The Preclinical Evaluation of a Second-Generation Antivenom for Treating Snake Envenoming in India
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties
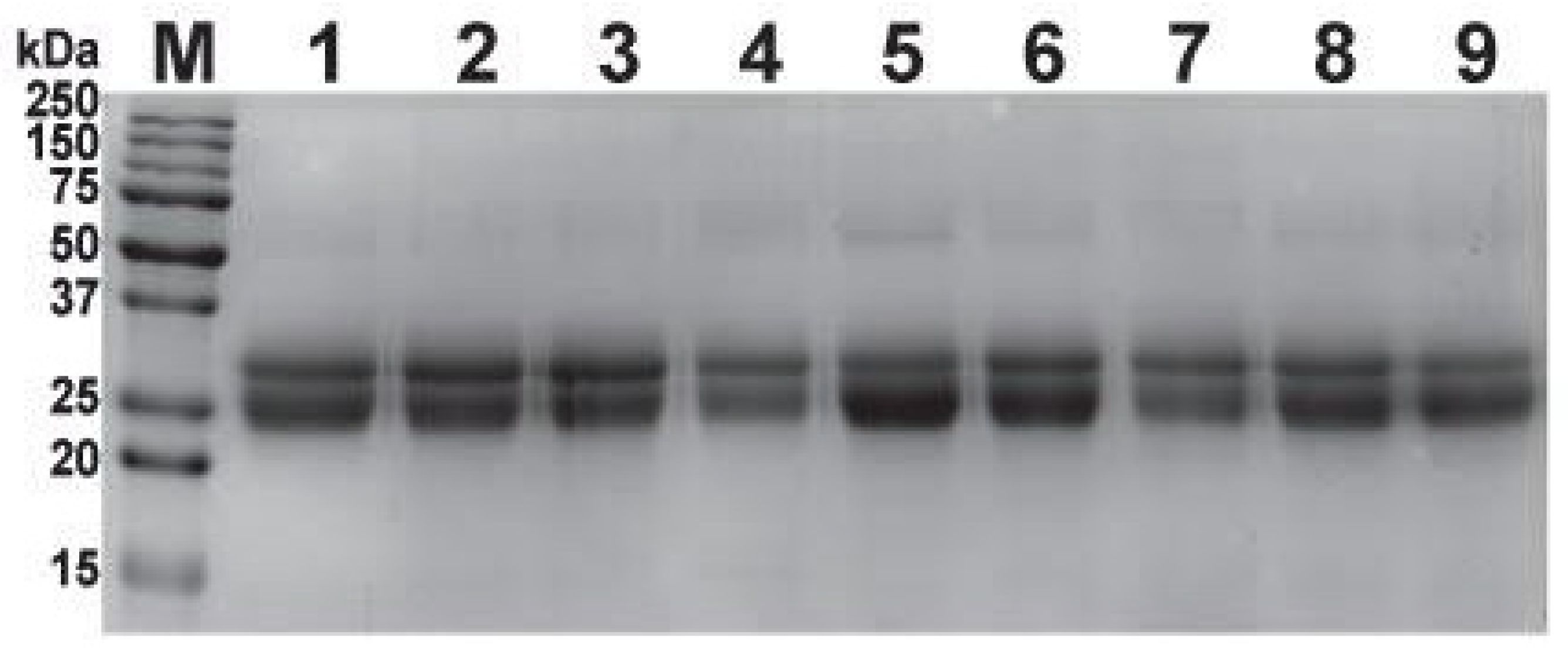
2.2. SDS-PAGE Profiling of Antivenoms
2.3. Size-Exclusion HPLC (SE-HPLC)
2.4. In Vitro Venom-Recognition
2.5. Immunochromatography
2.6. Mass Spectrometric Analyses of Antivenoms
2.7. Venom Neutralisation Potential of Antivenoms
2.8. Morbidity Assays
3. Discussion
3.1. Purification Steps Can Significantly Enhance the Dose-Effectiveness of Commercial Indian Antivenoms
3.2. The Purified Second-Generation Antivenom Exhibits Enhanced In Vitro Venom Recognition and In Vivo Venom Neutralisation Potential Compared to Its Conventional Antivenom Counterparts
3.3. The Second-Generation Antivenom also Exhibits Significant Improvements in the Morbidity Neutralisation Potential
3.4. The ‘Perfect’ Antidote: Formulation of India’s Next-Generation Antivenom Product
3.5. Limitations of the Study
4. Conclusions
5. Materials and Methods
5.1. Snake Venoms and Antivenoms
5.2. Chromatographic Purification
5.3. Physicochemical Characterisation
5.4. Protein Quantification
5.5. One Dimensional SDS-PAGE
5.6. Size-Exclusion HPLC (SE-HPLC)
5.7. Mass Spectrometry (LC-MS/MS)
5.8. Enzyme-Linked Immunosorbent Assay (ELISA)
5.9. Immunoblotting
5.10. Immunochromatography
5.11. The Intravenous Median Lethal Dose (LD50) of Venoms
5.12. Median Effective Dose (ED50) of Antivenoms
5.13. Preclinical Assays
5.14. Morbidity Neutralisation Tests
5.15. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef] [PubMed]
- Senji Laxme, R.; Khochare, S.; Attarde, S.; Suranse, V.; Iyer, A.; Casewell, N.R.; Whitaker, R.; Martin, G.; Sunagar, K. Biogeographic venom variation in Russell’s viper (Daboia russelii) and the preclinical inefficacy of antivenom therapy in snakebite hotspots. PLoS Negl. Trop. Dis. 2021, 15, e0009247. [Google Scholar] [CrossRef]
- Warrell, D.A.; Gutierrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar]
- Shashidharamurthy, R.; Kemparaju, K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int. Immunopharmacol. 2007, 7, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kalita, B.; Singh, S.; Patra, A.; Mukherjee, A.K. Quantitative proteomic analysis and antivenom study revealing that neurotoxic phospholipase A2 enzymes, the major toxin class of Russell’s viper venom from southern India, shows the least immuno-recognition and neutralization by commercial polyvalent antivenom. Int. J. Biol. Macromol. 2018, 118, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the ‘big four’: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Khochare, S.; Senji Laxme, R.R.; Attarde, S.; Dam, P.; Suranse, V.; Khaire, A.; Martin, G.; Captain, A. A Wolf in Another Wolf’s Clothing: Post-Genomic Regulation Dictates Venom Profiles of Medically-Important Cryptic Kraits in India. Toxins 2021, 13, 69. [Google Scholar] [CrossRef]
