Abstract
Immunotherapy against cancer and infectious disease holds the promise of high efficacy with minor side effects. Mucosal vaccines to protect against tumors or infections disease agents that affect the upper airways or the lung are still lacking, however. One mucosal vaccine candidate is the B-subunit of Shiga toxin, STxB. In this review, we compare STxB to other immunotherapy vectors. STxB is a non-toxic protein that binds to a glycosylated lipid, termed globotriaosylceramide (Gb3), which is preferentially expressed by dendritic cells. We review the use of STxB for the cross-presentation of tumor or viral antigens in a MHC class I-restricted manner to induce humoral immunity against these antigens in addition to polyfunctional and persistent CD4+ and CD8+ T lymphocytes capable of protecting against viral infection or tumor growth. Other literature will be summarized that documents a powerful induction of mucosal IgA and resident memory CD8+ T cells against mucosal tumors specifically when STxB-antigen conjugates are administered via the nasal route. It will also be pointed out how STxB-based vaccines have been shown in preclinical cancer models to synergize with other therapeutic modalities (immune checkpoint inhibitors, anti-angiogenic therapy, radiotherapy). Finally, we will discuss how molecular aspects such as low immunogenicity, cross-species conservation of Gb3 expression, and lack of toxicity contribute to the competitive positioning of STxB among the different DC targeting approaches. STxB thereby appears as an original and innovative tool for the development of mucosal vaccines in infectious diseases and cancer.
Keywords:
glycolipid-lectin; GL-Lect; endosomal escape; cross-presentation; tissue resident memory T cells; TRM; immune checkpoint; radiotherapy; chemotherapy; cytotoxic CD8+ T lymphocyte Key Contribution:
STxB is a vector for the delivery of antigenic peptides and proteins to dendritic cells. STxB-based vaccines induce cellular and humoral immunity in mucosal and periphaeral locations, including combinations with immune checkpoint inhibitors, chemo- and radiotherapy.
1. Shiga Toxin and Its Intracellular Trafficking
The bacterial Shiga toxin belongs to the family of AB5 toxins [1]. These are composed of a catalytic A-subunit and a homopentameric B-subunit which is made from five identical B-fragments. The B-subunits of AB5 toxins bind to glycans of cellular protein or lipids [2]. The cellular receptor of the B-subunit of Shiga toxin (abbreviated as STxB) is the glycosphingolipid globotriaosylceramide (Gb3 or CD77) [3]. Of note, STxB is needed not only for toxin binding to cells, but also for the trafficking of the catalytic A-subunits inside the cells [4] (Figure 1). With the help of STxB, the A-subunit of Shiga toxin is delivered into the cytosol where it inhibits protein biosynthesis by modifying ribosomal RNA. This leads to cell death and contributes to the overall pathology that is associated with Shiga toxin producing enterohemorrhagic Escherichia coli bacteria, which bring about hemolytic-uremic syndrome, the leading cause of pediatric renal failure [5,6], but which also poses health risks to adults [7].
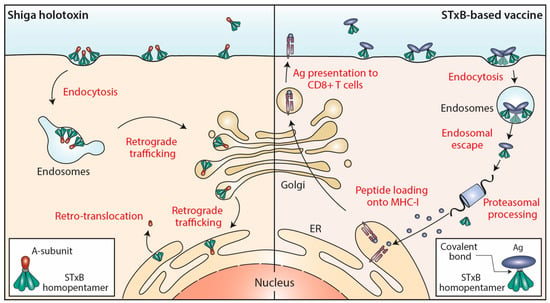
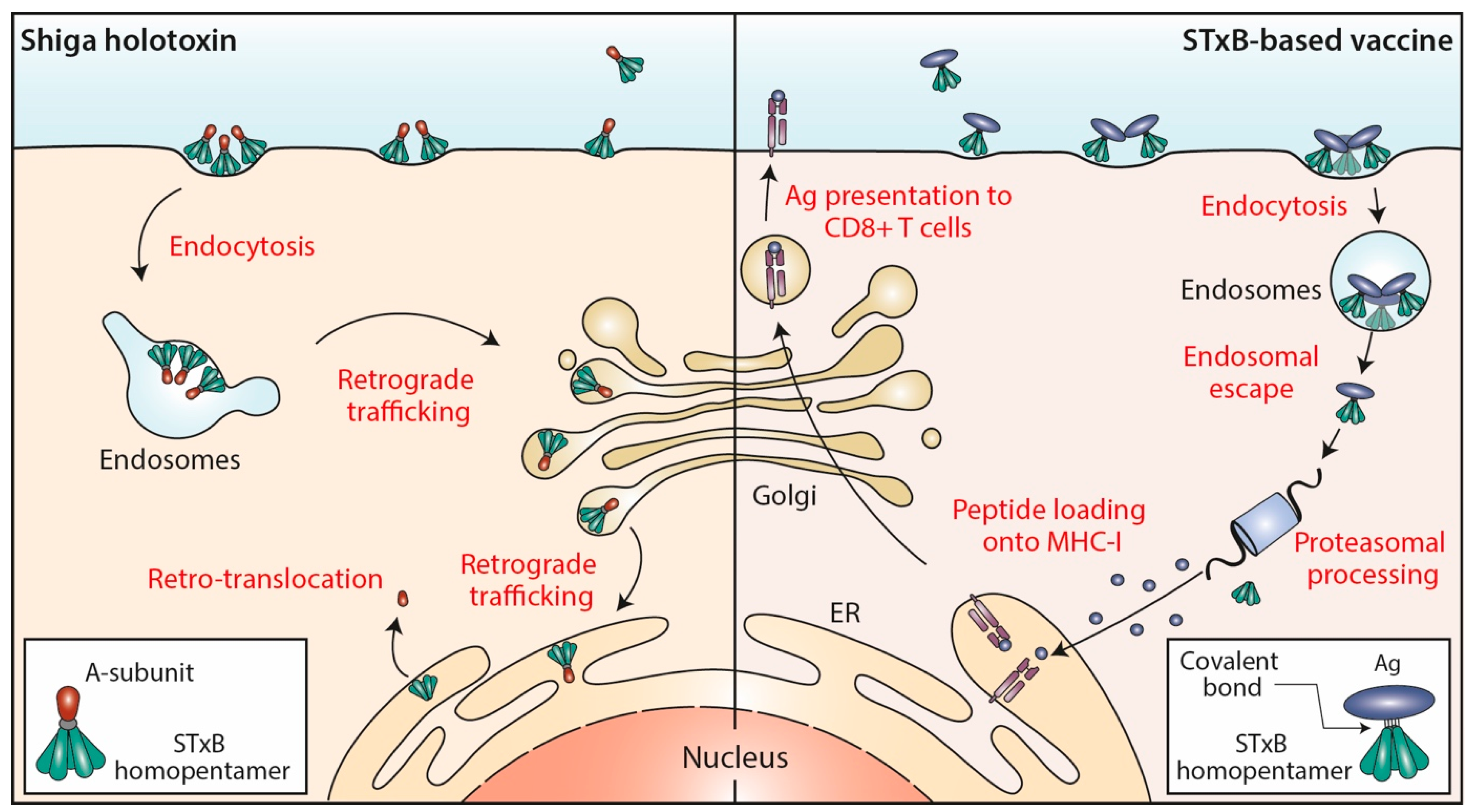
Figure 1.
STxB trafficking into cells. Left: Shiga holotoxin molecules are composed of a STxB homopentamer (green) and a catalytic A-subunit (red), which are non-covalently associated. STxB binds to the plasma membrane of target cells via the glycosphingolipid Gb3 (not shown). STxB induces an increment of spontaneous curvature, which upon membrane-mediated clustering of several toxin molecules leads to the formation of endocytic pits from which clathrin-independent carriers are generated for toxin trafficking to early endosomes. From there, the holotoxins are transported via the retrograde trafficking route to the endoplasmic reticulum (ER), via the Golgi apparatus. The catalytic A-subunit is then translocated to the cytosol where it inhibits protein biosynthesis by modifying ribosomal RNAs (not shown). Right: In STxB (green)-based vaccines, antigens (blue) are linked via covalent bonds to the vector. The endocytic process then operates as for Shiga holotoxin molecules. While STxB-antigen conjugates also undergo retrograde trafficking (not shown), a small fraction of them escapes from the lumen of endosomes to reach the cytosol (endosomal escape). Here, proteasomes process the antigens to generate antigenic peptides, that are then imported into the lumen of the ER (or of endo/phagosomal processing compartments; not shown) for loading onto MHC class I molecules and subsequent presentation at the plasma membrane to CD8+ T cells.
