pigs Finished on Food Waste. Korean J. Food Sci. Anim. Resour. 2017, 37, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Luthy, J.; Zweifel, U.; Schlatter, C. Metabolism [14C] Aflatoxin B, in Pigs. Food Cosmet. Toxicol. 1980, 18, 253–256. [Google Scholar] [CrossRef]
- Patterson, D.S.P. Metabolism as a factor in determining the toxic action of the aflatoxins in different animal species. Food Cosmet. Toxicol. 1973, 11, 287–294. [Google Scholar] [CrossRef]
- Tang, D.; Sauceda, J.C.; Lin, Z.; Ott, S.; Basova, E.; Goryacheva, I.; Biselli, S.; Lin, J.; Niessner, R.; Knopp, D. Magnetic nanogold microspheres-based lateral-flow immunodipstick for rapid detection of aflatoxin B2 in food. Biosens. Bioelectron. 2009, 25, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Furtado, R.M.; Pearson, A.M.; Hogberg, M.G.; Miller, E.R.; Gray, J.I.; Aust, S.D. Withdrawal Time Required for Clearance of Aflatoxins from Pig Tissues. J. Agric. Food Chem. 1982, 30, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, D.P.H.; Wong, J.J. Pharmacokinetics and excretionof aÑatoxins. In The Toxicology of Afatoxins; Eaton, D.L., Groopman, J.D., Eds.; Academic Press Inc.: San Diego, CA, USA, 1994; pp. 73–88. [Google Scholar]
- Thieu, N.Q.; Pettersson, H. Zearalenone, deoxynivalenol and aflatoxin B1and their metabolites in pig urine as biomarkers for mycotoxin exposure. Mycotoxin Res. 2009, 25, 59–66. [Google Scholar] [CrossRef] [PubMed]
- AFSSA. Review of mycotoxin-detoxifying agents used as feed additives: Mode of action, efficacy and feed/food safety. EFSA Support. Publ. 2009, 6, 1–192. [Google Scholar]
- Food and Drug Administration. CPG Sec. 683.100 Action Levels for Aflatoxins in Animal Feeds. 2015. Available online: https://www.fda.gov/iceci/compliancemanuals/ (accessed on 23 September 2022).
- Korley Kortei, N.; Akomeah Agyekum, A.; Akuamoa, F.; Baffour, V.K.; Wiisibie Alidu, H. Risk assessment and exposure to levels of naturally occurring aflatoxins in some packaged cereals and cereal based foods consumed in Accra, Ghana. Toxicol. Rep. 2019, 6, 34–41. [Google Scholar] [CrossRef]
- Lee, H.S.; Lindahl, J.; Nguyen-Viet, H.; Khong, N.V.; Nghia, V.B.; Xuan, H.N.; Grace, D. An investigation into aflatoxin M1in slaughtered fattening pigs and awareness of aflatoxins in Vietnam. BMC Vet. Res. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mupunga, I.; Mngqawa, P.; Katerere, D.R. Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Nakavuma, J.L.; Kirabo, A.; Bogere, P.; Nabulime, M.M.; Kaaya, A.N.; Gnonlonfin, B. Awareness of mycotoxins and occurrence of aflatoxins in poultry feeds and feed ingredients in selected regions of Uganda. Int. J. Food Contam. 2020, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Weaver, A.C. The Impact of Mycotoxins on Growth and Health of Swine. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2013; p. 211. [Google Scholar]
- Newbern, P.M.; Butler, W.H. Acute and Chronic Effects of Aflatoxin on the Liver of Domestic and Laboratory Animals: A Review. Cancer Res. 1969, 29, 236–250. [Google Scholar]
- Silvotti, L.; Petterino, C.; Bonomi, A.; Cabassi, E. Immunotoxicological effects on piglets of feeding sows diets containing aflatoxins. Vet. Rec. 1997, 141, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, A.; Bonomi, F.; Benedetti, S.; Mannino, S.; Monti, L.; Cattaneo, T.; Iametti, S. Binding of Aflatoxin M1 to Different Protein Fractions in Ovine and Caprine Milk. J. Dairy Sci. 2007, 90, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, J.H.; Choi, D.-K. Aflatoxins: Detection, Toxicity, and Biosynthesis. Biotechnol. Bioprocess Eng. 2007, 12, 585–593. [Google Scholar] [CrossRef]
- Jw, J.; Nm, W.; Hm, I.; Ss, A. Short Communication Aflatoxicosis Associated with Swine Stillbirth in the Aflatoxicosis Associated with Swine Stillbirth in the Piggery Farm University of Agriculture Makurdi. CTEB 2018, 13, 13–16. [Google Scholar] [CrossRef]
