Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A
Abstract
:1. Introduction
2. Results
2.1. Effects of AFM1 and OTA Alone and in Combination on the Lactate Dehydrogenase (LDH) and Cytokines Release from the Isolated Jejunal Tissues
2.2. Effects of AFM1 and OTA Alone and in Combination on the Gene Expression Pattern of Jejunal Tissue
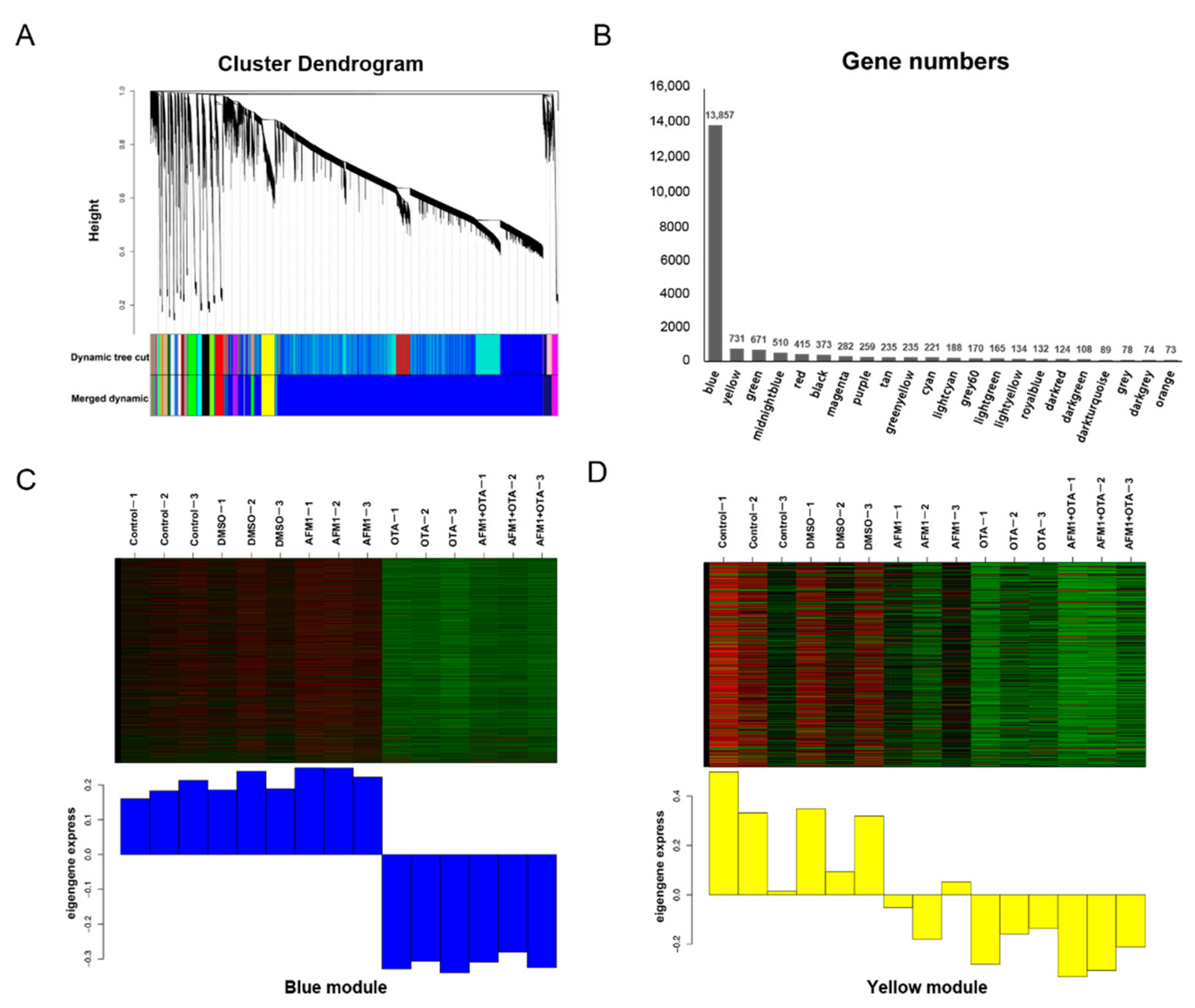
2.3. Pathway Analysis by WGCNA-ORA
2.4. Hub Genes Searching by PPI Analysis
2.5. Effects of AFM1 and OTA Alone and in Combination on the Viability and Cytokines Expression of FHs 74 Int Cells
2.6. NF-κB Acted as a Key Sensor in the Process of Inflammation Induced by OTA and AFM1 + OTA
2.7. ROS Mediated Inflammatory Responses Induced by OTA and AFM1 + OTA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Ex Vivo Culturing of Isolated Jejunal Tissues
4.3. Treatment of Isolated Jejunum Tissues with Individual and Combined AFM1 and OTA
4.4. RNA-seq and Bioinformatics Analysis
4.5. FHs 74 Int Cells Culturing and Treatment
4.6. RT-qPCR and ELISA Assays
4.7. Western Blotting Assay
4.8. Analysis of Intracellular ROS Concentration
4.9. Treatment of Antioxidant N-Acetyl-l-Cysteine (NAC) in the Presence of Toxins
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitt, J.I.; Basilico, J.C.; Abarca, M.L.; Lopez, C. Mycotoxins and toxigenic fungi. Med. Mycol. 2000, 38, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, M.; Juan-Garcia, A.; Molto, J.C.; Manes, J.; Juan, C. Evaluation of Mycotoxins in Infant Breast Milk and Infant Food, Reviewing the Literature Data. Toxins 2021, 13, 535. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Raiola, A.; Mañes, J.; Ritieni, A. Presence of mycotoxin in commercial infant formulas and baby foods from Italian market. Food Control 2014, 39, 227–236. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.C.; Manyes, L.; Meca, G.; Ritieni, A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015, 84, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.; Abia, W.; Braun, D.; Sarkanj, B.; Ayeni, K.; Oyedele, O.; Michael-Chikezie, E.; Ezekiel, V.; Mark, B.; Ahuchaogu, C.; et al. Comprehensive mycotoxin exposure biomonitoring in breastfed and non-exclusively breastfed Nigerian children. medRxiv 2020. [Google Scholar] [CrossRef]
- Amuzie, C.; Bandyopadhyay, R.; Bhat, R.; Black, R.; Burger, H.-M.; Cardwell, K.; Gelderblom, W.; Gong, Y.Y.; Groopman, J.; Kimanya, M.; et al. Mycotoxin Control in Low- and Middle-Income Countries; IARC Working Group Reports, No. 9; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Gong, Y.Y.; Watson, S.; Routledge, M. Aflatoxin Exposure and Associated Human Health Effects, a Review of Epidemiological Studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Assunção, R.; Silva, M.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Mitchell, N.J.; Gratz, J.; Houpt, E.R.; Gong, Y.; Egner, P.A.; Groopman, J.D.; Riley, R.T.; Showker, J.L.; Svensen, E.; et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ. Int. 2018, 115, 29–37. [Google Scholar] [CrossRef]
- De Santis, B.; Brera, C.; Mezzelani, A.; Soricelli, S.; Ciceri, F.; Moretti, G.; Debegnach, F.; Bonaglia, M.C.; Villa, L.; Molteni, M.; et al. Role of mycotoxins in the pathobiology of autism: A first evidence. Nutr. Neurosci. 2019, 22, 132–144. [Google Scholar] [CrossRef]