The endocytic and intracellular trafficking of STxB has been analyzed in some detail (Figure 1). At the plasma membrane, STxB reorganizes lipids, including its receptor glycolipid Gb3, in a way such that narrow tubular endocytic pits are formed [8] by exploiting a specific geometry of Gb3 binding sites on STxB [9] and its capacity to induce an asymmetric compressive stress onto the membrane leaflet to which it binds [10]. STxB-Gb3 complexes are then clustered by membrane-mediated mechanism, likely involving lipid fluctuation forces [11]. This mechanism of building endocytic pits without the need for the conventional clathrin machinery has been termed the glycolipid-lectin (GL-Lect) hypothesis [12,13]. This GL-Lect mechanism has been suggested to apply also for the structurally related glycolipid-binding B-subunit of cholera toxin [14,15,16].
The toxin-induced tubular endocytic pits then detach by scission from the plasma membrane to form clathrin-independent endocytic carriers [17]. This scission reaction involves the conventional pinchase dynamin [8] and also actin-driven domain boundary forces [18] and a mechanism that has been termed friction-driven scission in which the pulling of the molecular motor dynein on tubular endocytic pits that are scaffolded by the BAR domain protein endophilin leads to the thinning of their necks and to subsequent detachment [19,20]. The thereby generated clathrin-independent endocytic carriers are then targeted in a SNARE protein-dependent manner to early endosomes [21].
From early endosomes, STxB is delivered by retrograde transport to the endoplasmic reticulum, via the Golgi apparatus (reviewed in [22,23,24]) (Figure 1). From there, the catalytic A-subunit is translocated to the cytosol using the cellular retrotranslocation machinery [25].
2. Gb3 Expression and Membrane Translocation of STxB
In the healthy organism, the Gb3 glycolipid is found to be expressed in a limited number of tissues. In agreement with the fact that renal pathology is the most striking clinical manifestation that is associated with Shiga toxin, cells of microvascular glomeruli and proximal tubules have high Gb3 levels [26,27]. Gb3 is also found on microvascular endothelial cells, and in individuals who are infected with Shiga toxin producing E. coli strains, vascular endothelia of colon and brain are affected [28,29,30]. Platelets and erythrocytes also express Gb3 [31,32], as much as some immune cells such as germinal center B lymphocytes [33], monocytes, macrophages, and dendritic cells (DCs) [34].
DCs are key cells for the induction of primary immune responses, and notably also of CD8+ cytotoxic T lymphocytes (CTL) through the cytosolic processing and the cross-presentation of exogenous antigens (see below). It was therefore of interest when it was found that STxB (in some studies covalently coupled to cargo proteins such as antigens), in addition to reaching the retrograde trafficking route, also has the propensity to escape from the lumen of endosomes to reach the cytosolic compartment of DCs [34,35,36] (Figure 1). Using a quantitative assay, it was measured that roughly 0.5% of cell-associated STxB was translocated to the cytosol within 4 h incubation at 37 °C [37], which is in the range of the numbers that were described for other delivery systems [38]. The exact mechanism underlying this endosomal escape capacity remains very little understood.
3. Targeting of DCs: A Competitive Approach for Vaccine Development
3.1. A Brief History
Today’s marketed preventive vaccines induce antibodies that block different pathogens from infecting host cells. Prior to the success of RNA vaccines, most of these prophylactic vaccines were based on the administration of recombinant proteins. In other clinical situations such as chronic infections or cancer, other immune effectors such as CD8+ T lymphocytes must be mobilized for the development of therapeutic vaccines. At the end of the 1990s, apart from live attenuated vaccines and recombinant viruses which pose safety issues, there were no inactivated vaccines capable of inducing CD8+ T cells. Indeed, the recombinant proteins used for immunization were internalized in the endosomal pathway and processed peptides were presented to CD4+ T lymphocytes activating a humoral response. Different groups have therefore become interested in toxins because of their capacity to undergo endocytic trafficking and to deliver their catalytic subunits into the cytosol [39,40]. Access to the cytosol is indeed a prerequisite for the targeting of exogenous antigens into the HLA class I-restricted presentation pathway (cross-presentation) for their recognition by CD8+ T cells.
The teams of Claude Leclerc and Daniel Ladant were the first to show that a recombinant toxin derived from adenylate cyclase A produced by the bacterium Bordetella pertussis and incorporating a peptide derived from the lymphocytic choriomeningitis virus (LCMV) nucleoprotein triggered antigen cross-presentation to CD8+ T cells [41]. These teams also demonstrated that administration of recombinant cyclase A with a model peptide antigen was able to induce CD8+ T cells in mice [42]. Similarly, Pseudomonas exotoxin and anthrax toxin were then also shown to target antigens into the MHC class I presentation pathway [43] and to induce cytotoxic CD8+ T cells in mice [44,45,46,47].
In 1998, our teams published a first study showing that also the non-toxic STxB could be used to target antigens to the MHC class I-restricted pathway in human mononuclear cells and DCs [48]. Importantly, this presentation was shown to follow the proteasomal and transporter associated with antigen processing (TAP)-dependent pathway ensuring that with this vaccine strategy naturally occurring peptides are presented [34] (Figure 1).
Professional antigen presenting cells such as DCs express costimulatory molecules [49]. When cells that do not express these costimulatory molecules present HLA class I-restricted antigens to CD8+ T cells, this often leads to tolerance [50]. It was therefore of primary importance when we showed that Gb3 is preferentially expressed by DCs, and that STxB functions as a delivery tool for the in vivo targeting of antigens to DCs and for the improvement of immune responses, in particular by CD8+ T cells [34]. Consistently, we also demonstrated the role of DCs in the vaccine function of STxB [51].
3.2. Which DCs and Which Receptors to Target
In mice, DCs include DC1 (also known as Batf3-dependent, CD103+ tissue resident and CD8α+ lymphoid resident DCs), myeloid CD8α negative CD11b+DC2, and plasmacytoid DCs (B220+, Bst2+ and SiglecH+). In humans, a correspondence with this classification has been found with DC1 defined as CD141(BDCA-3)+ Clec9A (DNGR1)+CXR1+ DCs, DC2 identified as CD1c/BDCA-1+ DCs, and plasmacytoid DCs expressing BDCA2 (CD303+). For the skin, Langerhans cells and dermal DCs (CD14+) need to be added to this listing [52,53].
Initial studies showed that CD8α+DC in mice and BDCA3+ in humans had a higher cross-presentation capacity than the other subpopulations, thereby explaining their ability to preferentially induce CD8+ T cells [54,55]. On the contrary, CD8α negative DCs and Langerhans cells preferentially induce humoral responses [56]. Targeting DC2 via DCIR2 in mice [56,57] and Langerhans cells via langerin was more effective in activating follicular helper T cells and a humoral response than targeting DC1 [58,59].
In the light of these findings, targeting highly specific DC1 markers such as Clec9a and XCR1 appeared as the most promising strategy for CD8+ T-cell induction (Figure 2). However, converging evidence has since accumulated that has challenged this idea on a functional dichotomy of DCs. It was indeed shown that all DCs are capable of cross-presentation in humans, especially when antigens are targeted to early endosomes [60,61,62].