- Wilfred, E.G.; Dungworth, D.L.; Moulton, J.E. Pathologic Effects of Aflatoxin in Pigs. Vet. Pathol. 1968, 5, 370–384. [Google Scholar] [CrossRef]
- Dilkin, P.; Zorzete, P.; Mallmann, C.A.; Gomes, J.D.F.; Utiyama, C.E.; Oetting, L.L.; Corrêa, B. Toxicological effects of chronic low doses of aflatoxin B1 and fumonisin B1-containing Fusarium moniliforme culture material in weaned piglets. Food Chem. Toxicol. 2003, 41, 1345–1353. [Google Scholar] [CrossRef]
- Obuseh, F.A.; Jolly, P.E.; Jiang, Y.; Shuaib, F.M.B.; Waterbor, J.; Ellis, W.O.; Piyathilake, C.J.; Desmond, R.A.; Afriyie-Gyawu, E.; Phillips, T.D. Aflatoxin B1 albumin adducts in plasma and aflatoxin M1 in urine are associated with plasma concentrations of vitamins A and E. Int. J. Vitam. Nutr. Res. 2010, 80, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Marin, D.E.; Taranu, I.; Bunaciu, R.P.; Pascale, F.; Tudor, D.S.; Avram, N.; Sarca, M.; Cureu, I.; Criste, R.D.; Suta, V.; et al. Changes in performance, blood parameters, humoral and cellular immune responses in weanling piglets exposed to low doses of aflatoxin. J. Anim. Sci. 2002, 80, 1250–1257. [Google Scholar] [CrossRef]
- Meissonnier, G.M.; Pinton, P.; Laffitte, J.; Cossalter, A.M.; Gong, Y.Y.; Wild, C.P.; Bertin, G.; Galtier, P.; Oswald, I.P. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 2008, 231, 142–149. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Stec, J.A.N.; Mudzki, J.Ż.; Rachubik, J.Ł.A.W.; Szczotka, M. Effects of aflatoxin B1, ochratoxin A, patulin, citrinin, and zearalenone on the in vitro proliferation of pig blood lymphocytes. Bull. Vet. Inst. Pulawy 2009, 53, 129–134. [Google Scholar]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hintz, H.F.; Heitman, H.; Booth, A.N.; Gagne, W.E. Effects of aflatoxin on reproduction in swine. Proc. Soc. Exp. Biol. Med. 1967, 126, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.C.; Han, J.; Xiong, B.; Sun, S.C. Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis 2015, 30, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.M.; Stuart, B.P.; Crowell, W.A. Experimental aflatoxicosis in swine: Morphological and clinical pathological results. Can. J. Comp. Med. 1981, 45, 343–351. [Google Scholar] [PubMed]
- Ketterer, P.J.; Blaney, B.J.; Moore, C.J.; McInnes, I.S.; Cook, P.W. Field cases of aflatoxicosis in pigs. Aust. Vet. J. 1982, 59, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidin, Z.; Khatoon, A.; Numan, M. Mycotoxins in broilers: Pathological alterations induced by aflatoxins and ochratoxins, diagnosis and determination, treatment and control of mycotoxicosis. Worlds Poult. Sci. J. 2011, 67, 485–496. [Google Scholar] [CrossRef]
- Shivasharanappa, G.Y.; Mundas, S.; Rao, D.G.K.; Tikare, V.; Shridhar, N.B. Histopathological Changes in Pigs Exposed to Aflatoxin B1 During Pregnancy. Indian J. Anim. Res. 2013, 47, 386–391. [Google Scholar]
- Monson, M.; Coulombe, R.; Reed, K. Aflatoxicosis: Lessons from Toxicity and Responses to Aflatoxin B1 in Poultry. Agriculture 2015, 5, 742–777. [Google Scholar] [CrossRef] [Green Version]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Freitas, B.V.; Mota, M.M.; Del Santo, T.A.; Afonso, E.R.; Silva, C.C.; Utimi, N.B.P.; Barbosa, L.C.G.S.; Vilela, F.G.; Araujo, L.F. Mycotoxicosis in swine: A review. J. Anim. Vet. Adv. 2012, 2, 174–181. [Google Scholar]