- De Santis, B.; Raggi, M.E.; Moretti, G.; Facchiano, F.; Mezzelani, A.; Villa, L.; Bonfanti, A.; Campioni, A.; Rossi, S.; Camposeo, S.; et al. Study on the Association among Mycotoxins and other Variables in Children with Autism. Toxins 2017, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Katerere, D.; Shephard, G.; Faber, M. Infant malnutrition and chronic aflatoxicosis in Southern Africa: Is there a link? Int. J. Food Saf. Nutr. Public Health 2008, 1, 127–136. [Google Scholar] [CrossRef]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Mbuya, M.N.N.; Mutasa, K.; Kembo, G.; Stoltzfus, R.J.; for the Sanitation Hygiene Infant Nutrition Efficacy (SHINE) Trial Team. The Potential Role of Mycotoxins as a Contributor to Stunting in the SHINE Trial. Clin. Infect. Dis. 2015, 61, S733–S737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, L.; Wood, M.; Fisher, B.; Jaspan, H.; Sodora, D. T Cell Activation in South African HIV-Exposed Infants Correlates with Ochratoxin A Exposure. Front. Immunol. 2017, 8, 1857. [Google Scholar] [CrossRef] [Green Version]
- Alegbe, S.; Yakubu, S.; Olonitola, O.S.; Mukhtar, M. Assessing Aflatoxin M1 levels among lactating mothers’ in Damaturu Yobe state, Nigeria. Bayero J. Pure Appl. Sci. 2018, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Eshete, M.; Gebremedhin, S.; Alemayehu, F.R.; Taye, M.; Boshe, B.; Stoecker, B.J. Aflatoxin contamination of human breast milk and complementary foods in southern Ethiopia. Matern. Child Nutr. 2021, 17, e13081. [Google Scholar] [CrossRef]
- Hooshfar, S.; Khosrokhavar, R.; Yazdanpanah, H.; Eslamizad, S.; Kobarfard, F.; Nazari, F.; Kokaraki, V.; Kokkinakis, M.; Goumenou, M.; Tsitsimpikou, C.; et al. Health risk assessment of aflatoxin M1 in infant formula milk in IR Iran. Food Chem. Toxicol. 2020, 142, 111455. [Google Scholar] [CrossRef]
- Islam, F.; Das Trisha, A.; Hafsa, J.M.; Hasan, A.; Degen, G.H.; Ali, N. Occurrence of aflatoxin M1 in human breast milk in Bangladesh. Mycotoxin Res. 2021, 37, 241–248. [Google Scholar] [CrossRef]
- Kang’ethe, E.; Gatwiri, M.; Sirma, A.; Ouko, O.; Mburugu-Musoti, C.; Kitala, P.; Gitahi, N.; Nderitu, J.; Mung’atu, J.; Hietaniemi, V.; et al. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Qual. Saf. 2017, 1, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Cherkani-Hassani, A.; Ghanname, I.; Zinedine, A.; Sefrioui, H.; Qmichou, Z.; Mouane, N. Aflatoxin M1 prevalence in breast milk in Morocco: Associated factors and health risk assessment of newborns “CONTAMILK study”. Toxicon 2020, 187, 203–208. [Google Scholar] [CrossRef]
- Karayagiz Muslu, G.; Ozdemir, M. Occurrence of and Factors Associated With the Presence of Aflatoxin M1 in Breast Milk of Mothers in Fethiye, Turkey. Biol. Res. Nurs. 2020, 22, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Quevedo-Garza, P.A.; Amador-Espejo, G.G.; Salas-Garcia, R.; Ramos-Pena, E.G.; Trujillo, A.J. Aflatoxin M1 Determination in Infant Formulae Distributed in Monterrey, Mexico. Toxins 2020, 12, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Y.Y.; Turner, P.; Hall, A.J.; Wild, C.P. Aflatoxin exposure and impaired child growth in West Africa: An unexplored international public health burden. Mycotoxins Detect. Methods Manag. Public Health Agric. Trade 2008, 53–65. [Google Scholar] [CrossRef]