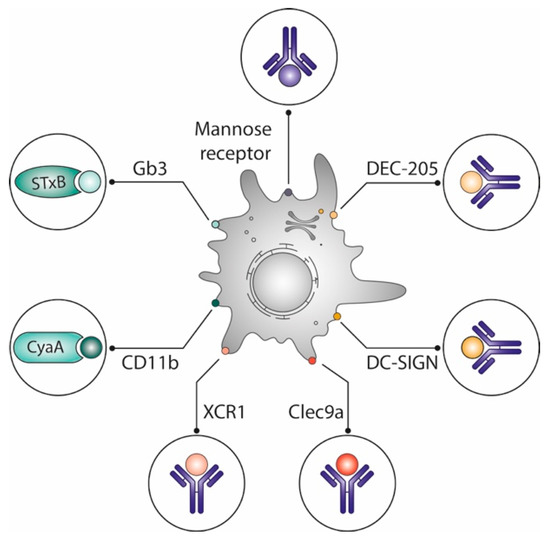
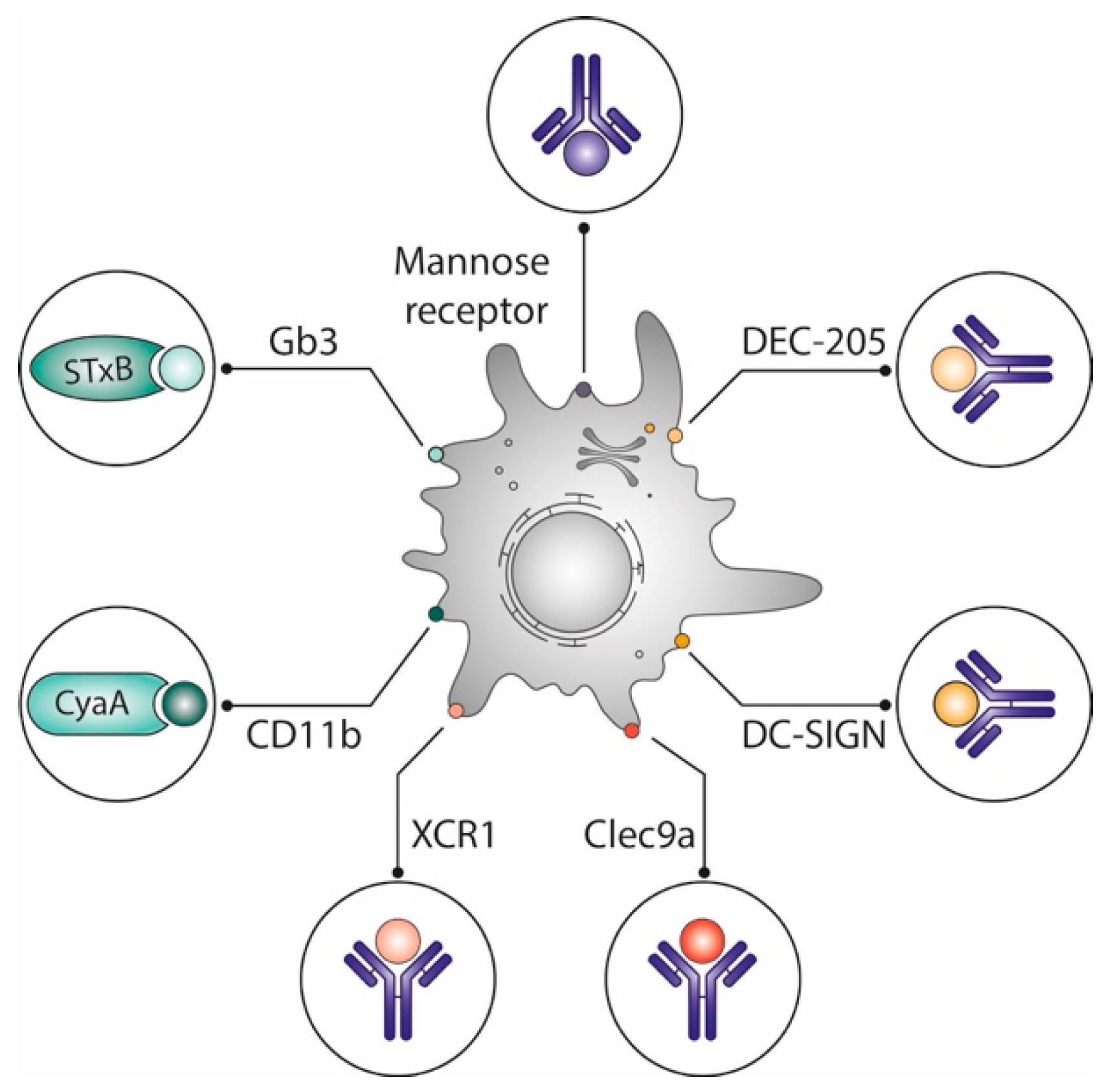
Figure 2.
Optimization of vaccines by delivering antigens to dendritic cells. Different vaccine delivery systems preferentially target antigens to dendritic cells, which are known for their capacity to prime naive T cells: Vectors derived from toxin subunits such as the non-toxic STxB, which binds to the glycosphingolipid Gb3, or adenylate cyclase A, which binds to CD11b; antibodies targeting lectins (DEC-205, DC Sign) or other surface markers (mannose receptor, XCR1, Clec9a) of which some are specifically expressed on DC subpopulations. See text for details.
In mice, STxB does not specifically target only CD8α+DCs [51], and adenylate cyclase A binds to the myeloid DC-specific CD11b [63]. Yet, both vectors potently induce CD8+ T cells. Other studies have also shown that engagement of different DC subpopulations as observed with STxB and other vectors is more effective for the induction of potent immune responses than targeting single DC subpopulations [64,65,66].
For the development of immunotherapy strategies, the structural homology and similarity of tissue expression patterns between humans and mice of the targeted DC surface markers also are important criteria to consider. For example, while in humans and mice DEC-205 is expressed at relatively high levels on myeloid blood DCs, the protein is, only in humans, also present on monocytes, B lymphocytes, NK cells, plasmacytoid blood DCs, and T lymphocytes [67,68,69]. Another example is the DC inhibitory receptor 2 (DCIR2, 33D1, Clec4A, CD367), which in mice is exclusively expressed by CD8-negative resident splenic DCs [56,70,71,72], while the protein is found on all blood DC subsets, monocytes, and granulocytes in humans [70,73,74]. It is therefore of note that the globotriose glycan to which STxB binds is structurally the same in all species, and that according to the current state of knowledge the expression pattern of the Gb3 glycolipid on DC populations is similar between species.
Considerations of differences between species also apply in the choice of adjuvants. For example, it has been reported that TLR9 is found on all major murine DC subsets, while the protein is only expressed by pDC in humans [75].
3.3. DC Maturation and Role of Adjuvants
Among the DC targeting vectors, some are known to induce DC maturation (e.g., anti-CD40, adenylate cyclase A, anti-Dectin-1) [76,77,78], while others do not (e.g., anti-DEC-205, anti-CD11c, anti-Clec9a, anti-Siglec H) [79,80,81,82]. When used without adjuvants or associated inflammatory stimuli, some of the latter, such as anti-DEC-205, even induce tolerance rather than activation of immune cells [80,81]. With anti-DEC-205, this has been used in mouse models to prevent the onset of diabetes [83] or of experimental autoimmune arthritis [84]. An anti-Siglec H antibody coupled to a Mog peptide similarly inhibits T-cell dependent autoimmune reactions in a murine EAE model when it is administered without adjuvant [85].
Other vectors like adenylate cyclase A that trigger DC maturation via the TLR4/TRIF pathway [77] induce CD8+ T cells without adjuvants. Vectors capable of inducing DC maturation, e.g., anti-CD40 or anti-dectin-1, have themselves been used as vaccine adjuvants [86,87].
One group reported a role for STxB in the maturation of DCs in the spleen and nasal-associated lymphoid tissue (NALT) [88,89]. However, this has not been seen by another team [90], and we did not observe any effect of endotoxin-free STxB on DC maturation [51]. Furthermore, we found that antigens must be conjugated to STxB for the induction of immune responses in mice [34]. If antigens and STxB are co-administrated, no immunomodulation is observed [34].
Unlike for anti-DEC-205, STxB-antigen conjugates induce cellular and humoral immune responses without the need for adjuvants [34,51,91]. Adjuvants such as αGalCer, CpG or poly(I:C) (a TLR3 ligand) nevertheless significantly improve specific CD8+ T-cell responses against antigens that are delivered via STxB, as one would expect for a delivery tool that does not itself activate DCs [92].
From a clinical perspective, the optimal combination of these vectors with adjuvants will result in specific immune response profiles. Thus, antigenic targeting by anti-Clec9A without associated adjuvant promotes proliferation and induction of antigen-specific regulatory CD4+ T cells, while coadministration of poly(I:C) or curdlan (Dectin-1 ligand) promotes the generation of antigen-specific Th1 or Th17 cells, respectively [93].
3.4. Systemic Immune Responses Induced by STxB and Other DC Targeting Vectors
DC targeting vectors are developed for the induction of CD8+ T cells (Figure 2). Indeed, many vectors (anti-CD40, anti-Clec9a, anti-DEC-205, adenylate cyclase A, anti-Dectin-1, anti-mannose receptor...), when coupled to different antigens have been shown to be more efficient than the non-vectorized antigens for the generation of CD8+ T cells in mouse models [78,94,95,96,97,98,99,100,101]. Some of these vectors (i.e., anti-CD40, anti-Clec9a, anti-Dec-205) have even been shown to induce CD8+ T cell in non-human primates [102,103,104,105,106].
In wild-type mice of different genetic backgrounds and in humanized mice, conjugates of STxB with antigenic peptides or proteins induce antigen-specific CD8+ T cells, which are detectable ex vivo and persistent over time [34,51,91,92,107]. Of note, only a few micrograms of STxB-antigen vaccine are required per mouse to induce CD8+ T cells when adjuvants are used, as opposed to the situation with non-vectorized antigens [92]. Similar observations have been reported for strategies that are based on targeting DEC-205 or XCR1 [97,108].
Most of these vectors also drive the presentation of antigen-derived peptides by HLA class II molecules to CD4+ T cells, thereby inducing antibodies with titers that are higher when compared to those obtained by vaccination with non-vectorized antigens. Some vectors (STxB, anti-XCR1, adenylate cyclase A) cause TH1 polarization with the production of IgG2a isotype antibodies [91,99,109], while others (anti-DEC-205, anti-Dectin 1) result in a mixed TH1/TH17 polarization [110,111,112]. Some vectors (anti-Lox-1, anti-Clec9a, anti-DCIR2) appear to be particularly effective in promoting antibody induction, which might be linked to their ability to activate follicular CD4+ T cell [103,113,114,115]. These mixed humoral and cellular responses provide additional arguments to suggest that the attempt to match the targeting of given types of DC with specific types of immune responses might be an oversimplification.