- Varga, J.; Péteri, Z.; Tábori, K.; Téren, J.; Vágvölgyi, C. Degradation of ochratoxin A and other mycotoxins by Rhizopus isolates. Int. J. Food Microbiol. 2005, 99, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Knabe, D.A.; Wu, G.; Dixon, J.B.; Barrientos Velázquez, A.L.; Deng, Y. Impacts of Aflatoxins on Swine Nutrition and Possible Measures of Amelioration. In Aflatoxin Control: Safeguarding Animal Feed with Calcium Smectite; ACSESS: Hoboken, NJ, USA, 2014; pp. 54–67. [Google Scholar] [CrossRef]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, W. Clustered patway genes in aflatoxins biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalili, M. A Review on Aflatoxins Reduction in Food. Iran. J. Health Saf. Environ. 2015, 3, 445–459. [Google Scholar] [CrossRef] [Green Version]
- Khadem, A.A.; Sharifi, S.D.; Barati, M.; Borji, M. Evaluation of the effectiveness of yeast, zeolite and active charcoal as aflatoxin absorbents in broiler diets. Glob. Vet. 2012, 8, 426–432. [Google Scholar]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 651–657. [Google Scholar] [CrossRef]
- Juglal, S.; Govinden, R.; Odhav, B. Spice Oils for the Control of Co-Occurring Mycotoxin-Producing Fungi. J. Food Prot. 2002, 65, 683–687. [Google Scholar] [CrossRef]
- Roze, L.V.; Hong, S.-Y.; Linz, J.E. Aflatoxin Biosynthesis: Current Frontiers. Annu. Rev. Food Sci. Technol. 2013, 4, 293–311. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Gras, M.A.; Palade, M.L.; Taranu, I. Comparative effect of ochratoxin A on inflammation and oxidative stress parameters in gut and kidney of piglets. Regul. Toxicol. Pharmacol. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, X.; Li, S.; Li, R.; Zhang, X. Curcumin confers hepatoprotection against AFB1-induced toxicity via activating autophagy and ameliorating inflammation involving Nrf2/HO-1 signaling pathway. Mol. Biol. Rep. 2018, 45, 1775–1785. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Cicero, A.F.G.; Sahebkar, A. Protective effects of curcumin against aflatoxicosis: A comprehensive review. J. Cell. Physiol. 2017, 233, 3552–3577. [Google Scholar] [CrossRef] [PubMed]
- Sayyari, A.; Fæste, C.K.; Hansen, U.; Uhlig, S.; Framstad, T.; Schatzmayr, D.; Sivertsen, T. Effects and biotransformation of the mycotoxin deoxynivalenol in growing pigs fed with naturally contaminated pelleted grains with and without the addition of Coriobacteriaceum DSM 11798. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 1394–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, R.G.; Bulgaru, C.; Untea, A.; Vlassa, M.; Filip, M.; Hermenean, A.; Marin, D.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The Effectiveness of Dietary Byproduct Antioxidants on Induced CYP Genes Expression and Histological Alteration in Piglets Liver and Kidney Fed with Aflatoxin B1 and Ochratoxin A. Toxins 2021, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Avramescu, S.; Marin, D.E.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The reduction of the combined effects of aflatoxin and ochratoxin a in piglet livers and kidneys by dietary antioxidants. Toxins 2021, 13, 648. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.G.; Rădulescu, A.L.; Georgescu, S.E.; Dinischiotu, A. Aflatoxins in Feed: Types, Metabolism, Health Consequences in Swine and Mitigation Strategies. Toxins 2022, 14, 853. https://doi.org/10.3390/toxins14120853
Popescu RG, Rădulescu AL, Georgescu SE, Dinischiotu A. Aflatoxins in Feed: Types, Metabolism, Health Consequences in Swine and Mitigation Strategies. Toxins. 2022; 14(12):853. https://doi.org/10.3390/toxins14120853
Chicago/Turabian StylePopescu, Roua Gabriela, Andreea Luminița Rădulescu, Sergiu Emil Georgescu, and Anca Dinischiotu. 2022. "Aflatoxins in Feed: Types, Metabolism, Health Consequences in Swine and Mitigation Strategies" Toxins 14, no. 12: 853. https://doi.org/10.3390/toxins14120853
APA StylePopescu, R. G., Rădulescu, A. L., Georgescu, S. E., & Dinischiotu, A. (2022). Aflatoxins in Feed: Types, Metabolism, Health Consequences in Swine and Mitigation Strategies. Toxins, 14(12), 853. https://doi.org/10.3390/toxins14120853