- Magoha, H.; Kimanya, M.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Risk of dietary exposure to aflatoxins and fumonisins in infants less than 6 months of age in Rombo, Northern Tanzania. Matern. Child Nutr. 2016, 12, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Okoth, S.A.; Ohingo, M. Dietary aflatoxin exposure and impaired growth in young children from Kisumu District, Kenya: Cross sectional study. Afr. J. Health Sci. 2004, 11, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.; Stoltzfus, R.; Prendergast, A. Food Chain Mycotoxin Exposure, Gut Health, and Impaired Growth: A Conceptual Framework. Adv. Nutr. 2012, 3, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Muñoz, K.; Degen, G. Ochratoxin A and its metabolites in urines of German adults—An assessment of variables in biomarker analysis. Toxicol. Lett. 2017, 275, 19–26. [Google Scholar] [CrossRef]
- Bondy, G.; Curran, I.; Coady, L.; Armstrong, C.; Bourque, C.; Bugiel, S.; Caldwell, D.; Kwong, K.; Lefebvre, D.; Maurice, C.; et al. A one-generation reproductive toxicity study of the mycotoxin ochratoxin A in Fischer rats. Food Chem. Toxicol. 2021, 153, 112247. [Google Scholar] [CrossRef]
- Hassan, A.; Sheashaa, H.; Fattah, M.; Ibrahim, A.; Gaber, O.; Sobh, M. Study of Ochratoxin A as an Environmental Risk That Causes Renal Injury in Breast-Fed Egyptian Infants. Pediatr. Nephrol. 2006, 21, 102–105. [Google Scholar] [CrossRef]
- Khoshnamvand, Z.; Nazari, F.; Mehrasbi, M.; Hosseini, M.-J. Occurrence and Safety Evaluation of Ochratoxin A in Cereal-based Baby Foods Collected from Iranian Retail Market. J. Food Sci. 2019, 84, 695–700. [Google Scholar] [CrossRef]
- Cappozzo, J.; Jackson, L.; Lee, H.J.; Zhou, W.; Al-Taher, F.; Zweigenbaum, J.; Ryu, D. Occurrence of Ochratoxin A in Infant Foods in the United States. J. Food Prot. 2017, 80, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Fu, X.; Zhu, M. Ex vivo gut culture for studying differentiation and migration of small intestinal epithelial cells. Open Biol. 2018, 8, 170256. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, J.; Fein, K.; Chaudhary, N.; Doerfler, R.; Newby, A.; Whitehead, K. The enhanced intestinal permeability of infant mice enables oral protein and macromolecular absorption without delivery technology. Int. J. Pharm. 2021, 593, 120120. [Google Scholar] [CrossRef] [PubMed]
- Gasparoni, A.; Ciardelli, L.; Avanzini, A.; Castellazzi, A.; Carini, R.; Rondini, G.; Chirico, G. Age-Related Changes in Intracellular Th1/Th2 Cytokine Production, Immunoproliferative T Lymphocyte Response and Natural Killer Cell Activity in Newborns, Children and Adults. Biol. Neonate 2003, 84, 297–303. [Google Scholar] [CrossRef]
- Leushacke, M.; Barker, N. Ex vivo culture of the intestinal epithelium: Strategies and applications. Gut 2014, 63, 1345–1354. [Google Scholar] [CrossRef]
- Zheng, N.; Gao, Y.; Zhu, W.; Meng, D.; Walker, W. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020, 15, e0229283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwood, B.; Wood, D.; Yuan, C.; Nicolas, J.; Plaen, I.; Farrow, K.; Md, P.; Turner, J.; Hunter, C. A Role for cAMP and Protein Kinase A in Experimental Necrotizing Enterocolitis. Am. J. Pathol. 2016, 187, 401–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Martins, G.R.; Gelaleti, G.B.; Moschetta, M.G.; Maschio-Signorini, L.B.; Zuccari, D.A.P.d.C. Proinflammatory and Anti-Inflammatory Cytokines Mediated by NF-κB Factor as Prognostic Markers in Mammary Tumors. Mediat. Inflamm. 2016, 2016, 9512743. [Google Scholar] [CrossRef] [Green Version]