3.5. Protection against Viral Infection and Tumor Growth
In different preclinical models of wild-type or humanized mice it was shown that systemic administration of STxB-antigen conjugates inhibits tumor growth both in the context of prophylactic or therapeutic vaccination [51,107,116]. In most of these models, protection was conferred by CD8+ T cells. STxB-antigen conjugates also protect against infection caused by a smallpox-derived virus [92] or against bacterial infection caused by Boretella pertussis [117]. Interestingly, coupling of a STxB-related toxin, STx2b, to a clostridium perfringens-derived enterotoxin enhances the humoral response against enterotoxin and provides protection against this pathogenic product [118]. It was also found that vectors targeting DEC-205, Clec9a, XCR1, CD11b (via adenylate cyclase A), or CD40 conferred protection against infectious disease [109,119,120,121,122,123] and tumor growth [96,97,98,124,125,126,127,128,129,130].
4. STxB Functions as a Mucosal Delivery Vector
4.1. The Mucosal Immune System and Its Specific Effectors
The mucosal immune system, also called MALT (mucosa-associated lymphoid tissue), is an integrated and well-organized architecture covering the lung, head and neck, digestive and genital mucosa. It is made of lymphoid follicles that are associated with a layer of T, B, and antigen presenting cells. These immune cells, which are close to the epithelium and M cells, represent between 10 and 20% of the epithelial barrier. M cells play an important role in the internalization and transfer of antigens to DCs [131]. A first priming of immune responses takes place at this follicle-associated epithelium (FAE), which is also called mucosal inductive site. Thereby induced immune cells reach the adjacent lymph nodes upon which they return to the very mucosa in which they had been generated [132,133].
Compared to the immune response induced by peripheral lymph node priming, the mucosal immune response is characterized by two immune effectors that are specifically found in mucosal tissues: secretory IgA and resident memory T cells [134]. In contrast to IgA in serum, secretory (s)-IgA antibodies are produced locally in the mucosa and are more resistant to bacterial enzymes. sIgAs are the key immune effector molecules in the mucosa. After binding to polymeric immunoglobulin (Ig) receptors (pIgRs), sIgAs are transported across mucosal epithelial cells to the intestinal lumen or other mucosa. Only mucosal and not systemic immunization pathways can generate them [135,136]. Their presence in mucosal sites is associated with optimal vaccine protection against viral infections [137,138].
More recently, a new non-recirculating lineage of T cells has been described in mucosal tissues, which were termed tissue resident memory (TRM) T cells [139,140]. TRM specifically differentiate in the mucosal tissue and are not found in the blood. They express the CD103 marker which binds to epithelial cell-specific E-cadherin. TRM are thought to play an immunosurveillance role in the mucosa. Their presence in the vicinity of the epithelium allows them to act rapidly in the event of infection and to promote the swift recruitment of new effectors without the need for a lengthy T-cell differentiation process in lymph nodes [141]. TRM have also been found in tumors especially in mucosal localization and are associated with a favorable prognosis [142,143]. As for sIgAs, mucosal routes of immunization are more efficient in inducing TRM than conventional systemic routes [144].
4.2. STxB—The First Non-Live Mucosal Delivery Vector That Induces TRM
We showed that intranasal immunization with conjugates between STxB and the E7 protein from human papilloma virus 16 (HPV16) is more effective in inducing mucosal IgAs and anti-E7 CD8+ T cells in the lung than intramuscular immunization [145]. Intranasal STxB-E7 immunization promotes intratumoral CD8+ T cell recruitment and the regression of E7-expressing tumor in the lung or head and neck mucosa. In contrast, intramuscular immunization with STxB-E7 induces CD8+ T cells in blood and spleen, but not in the lung and has no significant effect on the growth of a tumor xenograft in the tongue. The intranasally induced CD8+ T cells express CD103 and CD49a and have a TRM phenotype. Of note, these cells are not induced when STxB-E7 is injected via the intramuscular route of immunization [145,146].
In a series of experiments based on the elimination of TRM, the blocking of their differentiation or migration, or their isolation by parabiosis, we have clearly shown their role in the inhibition of tumor growth after immunization of mice with different STxB-antigen conjugates [146]. More recently, we have shown that TRM preferentially express the chemokine receptor CXCR6, when compared to effector CD8+ T cells [147]. Immunization via the intranasal route and not the intramuscular route allows to induce the chemokine CXCL16 in the lung, which could explain the recruitment of TRM [147].
These studies demonstrate for the first time that a protein-based vector targeting DCs induces TRM, and that the nasal immunization route is required for this. Earlier work had already pointed to the possibility that STxB might act as a mucosal delivery vector. Indeed, a STxB fusion protein with a rotavirus NS4 polypeptide was shown to increase intestinal IgA concentrations and serum IgG when administered orally, and to protect breastfeeding pups against diarrhea after an infectious challenge [148].
4.3. Other Mucosal Vaccination Strategies
Preparations based on vesicular stomatitis virus (VSV), adenovirus 26 (ADV26), or modified vaccinia virus Ankara have enabled the commercialization of vaccines against Ebola virus [149,150]. Intranasal administration of recombinant preparations based on cytomegalovirus (CMV)-derived viruses, influenza virus, ADV, VSV have been shown to induce IgA and TRM in different mucosal locations [151,152,153,154]. In a preclinical model of infection with SARS-CoV-2, a recombinant chimpanzee ADV (ChAdOx1)-encoding SARS-CoV-2 Spike administered nasally or subcutaneously was shown to protect against lung infection after a viral challenge, but only intranasal administration of the vaccine protects against upper airway infection. This protection is associated with the preferential induction of local mucosal IgA and TRM [155].
Few non-live vectors have been tested for their ability to deliver antigens via the mucosal route. For example, conjugates between the non-toxic B-subunit of cholera toxin and bacterial or viral antigens increase antigen-specific IgA compared to non-vectorized antigen when they are administered nasally or sublingually [156,157]. Upon nasal or subcutaneous administration, a scFv directed against DEC-205 and coupled to a parasite antigen increases IgA concentrations in nasal washings as well as a CD4+ T-cell response in the spleen, allowing partial protection against a parasite challenge [158].
More generally, two main mucosal delivery tools are developed for vaccine:
- (i)
- Lactic acid bacteria (LAB) that include Lactobacillus spp., Lactococcus spp., and Streptococcus spp. LAB are generally recognized as safe and considered as transiting and non-invasive bacteria [159,160].
- (ii)
- Nanoparticles, i.e., (a) polysaccharide-based natural polymers such as chitosan, pullulan, alginate, inulin, hyaluronic acid, maltodextrin; (b) lipid-based delivery systems (i.e., cationic liposomes, virions, archaeological bodies, small cochlea, and immunostimulating complexes); (c) synthetic polymeric nanoparticles (poly(lactic-co-glycolic acid), polycaprolactone, polyahydrides, polyphosphazene). These polymers have the advantage of being biodegradable.
After mucosal administration, LABs and nanoparticles generate mucosal responses against entrapped antigens [161,162,163,164]. To improve their efficacy, LABs such as lactobacillus have been coupled with DC targeting peptides; alternatively, complement C3d3, anti-CD205, anti-CD11c, or neonatal Fc receptors (FcRn) have been expressed at their surface [165,166,167,168]. Nanoparticles such as poly(lactic-co-glycolic acid) and liposomes have also been functionalized with anti-DEC-205 [169], anti-CD40 [170], anti-mannose receptor [171], or anti-CD11c [172] antibodies to target them to DCs. These elegant strategies, which combine mucosal delivery, DC targeting, and the possibility to incorporate multiple cargo molecules are up until now limited by issues related to reproducibility of their synthesis and scale up for clinical application.
Regarding RNA vaccines, their direct intranasal administration without encapsulation does not lead to the induction of a mucosal immune response [173]. Some studies show that their encapsulation as nanoparticles, cationic liposome/protamine complexes (LPC), or mannose-conjugated lipid nanoparticles generate cellular responses that inhibit tumor growth [173,174,175]. Xun Sun’s group demonstrated that cationic cyclodextrin-polyethylenimine 2k conjugates (CP 2k) which are complexed with anionic mRNA-encoding HIV gp120 induce strong systemic and mucosal anti-HIV immune responses [176]. Nevertheless, toxicity problems have been reported with polyethyleneimine and lipid nanoparticles when these are injected via the nasal route [177,178,179]. Improving the benefit-risk balance and the efficacy of these mucosal RNA vaccines is the subject of numerous ongoing studies.