- Rashmi, U.; Khochare, S.; Attarde, S.; Laxme, R.S.; Suranse, V.; Martin, G.; Sunagar, K. Remarkable intrapopulation venom variability in the monocellate cobra (Naja kaouthia) unveils neglected aspects of India’s snakebite problem. J. Proteom. 2021, 242, 104256. [Google Scholar] [CrossRef] [PubMed]
- Attarde, S.; Khochare, S.; Iyer, A.; Dam, P.; Martin, G.; Sunagar, K. Venomics of the Enigmatic Andaman Cobra (Naja sagittifera) and the Preclinical Failure of Indian Antivenoms in Andaman and Nicobar Islands. Front. Pharmacol. 2021, 12, 768210. [Google Scholar] [CrossRef]
- Deka, A.; Reza, M.A.; Faisal Hoque, K.M.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.K.; Maity, C.R. Biochemical composition, lethality and pathophysiology of venom from two cobras—Naja naja and N. kaouthia. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 131, 125–132. [Google Scholar] [CrossRef]
- Faiz, M.A.; Ahsan, M.F.; Ghose, A.; Rahman, M.R.; Amin, R.; Hossain, M.; Tareq, M.N.U.; Jalil, M.A.; Kuch, U.; Theakston, R.D.G.; et al. Bites by the Monocled cobra, Naja kaouthia, in Chittagong Division, Bangladesh: Epidemiology, Clinical Features of Envenoming and Management of 70 Identified Cases. Am. J. Trop. Med. Hyg. 2017, 96, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Squaiella-Baptistao, C.C.; Magnoli, F.C.; Marcelino, J.R.; Sant’Anna, O.A.; Tambourgi, D.V. Quality of horse F(ab’)2 antitoxins and anti-rabies immunoglobulins: Protein content and anticomplementary activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 16. [Google Scholar] [CrossRef] [PubMed]
- Squaiella-Baptistao, C.C.; Marcelino, J.R.; Ribeiro da Cunha, L.E.; Gutierrez, J.M.; Tambourgi, D.V. Anticomplementary activity of horse IgG and F(ab’)2 antivenoms. Am. J. Trop. Med. Hyg. 2014, 90, 574–584. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Z.; Huang, Y.; Lopes-Virella, M.F.; Virella, G. F(ab’)2 fragments of anti-oxidized LDL IgG attenuate vascular inflammation and atherogenesis in diabetic LDL receptor-deficient mice. Clin. Immunol. 2016, 173, 50–56. [Google Scholar] [CrossRef]
- Ramos-Cerrillo, B.; de Roodt, A.R.; Chippaux, J.P.; Olguin, L.; Casasola, A.; Guzman, G.; Paniagua-Solis, J.; Alagon, A.; Stock, R.P. Characterization of a new polyvalent antivenom (Antivipmyn Africa) against African vipers and elapids. Toxicon 2008, 52, 881–888. [Google Scholar] [CrossRef]
- Tan, C.H.; Liew, J.L.; Tan, K.Y.; Tan, N.H. Assessing SABU (Serum Anti Bisa Ular), the sole Indonesian antivenom: A proteomic analysis and neutralization efficacy study. Sci. Rep. 2016, 6, 37299. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.Y.; Ng, T.S.; Bourges, A.; Ismail, A.K.; Maharani, T.; Khomvilai, S.; Sitprija, V.; Tan, N.H.; Tan, C.H. Geographical variations in king cobra (Ophiophagus hannah) venom from Thailand, Malaysia, Indonesia and China: On venom lethality, antivenom immunoreactivity and in vivo neutralization. Acta Trop. 2020, 203, 105311. [Google Scholar] [CrossRef]