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Ossa, J.C.; Yanez, D.; Valenzuela, R.; Gallardo, P.; Lucero, Y.; Farfan, M.J. Intestinal Inflammation in Chilean Infants Fed With Bovine Formula vs. Breast Milk and Its Association With Their Gut Microbiota. Front. Cell Infect. Microbiol. 2018, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Arevalo, A.; Stiemsma, L.; Dimitriu, P.; Chico, M.E.; Loor, S.; Vaca, M.; Boutin, R.C.T.; Morien, E.; Jin, M.; et al. Associations between infant fungal and bacterial dysbiosis and childhood atopic wheeze in a nonindustrialized setting. J. Allergy Clin. Immunol. 2018, 142, 424–434.e410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffeis, C.; Martina, A.; Corradi, M.; Quarella, S.; Nori, N.; Torriani, S.; Plebani, M.; Contreas, G.; Felis, G.E. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Orivuori, L.; Mustonen, K.; de Goffau, M.C.; Hakala, S.; Paasela, M.; Roduit, C.; Dalphin, J.C.; Genuneit, J.; Lauener, R.; Riedler, J.; et al. High level of fecal calprotectin at age 2 months as a marker of intestinal inflammation predicts atopic dermatitis and asthma by age 6. Clin. Exp. Allergy 2015, 45, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Li, Y.; Li, M.; Chen, X.; Yuan, D.; Tang, M.; Xu, K. Baicalin alleviates deoxynivalenol-induced intestinal inflammation and oxidative stress damage by inhibiting NF-kappaB and increasing mTOR signaling pathways in piglets. Food Chem. Toxicol. 2020, 140, 111326. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Yuan, D.; Liao, P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-kappaB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 2021, 289, 117865. [Google Scholar] [CrossRef]
- Ying, C.; Hong, W.; Nianhui, Z.; Chunlei, W.; Kehe, H.; Cuiling, P. Nontoxic concentrations of OTA aggravate DON-induced intestinal barrier dysfunction in IPEC-J2 cells via activation of NF-kappaB signaling pathway. Toxicol. Lett. 2019, 311, 114–124. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lai, Y.H.; Hsiao, F.S.; Cheng, Y.H. Effects of Deoxynivalenol and Mycotoxin Adsorbent Agents on Mitogen-Activated Protein Kinase Signaling Pathways and Inflammation-Associated Gene Expression in Porcine Intestinal Epithelial Cells. Toxins 2021, 13, 301. [Google Scholar] [CrossRef]
- Ruan, D.; Wang, W.C.; Lin, C.X.; Fouad, A.M.; Chen, W.; Xia, W.G.; Wang, S.; Luo, X.; Zhang, W.H.; Yan, S.J.; et al. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal 2019, 13, 42–52. [Google Scholar] [CrossRef]
- Tong, C.; Li, P.; Yu, L.-H.; Li, L.; Li, K.; Chen, Y.; Yang, S.-H.; Long, M. Selenium-rich yeast attenuates ochratoxin A-induced small intestinal injury in broiler chickens by activating the Nrf2 pathway and inhibiting NF-KB activation. J. Funct. Foods 2020, 66, 103784. [Google Scholar] [CrossRef]