5. STxB in Combination with Other Cancer Treatment Modalities
Apart from a few positive clinical signals of therapeutic HPV vaccines in pre-neoplastic cervical lesions, no therapeutic vaccine has demonstrated sufficient efficacy in patients with advanced cancer or chronic infection (e.g., HIV) to change clinical practice [140]. An in-depth investigation of the tumor microenvironment has revealed the existence of immunosuppressive mechanisms that likely explain the failure of therapeutic vaccines in advanced stage cancers [180]. Indeed, T cells that migrate into tumors quickly become exhausted and express inhibitory receptors like PD-1. Blocking the interaction between PD-1 and PD-L1 has led to the success of immunotherapy in many clinical indications [181,182]. Second generation immunotherapy protocols are therefore developed in which an inhibition of the PD-1/PD-L1 pathway is combined with vaccines or conventional treatments [183].
In preclinical models, we were one of the first teams to show that this combinatorial approach might indeed be successful [184]. In mice with HPV E7-expressing tumors, administration of either a STxB-E7 vaccine or an anti-PD-1 antibody led to only a partial therapeutic response. In contrast, the combination of both induced total tumor regression [184]. The value of combining a STxB-based vaccine with anti-PD-1 antibodies (and the local injection of IFNα) was also confirmed by another group [185,186].
Regulatory T cells are another type of the immunosuppressive cells in the tumor microenvironment that counteract vaccine efficacy. We have shown that the combination of a Treg inhibitor targeting the CCR4 pathway with a vaccine composed of STxB coupled to self-antigens overcomes tolerance and allows to eliminate tumors that express these self-antigens [187]. This combination proved to be effective in inhibiting the growth of numerous tumors (i.e., melanoma, colon cancer, and lung cancer). A similar synergistic effect was observed in the presence of a mTor pathway inhibitor [188].
In many clinical indications, a therapeutic vaccine would need to be combined with conventional treatments such as radiotherapy or chemotherapy. In collaboration with Eric Deutsch’s group, we have shown in a head and neck cancer model that radiotherapy increases the effect of a STxB-E7 vaccine by making endothelial cells more permissive to infiltration by CD8+ T cells [189].
As summarized above and also in other studies [190,191], the STxB vector has been used reproducibly by independent groups for the preclinical development of immunotherapy applications. These studies support the design of clinical trials including STxB-based vaccines in 2nd generation immunotherapy strategies.
6. Potential Limitations of STxB
6.1. Intrinsic Immunogenicity and Toxicity
One of the potential problems with the use of a vector derived from an exogenous protein is the presence of pre-existing antibodies or the development of a neutralizing immune response against the vector. For STxB, we have addressed these aspects in mice. Upon immunization of mice with STxB-antigen conjugates, an antibody response is observed against STxB, which is 100-fold lower than the one directed against the antigen itself, however [92]. Moreover, anti-STxB antibodies do not interfere with the induction of a CD8+ T-cell response against the antigen. Indeed, the intensity of the CD8+ T response increases in the same animal with repetitive immunizations, and if an animal is pre-immunized with non-antigen-coupled STxB at high doses, the CD8+ T-cell response is not diminished upon vaccination with STxB-antigen conjugates, compared to mice that were not pre-immunized [92].
The low immunogenicity of STxB is also observed in humans. Indeed, in serum samples from 30 patients with hemolytic-uremic syndrome (HUS), caused by Shiga toxin producing E. coli strain O157:H7, no antibodies against the toxin could be detected [192]. In other studies, on clinical samples, antibodies against Shiga toxin were present, but not against STxB, or only with a very low frequency of 1.3% [193,194]. We also detected the presence of anti-STxB antibodies in only 2 out of 30 serum samples of patients with HUS. Furthermore, our teams also did not find antibodies against STxB in sera of healthy subjects [192], and the frequency of antibodies directed against holotoxin was found to be about 1.8% in healthy subjects [195]. These studies demonstrate the low immunogenicity of STxB and the absence of pre-existing antibodies against this protein in humans. This low intrinsic immunogenicity gives STxB an important advantage over viral vectors.
Different approaches have been used to address a potential toxicity of STxB. Using a classical screening test for toxicity, i.e., the rabbit reticulocyte lysate system, no inhibition of globin synthesis was observed with up to 100 µg per reaction of Shiga toxin that by mutation was rendered devoid of enzymatic activity [196]. Mice treated intraperitoneally with toxoids of STx1 or STx2 whose catalytic sites were mutated at protein concentrations equivalent to more than 100 times the ones used for immunization showed no ill effects [197]. In another study, mice were given STxB doses as high as 200 mg/kg [198] or 220 µg per mouse [88]. Yet, no signs of clinical toxicity were observed, including mucosal sites. In our immunization experiments, mice were given three doses of up to 80 μg of STxB at 3-week intervals, which corresponds to 8 mg of STxB per kg of mouse weight. These mice did not exhibit any signs of clinical morbidity with a follow-up of 6 months (Ref. [51] and unpublished).
6.2. Production
For all studies that have been discussed above on STxB as an antigen delivery tool, the protein was purified from bacteria, which are its natural hosts. STxB is obtained in amounts that are typical for proteins which are expressed in the periplasmic space. STxB has recently also been chemically synthesized and refolded in vitro [199]. Whether this type of material can also be used to obtain functional vaccine conjugates remains to be tested.
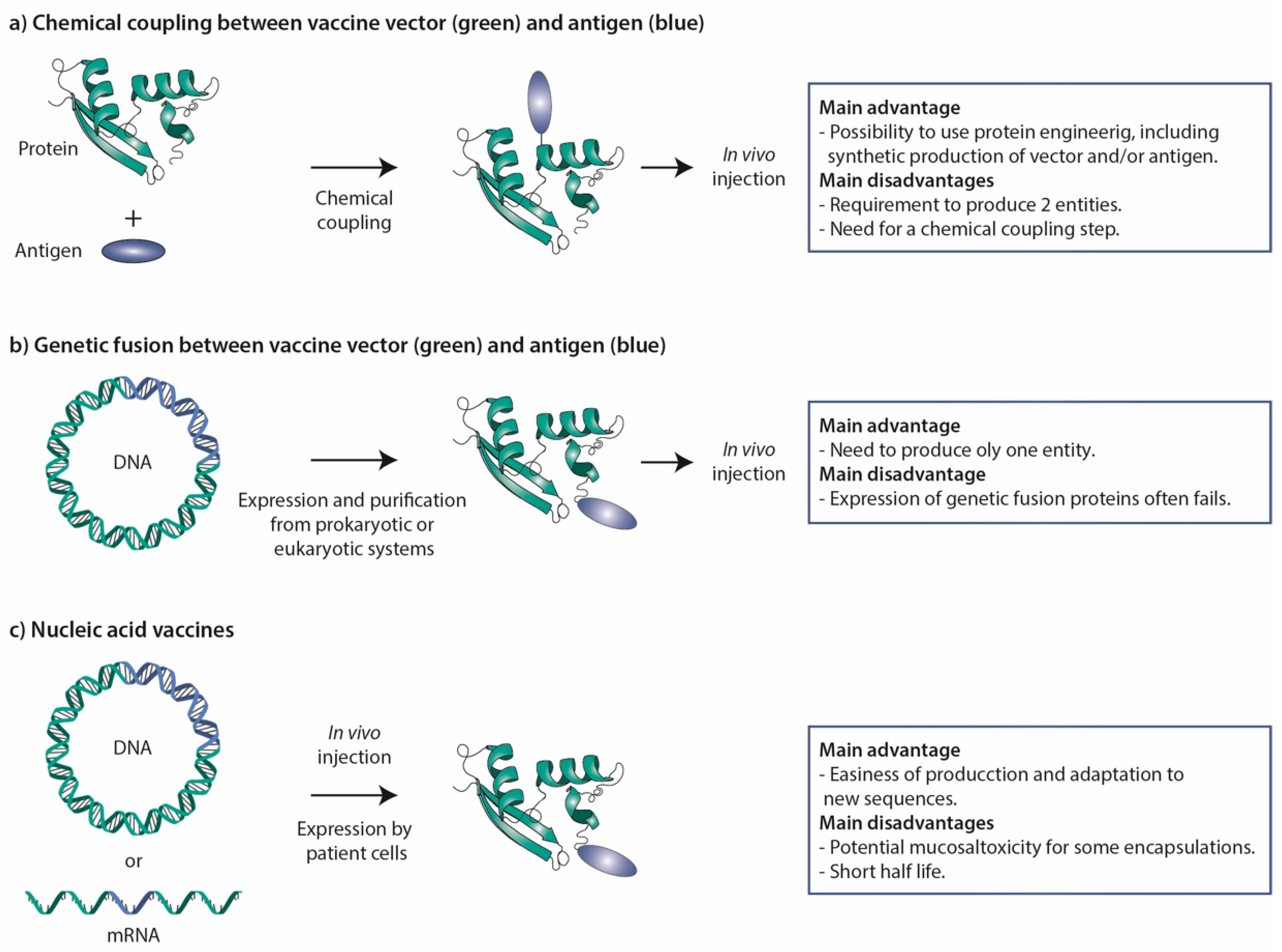
Antigenic peptides and proteins can in principle be genetically fused or chemically coupled to STxB (Figure 3). Even if successful in a few cases [48,148], genetic fusions in most cases fail to be found at significant levels in the bacterial periplasm. A chemical coupling approach was therefore favored in most studies (Figure 3).