- Calvete, J.J.; Rodríguez, Y.; Quesada-Bernat, S.; Pla, D. Toxin-resolved antivenomics-guided assessment of the immunorecognition landscape of antivenoms. Toxicon 2018, 148, 107–122. [Google Scholar] [CrossRef]
- Pla, D.; Gutierrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef]
- Oh, A.M.F.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Venom proteome of Bungarus sindanus (Sind krait) from Pakistan and in vivo cross-neutralization of toxicity using an Indian polyvalent antivenom. J. Proteom. 2019, 193, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.M.F.; Tan, C.H.; Ariaranee, G.C.; Quraishi, N.; Tan, N.H. Venomics of Bungarus caeruleus (Indian krait): Comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J. Proteom. 2017, 164, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, C.H.; Tan, N.H. Venom and Purified Toxins of the Spectacled Cobra (Naja naja) from Pakistan: Insights into Toxicity and Antivenom Neutralization. Am. J. Trop. Med. Hyg. 2016, 94, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. World Health Organisation Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Herrera, M.; Meneses, F.; Gutiérrez, J.M.; León, G. Development and validation of a reverse phase HPLC method for the determination of caprylic acid in formulations of therapeutic immunoglobulins and its application to antivenom production. Biologicals 2009, 37, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Collaco, R.C.; Randazzo-Moura, P.; Tamascia, M.L.; da Silva, I.R.; Rocha, T.; Cogo, J.C.; Hyslop, S.; Sanny, C.G.; Rodrigues-Simioni, L. Bothrops fonsecai snake venom activities and cross-reactivity with commercial bothropic venom. Comp. Biochem. Physiol. C Toxicol. Pharm. 2017, 191, 86–100. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Casewell, N.R.; Cook, D.A.; Wagstaff, S.C.; Nasidi, A.; Durfa, N.; Wuster, W.; Harrison, R.A. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom. PLoS Negl. Trop. Dis. 2010, 4, e851. [Google Scholar] [CrossRef] [Green Version]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Mendonca-da-Silva, I.; Magela Tavares, A.; Sachett, J.; Sardinha, J.F.; Zaparolli, L.; Gomes Santos, M.F.; Lacerda, M.; Monteiro, W.M. Safety and efficacy of a freeze-dried trivalent antivenom for snakebites in the Brazilian Amazon: An open randomized controlled phase IIb clinical trial. PLoS Negl. Trop. Dis. 2017, 11, e0006068. [Google Scholar] [CrossRef]