- Gao, Y.; Ye, Q.; Bao, X.; Huang, X.; Wang, J.; Zheng, N. Transcriptomic and proteomic profiling reveals the intestinal immunotoxicity induced by aflatoxin M1 and ochratoxin A. Toxicon 2020, 180, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Zhu, Q.; Lu, F. Comparative Transcriptome Analysis Reveals the Protective Mechanism of Glycyrrhinic Acid for Deoxynivalenol-Induced Inflammation and Apoptosis in IPEC-J2 Cells. Oxidative Med. Cell. Longev. 2020, 2020, 5974157. [Google Scholar] [CrossRef] [PubMed]
- Leong, H.S.; Kipling, D. Text-based over-representation analysis of microarray gene lists with annotation bias. Nucleic Acids Res. 2009, 37, e79. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol Induces Inflammation in IPEC-J2 Cells by Activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Gao, Y.; Yan, Q.; Bao, X.; Zhao, S.; Wang, J.; Zheng, N. Transcriptome Analysis of Ochratoxin A-Induced Apoptosis in Differentiated Caco-2 Cells. Toxins 2019, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Concetti, J.; Wilson, C.L. NFKB1 and Cancer: Friend or Foe? Cells 2018, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, C.; Foxwell, B.M.; Feldmann, M. RelB/p50 regulates CCL19 production, but fails to promote human DC maturation. Eur. J. Immunol. 2009, 39, 2215–2223. [Google Scholar] [CrossRef]
- Moriwaki, K.; Balaji, S.; McQuade, T.; Malhotra, N.; Kang, J.; Chan, F.K.-M. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 2014, 41, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus plantarum C88 protects against aflatoxin B(1)-induced liver injury in mice via inhibition of NF-κB-mediated inflammatory responses and excessive apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Inflammatory Injury in IPEC-J2 Cells via NF-κB Signaling Pathway. Toxins 2019, 11, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Bbosa, G.; Kitya, D.; Lubega, A.; Ogwal-Okeng, J.; Anokbonggo, W.; Kyegombe, D. Review of the Biological and Health Effects of Aflatoxins on Body Organs and Body Systems. Aflatoxins Recent Adv. Future Prospect. 2013, 3, 239–265. [Google Scholar]
- Li, H.; Li, S.; Yang, H.; Wang, Y.; Wang, J.; Zheng, N. l-Proline Alleviates Kidney Injury Caused by AFB1 and AFM1 through Regulating Excessive Apoptosis of Kidney Cells. Toxins 2019, 11, 226. [Google Scholar] [CrossRef] [Green Version]
- Boesch-Saadatmandi, C.; Loboda, A.; Jozkowicz, A.; Huebbe, P.; Blank, R.; Wolffram, S.; Dulak, J.; Rimbach, G. Effect of ochratoxin A on redox-regulated transcription factors, antioxidant enzymes and glutathione-S-transferase in cultured kidney tubulus cells. Food Chem. Toxicol. 2008, 46, 2665–2671. [Google Scholar] [CrossRef]
- Cavin, C.; Delatour, T.; Marin-Kuan, M.; Holzhäuser, D.; Higgins, L.; Bezençon, C.; Guignard, G.; Junod, S.; Richoz-Payot, J.; Gremaud, E.; et al. Reduction in Antioxidant Defenses may Contribute to Ochratoxin A Toxicity and Carcinogenicity. Tox-Icological Sci. 2007, 96, 30–39. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, Y.; Wan, X.; Pan, M.-H.; Xu, Y.; Sun, S.-C. Melatonin ameliorates ochratoxin A-induced oxidative stress and apoptosis in porcine oocytes. Environ. Pollut. 2020, 256, 113374. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Shi, X.-Z.; Lindholm, P.F.; Sarna, S.K. NF-κB activation by oxidative stress and inflammation suppresses contractility in colonic circular smooth muscle cells. Gastroenterology 2003, 124, 1369–1380. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajjhala, P.R.; Mirams, R.E.; Hill, J.M. Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J. Biol. Chem. 2012, 287, 41732–41743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Kalyanasundaram, R. Therapeutic implications of inflammasome in inflammatory bowel disease. FASEB J. 2021, 35, e21439. [Google Scholar] [CrossRef]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Domińska, K.; Urbanek, K.A.; Piastowska-Ciesielska, A.W. ERβ and NFκB—Modulators of Zearalenone-Induced Oxidative Stress in Human Prostate Cancer Cells. Toxins 2020, 12, 199. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wen, J.; Zhu, J.; Zhang, T.; Deng, Y.; Jiang, J. Aromatic hydrocarbon receptor regulates chicken cytochrome P450 1A5 transcription: A novel insight into T-2 toxin-induced gene expression and cytotoxicity in LMH cells. Biochem. Pharmacol. 2019, 168, 319–329. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-Analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Tavares, A.; Alvito, P.; Loureiro, S.; Louro, H.; Silva, M. Multi-mycotoxin determination and in vitro combined cytotoxic effects of aflatoxin M1 and ochratoxin A. World Mycotoxin J. 2013, 6, 375–388. [Google Scholar] [CrossRef]
- Meng, D.; Zhu, W.; Shi, H.N.; Lu, L.; Wijendran, V.; Xu, W.; Walker, W.A. Toll-like receptor-4 in human and mouse colonic epithelium is developmentally regulated: A possible role in necrotizing enterocolitis. Pediatr. Res. 2015, 77, 416–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]







| Module | Block | Pathway |
|---|---|---|
| Yellow | Up | Cytokine-Cytokine receptor interaction (KEGG) GPCR ligand binding (Reactome) |
| Down | Tight junction (KEGG) Tight junction interactions (Reactome) | |
| Blue up | 1 | Cytokine-Cytokine receptor interaction (KEGG) Cytokine signaling in immune system (Reactome) |
| 2 | Neuroactive ligand-receptor interaction (KEGG) GPCR signaling (Reactome) | |
| 3 | Ribosome (KEGG) Translation (Reactome) | |
| 4 | Oxidative phosphorylation (KEGG) Respiratory electron transport (Reactome) | |
| 5 | Calcium signaling pathway (KEGG) Muscle contraction (Reactome) | |
| 6 | Systemic lupus erythematosus (KEGG) DNA methylation (Reactome) | |
| 7 | ECM-receptor interaction (KEGG) ECM proteoglycans (Reactome) | |
| 8 | Folate biosynthesis (KEGG) Post-translational modification (Reactome) | |
| 9 | Phagosome (KEGG) Golgi-to-ER retrograde transport (Reactome) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-W.; Gao, Y.-N.; Huang, S.-N.; Wang, J.-Q.; Zheng, N. Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A. Toxins 2022, 14, 173. https://doi.org/10.3390/toxins14030173
Wang Z-W, Gao Y-N, Huang S-N, Wang J-Q, Zheng N. Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A. Toxins. 2022; 14(3):173. https://doi.org/10.3390/toxins14030173
Chicago/Turabian StyleWang, Zi-Wei, Ya-Nan Gao, Sheng-Nan Huang, Jia-Qi Wang, and Nan Zheng. 2022. "Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A" Toxins 14, no. 3: 173. https://doi.org/10.3390/toxins14030173
APA StyleWang, Z.-W., Gao, Y.-N., Huang, S.-N., Wang, J.-Q., & Zheng, N. (2022). Ex Vivo and In Vitro Studies Revealed Underlying Mechanisms of Immature Intestinal Inflammatory Responses Caused by Aflatoxin M1 Together with Ochratoxin A. Toxins, 14(3), 173. https://doi.org/10.3390/toxins14030173