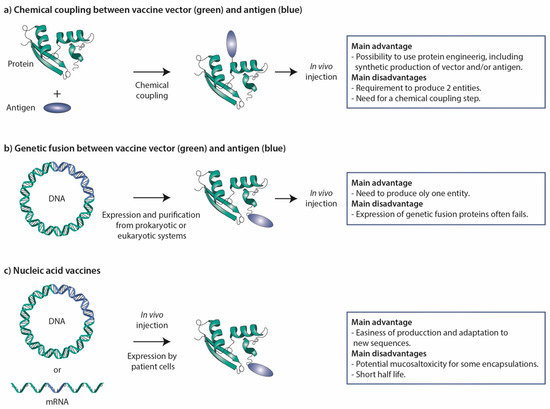
Figure 3.
Vaccine strategies. (a) Chemical coupling. In this procedure, vector and antigen are produced in parallel and then chemically coupled to generate the vaccine. This approach has been extensively used for STxB. (b) Genetic fusion. Vector and antigen are genetically fused at the cDNA level, and then expressed in and purified from prokaryotic or eukaryotic cell systems. In some cases, this approach has been used for STxB, but often corresponding fusion proteins could not be obtained. (c) Nucleic acid vaccines. Fusion proteins between vectors and antigens are expressed from DNA or mRNA molecules that are directly injected into the organism. These vaccine molecules are thereby produced by the cells of the organism receiving the vaccine. The main advantages and disadvantages of the different strategies are listed to the right.
For chemical crosslinking, a variant was designed in which a cysteine was added to the C-terminus of each B-fragment. It turns out that despite this supernumerary cysteine, the intrachain disulfide bond at the level of each B-fragment still forms with high specificity. This variant, termed STxB/Cys, is expressed and purified with similar efficiency as wild-type STxB, conserves the Gb3 binding and intracellular transport characteristics of the wild-type protein, and lacks toxicity. It has been conjugated to a large variety of molecular entities from fluorophores [200] and radioelements [201,202] to peptides [145], full-size proteins [36,91] and liposomes [203].
7. Conclusions
STxB appears to be a competitive DC-targeting vector for CD8+ T-cell induction, which remains a challenge in vaccinology. Other vectors such as adenylate cyclase A from Bordetella pertussis or anti-CD40 antibodies have also demonstrated their ability to induce CD8+ T cells. However, in a randomized phase II clinical trial no significant difference compared to placebo was observed in viral clearance in women with HPV16/18 cervical lesions vaccinated with the GTL001 vaccine composed of a recombinant HPV16–18 adenylate cyclase vaccine [204]. The use of anti-CD40 antibodies has been hampered by clinical toxicity [205,206]. Other recent data have positioned STxB as the first non-live mucosal vector capable of inducing mucosal IgA immunity and mucosal TRM, which play key roles in controlling pathogens and in anti-tumor immunosurveillance [145,146]. In contrast, other non-live vectors such as DEC205 ligands, nanoparticles, and mRNAs, when administered via the mucosal route, fail to induce mucosal immunity. Furthermore, the expression of DEC205 differs depending on species; it is less specific of cDC1 in humans, and ligands of DEC205 as delivery vectors induce tolerance [67,81]. Regarding functionalized nanoparticles, problems have been observed concerning the reproducibility of their synthesis and the feasibility of their industrial scale up, while mucosal administration of mRNA encapsulated in lipid nanoparticle appears to be toxic [173,177,207]. Finally, the low immunogenicity of STxB, its lack of toxicity, binding to a receptor that is conserved across species, and the reproducibility of vaccine efficacy that was obtained with this vector by different independent groups [91,185,186,190,191] make it an attractive antigen delivery tool, particularly for the development of mucosal vaccines in infectious diseases and cancer. Its potential use in the context of nucleic acid vaccines awaits further exploration (Figure 3).
Author Contributions
Writing and funding acquisition, E.T. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the Tartour team was funded by grants from Fondation ARC (Program Signit), INCA (contract n° 2019-1-PLBIO-05-1), Foncer contre le Cancer, SIRIC CARPEM, Labex Immuno-Oncology, and in the Johannes teams by grants from Cancéropôle Ile-de-France, Institut Carnot Curie Cancer, Institut National du Cancer (INCa, contract n° 2019-1-PLBIO-05-1), Mizutani Foundation for Glycosciences (n° 200014), PSL Valorisation and Institut de Convergence Qlife prematuration program (Q-life ANR-17-CONV-0005), Agence National de la Recherche (ANR-19-CE13-0001-01, ANR-20-CE15-0009-01), and Fondation pour la Recherche Médicale (EQU202103012926).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the Cell and Tissue Imaging (PICT-IBiSA) and Nikon Imaging Centre, Institut Curie, member of the French National Research Infrastructure France-BioImaging (ANR10-INBS-04) for microscopy use. The Johannes team is member of Labex Cell(n)Scale (ANR-11-LABX-0038) and Idex Paris Sciences et Lettres (ANR-10-IDEX-0001-02).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merritt, E.A.; Hol, W.G. AB5 toxins. Curr. Opin. Struct. Biol. 1995, 5, 165–171. [Google Scholar] [CrossRef]
- Beddoe, T.; Paton, A.W.; Le Nours, J.; Rossjohn, J.; Paton, J.C. Structure, biological functions and applications of the AB5 toxins. Trends Biochem. Sci. 2010, 35, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Jacewicz, M.; Clausen, H.; Nudelman, E.; Donohue-Rolfe, A.; Keusch, G.T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 1986, 163, 1391–1404. [Google Scholar] [CrossRef]
- Johannes, L.; Romer, W. Shiga toxins—From cell biology to biomedical applications. Nat. Rev. Microbiol. 2010, 8, 105–116. [Google Scholar] [CrossRef]
- Tarr, P.I.; Gordon, C.A.; Chandler, W.L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005, 365, 1073–1086. [Google Scholar] [CrossRef]
- Karmali, M.A. Infection by Shiga toxin-producing Escherichia coli: An overview. Mol. Biotechnol. 2004, 26, 117–122. [Google Scholar] [CrossRef]
- Karch, H.; Denamur, E.; Dobrindt, U.; Finlay, B.B.; Hengge, R.; Johannes, L.; Ron, E.Z.; Tonjum, T.; Sansonetti, P.J.; Vicente, M. The enemy within us: Lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol. Med. 2012, 4, 841–848. [Google Scholar] [CrossRef]
- Romer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windschiegl, B.; Tenza, D.; Aly, M.R.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Hansen, A.G.; Johannes, L.; Khandelia, H.; Shillcock, J.C.; Kumar, P.B.; Ipsen, J.H. Membrane invagination induced by Shiga toxin B-subunit: From molecular structure to tube formation. Soft Matter 2016, 12, 5164–5171. [Google Scholar] [CrossRef]
- Watkins, E.B.; Majewski, J.; Chi, E.Y.; Gao, H.; Florent, J.C.; Johannes, L. Shiga Toxin Induces Lipid Compression: A Mechanism for Generating Membrane Curvature. Nano Lett. 2019, 19, 7365–7369. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Gao, H.; Arumugam, S.; Becken, U.; Bassereau, P.; Florent, J.C.; Ipsen, J.H.; Johannes, L.; Shillcock, J.C. Mechanism of Shiga Toxin Clustering on Membranes. ACS Nano 2017, 11, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Wunder, C.; Shafaq-Zadah, M. Glycolipids and Lectins in Endocytic Uptake Processes. J. Mol. Biol. 2016, 428, 4792–4818. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L. Shiga Toxin—A Model for Glycolipid-Dependent and Lectin-Driven Endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef]
- Ewers, H.; Romer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pezeshkian, W.; Nabo, L.J.; Ipsen, J.H. Cholera toxin B subunit induces local curvature on lipid bilayers. FEBS Open Bio 2017, 7, 1638–1645. [Google Scholar] [CrossRef]