- Ainsworth, S.; Menzies, S.K.; Casewell, N.R.; Harrison, R.A. An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management. PLoS Negl. Trop. Dis. 2020, 14, e0008579. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Kondo, S.; Ikezawa, H.; Murata, R.; Ohsaka, A. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960, 13, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Manufacturer | Batch No. | Manufacturing (M) and Expiry (E) Dates | Formulation | Protein Content (mg/mL) | Appearance | Colour after Reconstitution | Odour | pH | Reconstitution Time |
|---|---|---|---|---|---|---|---|---|---|
| Second-generation’ antivenom | |||||||||
| Serum Institute of India (SIIPL-01) | SIIPL-01 | NA | F(ab’)2 | 39.78 ± 0.98 | Amorphous powder | Opaque | Odourless | 5.74 | 50 s |
| Serum Institute of India (SIIPL-02) | SIIPL-02 | NA | F(ab’)2 | 29.33 ± 7.87 | Amorphous powder | Clear colourless | Weak | 6.13 | 1.10 min |
| Serum Institute of India (SIIPL-03) | SIIPL-03 | NA | F(ab’)2 | 14.29 ± 0.88 | Amorphous powder | Clear colourless | Weak | 5.80 | 30 s |
| Conventional Indian antivenoms | |||||||||
| Premium Serums & Vaccines Pvt. Ltd. (Premium Serum) | ASVS(1)Ly-015 | NA | F(ab’)2 | 16.79 ± 4.26 | Amorphous powder | Slight yellow | Strong | 6.25 | 2.46 min |
| VINS Bioproducts Ltd. (VINS) | 01AS18067 | M: 11/2018 E: 10/2022 | F(ab’)2 | 19.34 ± 7.38 | Amorphous powder | Clear colourless | Weak | 6.49 | 5.23 min |
| Bharat Serums and Vaccines Ltd. (Bharat Serums) | A05318020 | M: 01/2018 E: 12/2021 | F(ab’)2 | 29.63 ± 10.8 | Amorphous powder | Opaque | Strong | 6.37 | 44 s |
| Haffkine BioPharmaceutical Corporation Ltd. (Haffkine) | AS180611 | M: 06/2018 E: 11/2022 | F(ab’)2 | 30.50 ± 5.97 | Amorphous powder | Pale yellow | Weak | 6.40 | 1.17 min |
| Virchow Biotech Private Ltd. (Virchow) | PAS00718 | M: 09/2018 E: 08/2022 | F(ab’)2 | 19.54 ± 3.86 | Amorphous powder | Clear colourless | Weak | 6.63 | 2.10 min |
| Biological E. Ltd. (Biological E.) | BAS00218 | NA | F(ab’)2 | 34.48 ± 6.71 | Amorphous powder | Clear colourless | Weak | 6.19 | 1.32 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attarde, S.; Iyer, A.; Khochare, S.; Shaligram, U.; Vikharankar, M.; Sunagar, K. The Preclinical Evaluation of a Second-Generation Antivenom for Treating Snake Envenoming in India. Toxins 2022, 14, 168. https://doi.org/10.3390/toxins14030168
Attarde S, Iyer A, Khochare S, Shaligram U, Vikharankar M, Sunagar K. The Preclinical Evaluation of a Second-Generation Antivenom for Treating Snake Envenoming in India. Toxins. 2022; 14(3):168. https://doi.org/10.3390/toxins14030168
Chicago/Turabian StyleAttarde, Saurabh, Ashwin Iyer, Suyog Khochare, Umesh Shaligram, Mayur Vikharankar, and Kartik Sunagar. 2022. "The Preclinical Evaluation of a Second-Generation Antivenom for Treating Snake Envenoming in India" Toxins 14, no. 3: 168. https://doi.org/10.3390/toxins14030168