- Kabbani, A.M.; Raghunathan, K.; Lencer, W.I.; Kenworthy, A.K.; Kelly, C.V. Structured clustering of the glycosphingolipid GM1 is required for membrane curvature induced by cholera toxin. Proc. Natl. Acad. Sci. USA 2020, 117, 14978–14986. [Google Scholar] [CrossRef]
- Kirkham, M.; Fujita, A.; Chadda, R.; Nixon, S.J.; Kurzchalia, T.V.; Sharma, D.K.; Pagano, R.E.; Hancock, J.F.; Mayor, S.; Parton, R.G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 2005, 168, 465–476. [Google Scholar] [CrossRef]
- Romer, W.; Pontani, L.L.; Sorre, B.; Rentero, C.; Berland, L.; Chambon, V.; Lamaze, C.; Bassereau, P.; Sykes, C.; Gaus, K.; et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell 2010, 140, 540–553. [Google Scholar] [CrossRef]
- Renard, H.F.; Simunovic, M.; Lemiere, J.; Boucrot, E.; Garcia-Castillo, M.D.; Arumugam, S.; Chambon, V.; Lamaze, C.; Wunder, C.; Kenworthy, A.K.; et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 2015, 517, 493–496. [Google Scholar] [CrossRef]
- Simunovic, M.; Manneville, J.B.; Renard, H.F.; Evergren, E.; Raghunathan, K.; Bhatia, D.; Kenworthy, A.K.; Voth, G.A.; Prost, J.; McMahon, H.T.; et al. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell 2017, 170, 172–184.e11. [Google Scholar] [CrossRef]
- Renard, H.F.; Garcia-Castillo, M.D.; Chambon, V.; Lamaze, C.; Johannes, L. Shiga toxin stimulates clathrin-independent endocytosis of the VAMP2, VAMP3 and VAMP8 SNARE proteins. J. Cell Sci. 2015, 128, 2891–2902. [Google Scholar] [PubMed]
- Johannes, L.; Wunder, C. Retrograde transport. Encycl. Cell Biol. 2016, 2, 433–441. [Google Scholar]
- Sandvig, K.; Skotland, T.; van Deurs, B.; Klokk, T.I. Retrograde transport of protein toxins through the Golgi apparatus. Histochem. Cell Biol. 2013, 140, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Linstedt, A.D. Retrograde trafficking of AB(5) toxins: Mechanisms to therapeutics. J. Mol. Med. 2013, 91, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.A.; Lord, J.M. How ricin and Shiga toxin reach the cytosol of target cells: Retrotranslocation from the endoplasmic reticulum. Curr. Top Microbiol. Immunol. 2012, 357, 19–40. [Google Scholar]
- Ergonul, Z.; Clayton, F.; Fogo, A.B.; Kohan, D.E. Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr. Nephrol. 2003, 18, 246–253. [Google Scholar] [CrossRef]
- Lingwood, C.A. Verotoxin-binding in human renal sections. Nephron 1994, 66, 21–28. [Google Scholar] [CrossRef]
- Ohmi, K.; Kiyokawa, N.; Takeda, T.; Fujimoto, J. Human microvascular endothelial cells are strongly sensitive to Shiga toxins. Biochem. Biophys. Res. Commun. 1998, 251, 137–141. [Google Scholar] [CrossRef]
- Ren, J.; Utsunomiya, I.; Taguchi, K.; Ariga, T.; Tai, T.; Ihara, Y.; Miyatake, T. Localization of verotoxin receptors in nervous system. Brain Res. 1999, 825, 183–188. [Google Scholar] [CrossRef]
- Obata, F.; Tohyama, K.; Bonev, A.D.; Kolling, G.L.; Keepers, T.R.; Gross, L.K.; Nelson, M.T.; Sato, S.; Obrig, T.G. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J. Infect. Dis. 2008, 198, 1398–1406. [Google Scholar] [CrossRef]
- Cooling, L.L.; Walker, K.E.; Gille, T.; Koerner, T.A. Shiga toxin binds human platelets via globotriaosylceramide (Pk antigen) and a novel platelet glycosphingolipid. Infect. Immun. 1998, 66, 4355–4366. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, R.; Carlier, K.; Wiels, J.; Levery, S.B.; Stroud, M.; Cedergren, B.; Nilsson Sojka, B.; Bennett, E.P.; Jersild, C.; Clausen, H. Cloning and expression of the histo-blood group Pk UDP-galactose: Ga1beta-4G1cbeta1-cer alpha1, 4-galactosyltransferase. Molecular genetic basis of the p phenotype. J. Biol. Chem. 2000, 275, 16723–16729. [Google Scholar] [CrossRef] [PubMed]
- Mangeney, M.; Richard, Y.; Coulaud, D.; Tursz, T.; Wiels, J. CD77: An antigen of germinal center B cells entering apoptosis. Eur. J. Immunol. 1991, 21, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Haicheur, N.; Bismuth, E.; Bosset, S.; Adotevi, O.; Warnier, G.; Lacabanne, V.; Regnault, A.; Desaymard, C.; Amigorena, S.; Ricciardi-Castagnoli, P.; et al. The B subunit of Shiga toxin fused to a tumor antigen elicits CTL and targets dendritic cells to allow MHC class I-restricted presentation of peptides derived from exogenous antigens. J. Immunol. 2000, 165, 3301–3308. [Google Scholar] [CrossRef]
- Falguieres, T.; Mallard, F.; Baron, C.; Hanau, D.; Lingwood, C.; Goud, B.; Salamero, J.; Johannes, L. Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 2001, 12, 2453–2468. [Google Scholar] [CrossRef]
- Garcia-Castillo, M.D.; Tran, T.; Bobard, A.; Renard, H.F.; Rathjen, S.J.; Dransart, E.; Stechmann, B.; Lamaze, C.; Lord, M.; Cintrat, J.C.; et al. Retrograde transport is not required for cytosolic translocation of the B-subunit of Shiga toxin. J. Cell Sci. 2015, 128, 2373–2387. [Google Scholar] [CrossRef]
- Lucchino, M.; Billet, A.; Bai, S.K.; Dransart, E.; Hadjerci, J.; Schmidt, F.; Wunder, C.; Johannes, L. Absolute Quantification of Drug Vector Delivery to the Cytosol. Angew. Chem. Int. Ed. 2021, 60, 14824–14830. [Google Scholar] [CrossRef]
- Johannes, L.; Lucchino, M. Current Challenges in Delivery and Cytosolic Translocation of Therapeutic RNAs. Nucleic Acid Ther. 2018, 28, 178–193. [Google Scholar] [CrossRef]
- Smith, D.C.; Lord, J.M.; Roberts, L.M.; Tartour, E.; Johannes, L. 1st class ticket to class I: Protein toxins as pathfinders for antigen presentation. Traffic 2002, 3, 697–704. [Google Scholar] [CrossRef]
- Goletz, T.J.; Klimpel, K.R.; Leppla, S.H.; Keith, J.M.; Berzofsky, J.A. Delivery of antigens to the MHC class I pathway using bacterial toxins. Hum. Immunol. 1997, 54, 129–136. [Google Scholar] [CrossRef]
- Sebo, P.; Fayolle, C.; d’Andria, O.; Ladant, D.; Leclerc, C.; Ullmann, A. Cell-invasive activity of epitope-tagged adenylate cyclase of Bordetella pertussis allows in vitro presentation of a foreign epitope to CD8+ cytotoxic T cells. Infect. Immun. 1995, 63, 3851–3857. [Google Scholar] [CrossRef] [PubMed]
- Fayolle, C.; Sebo, P.; Ladant, D.; Ullmann, A.; Leclerc, C. In vivo induction of CTL responses by recombinant adenylate cyclase of Bordetella pertussis carrying viral CD8+ T cell epitopes. J. Immunol. 1996, 156, 4697–4706. [Google Scholar] [PubMed]
- Goletz, T.J.; Klimpel, K.R.; Arora, N.; Leppla, S.H.; Keith, J.M.; Berzofsky, J.A. Targeting HIV proteins to the major histocompatibility complex class I processing pathway with a novel gp120-anthrax toxin fusion protein. Proc. Natl. Acad. Sci. USA 1997, 94, 12059–12064. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.C.; Arthur, J.F.; Landucci, G.R.; Forthal, D.N.; Theuer, C.P. CD8+ T-cell mediated tumor protection by Pseudomonas exotoxin fused to ovalbumin in C57BL/6 mice. Surgery 2003, 133, 404–410. [Google Scholar] [CrossRef]
- Liao, C.W.; Chen, C.A.; Lee, C.N.; Su, Y.N.; Chang, M.C.; Syu, M.H.; Hsieh, C.Y.; Cheng, W.F. Fusion protein vaccine by domains of bacterial exotoxin linked with a tumor antigen generates potent immunologic responses and antitumor effects. Cancer Res. 2005, 65, 9089–9098. [Google Scholar] [CrossRef]
- Lu, Y.; Friedman, R.; Kushner, N.; Doling, A.; Thomas, L.; Touzjian, N.; Starnbach, M.; Lieberman, J. Genetically modified anthrax lethal toxin safely delivers whole HIV protein antigens into the cytosol to induce T cell immunity. Proc. Natl. Acad. Sci. USA 2000, 97, 8027–8032. [Google Scholar] [CrossRef]
- Shaw, C.A.; Starnbach, M.N. Antigen delivered by anthrax lethal toxin induces the development of memory CD8+ T cells that can be rapidly boosted and display effector functions. Infect. Immun. 2008, 76, 1214–1222. [Google Scholar] [CrossRef]
- Lee, R.S.; Tartour, E.; van der Bruggen, P.; Vantomme, V.; Joyeux, I.; Goud, B.; Fridman, W.H.; Johannes, L. Major histocompatibility complex class I presentation of exogenous soluble tumor antigen fused to the B-fragment of Shiga toxin. Eur. J. Immunol. 1998, 28, 2726–2737. [Google Scholar] [CrossRef]
- Oudard, S.; Benhamouda, N.; Escudier, B.; Ravel, P.; Tran, T.; Levionnois, E.; Negrier, S.; Barthelemy, P.; Berdah, J.F.; Gross-Goupil, M.; et al. Decrease of Pro-Angiogenic Monocytes Predicts Clinical Response to Anti-Angiogenic Treatment in Patients with Metastatic Renal Cell Carcinoma. Cells 2021, 11, 17. [Google Scholar] [CrossRef]
- Palle, J.; Hirsch, L.; Lapeyre-Prost, A.; Malka, D.; Bourhis, M.; Pernot, S.; Marcheteau, E.; Voron, T.; Castan, F.; Lacotte, A.; et al. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers 2021, 13, 5562. [Google Scholar] [CrossRef]
- Vingert, B.; Adotevi, O.; Patin, D.; Jung, S.; Shrikant, P.; Freyburger, L.; Eppolito, C.; Sapoznikov, A.; Amessou, M.; Quintin-Colonna, F.; et al. The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur. J. Immunol. 2006, 36, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., 3rd; Murphy, K.M. Models of dendritic cell development correlate ontogeny with function. Adv. Immunol. 2019, 143, 99–119. [Google Scholar] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Bachem, A.; Guttler, S.; Hartung, E.; Ebstein, F.; Schaefer, M.; Tannert, A.; Salama, A.; Movassaghi, K.; Opitz, C.; Mages, H.W.; et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J. Exp. Med. 2010, 207, 1273–1281. [Google Scholar] [CrossRef]
- Reuter, A.; Panozza, S.E.; Macri, C.; Dumont, C.; Li, J.; Liu, H.; Segura, E.; Vega-Ramos, J.; Gupta, N.; Caminschi, I.; et al. Criteria for dendritic cell receptor selection for efficient antibody-targeted vaccination. J. Immunol. 2015, 194, 2696–2705. [Google Scholar] [CrossRef]
- Dudziak, D.; Kamphorst, A.O.; Heidkamp, G.F.; Buchholz, V.R.; Trumpfheller, C.; Yamazaki, S.; Cheong, C.; Liu, K.; Lee, H.W.; Park, C.G.; et al. Differential antigen processing by dendritic cell subsets in vivo. Science 2007, 315, 107–111. [Google Scholar] [CrossRef]
- Soares, H.; Waechter, H.; Glaichenhaus, N.; Mougneau, E.; Yagita, H.; Mizenina, O.; Dudziak, D.; Nussenzweig, M.C.; Steinman, R.M. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007, 204, 1095–1106. [Google Scholar] [CrossRef]
- Kervevan, J.; Bouteau, A.; Lanza, J.S.; Hammoudi, A.; Zurawski, S.; Surenaud, M.; Dieudonne, L.; Bonnet, M.; Lefebvre, C.; Hocini, H.; et al. Targeting human langerin promotes HIV-1 specific humoral immune responses. PLoS Pathog. 2021, 17, e1009749. [Google Scholar] [CrossRef]
- Bouteau, A.; Kervevan, J.; Su, Q.; Zurawski, S.M.; Contreras, V.; Dereuddre-Bosquet, N.; Le Grand, R.; Zurawski, G.; Cardinaud, S.; Levy, Y.; et al. DC Subsets Regulate Humoral Immune Responses by Supporting the Differentiation of Distinct Tfh Cells. Front. Immunol. 2019, 10, 1134. [Google Scholar] [CrossRef]
- Segura, E.; Durand, M.; Amigorena, S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organ-resident dendritic cells. J. Exp. Med. 2013, 210, 1035–1047. [Google Scholar] [CrossRef]
- Cohn, L.; Chatterjee, B.; Esselborn, F.; Smed-Sorensen, A.; Nakamura, N.; Chalouni, C.; Lee, B.C.; Vandlen, R.; Keler, T.; Lauer, P.; et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J. Exp. Med. 2013, 210, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Smed-Sorensen, A.; Cohn, L.; Chalouni, C.; Vandlen, R.; Lee, B.C.; Widger, J.; Keler, T.; Delamarre, L.; Mellman, I. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood 2012, 120, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Guermonprez, P.; Khelef, N.; Blouin, E.; Rieu, P.; Ricciardi-Castagnoli, P.; Guiso, N.; Ladant, D.; Leclerc, C. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J. Exp. Med. 2001, 193, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, D.; Sammicheli, C.; Tavarini, S.; Nuti, S.; Frigimelica, E.; Manetti, A.G.; Nuccitelli, A.; Aprea, S.; Valentini, S.; Borgogni, E.; et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood 2009, 113, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Kastenmuller, K.; Wille-Reece, U.; Lindsay, R.W.; Trager, L.R.; Darrah, P.A.; Flynn, B.J.; Becker, M.R.; Udey, M.C.; Clausen, B.E.; Igyarto, B.Z.; et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Investig. 2011, 121, 1782–1796. [Google Scholar] [CrossRef]
- Oh, J.Z.; Kurche, J.S.; Burchill, M.A.; Kedl, R.M. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood 2011, 118, 3028–3038. [Google Scholar] [CrossRef]
- Kato, M.; McDonald, K.J.; Khan, S.; Ross, I.L.; Vuckovic, S.; Chen, K.; Munster, D.; MacDonald, K.P.; Hart, D.N. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int. Immunol. 2006, 18, 857–869. [Google Scholar] [CrossRef]
- Witmer-Pack, M.D.; Swiggard, W.J.; Mirza, A.; Inaba, K.; Steinman, R.M. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. II. Expression in situ in lymphoid and nonlymphoid tissues. Cell. Immunol. 1995, 163, 157–162. [Google Scholar] [CrossRef]
- Inaba, K.; Swiggard, W.J.; Inaba, M.; Meltzer, J.; Mirza, A.; Sasagawa, T.; Nussenzweig, M.C.; Steinman, R.M. Tissue distribution of the DEC-205 protein that is detected by the monoclonal antibody NLDC-145. I. Expression on dendritic cells and other subsets of mouse leukocytes. Cell. Immunol. 1995, 163, 148–156. [Google Scholar] [CrossRef]
- Heidkamp, G.F.; Neubert, K.; Haertel, E.; Nimmerjahn, F.; Nussenzweig, M.C.; Dudziak, D. Efficient generation of a monoclonal antibody against the human C-type lectin receptor DCIR by targeting murine dendritic cells. Immunol. Lett. 2010, 132, 69–78. [Google Scholar] [CrossRef]
- Henriques, H.R.; Rampazo, E.V.; Goncalves, A.J.; Vicentin, E.C.; Amorim, J.H.; Panatieri, R.H.; Amorim, K.N.; Yamamoto, M.M.; Ferreira, L.C.; Alves, A.M.; et al. Targeting the non-structural protein 1 from dengue virus to a dendritic cell population confers protective immunity to lethal virus challenge. PLoS Negl. Trop. Dis. 2013, 7, e2330. [Google Scholar] [CrossRef] [PubMed]
- Idoyaga, J.; Fiorese, C.; Zbytnuik, L.; Lubkin, A.; Miller, J.; Malissen, B.; Mucida, D.; Merad, M.; Steinman, R.M. Specialized role of migratory dendritic cells in peripheral tolerance induction. J. Clin. Investig. 2013, 123, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.E.; Fournier, N.; Garcia, E.; Valladeau, J.; Durand, I.; Pin, J.J.; Zurawski, S.M.; Patel, S.; Abrams, J.S.; Lebecque, S.; et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 1999, 163, 1973–1983. [Google Scholar] [PubMed]
- Klechevsky, E.; Flamar, A.L.; Cao, Y.; Blanck, J.P.; Liu, M.; O’Bar, A.; Agouna-Deciat, O.; Klucar, P.; Thompson-Snipes, L.; Zurawski, S.; et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 2010, 116, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, M.; Tacken, P.J.; Figdor, C.G. Targeting dendritic cells—Why bother? Blood 2013, 121, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, M.; Beverley, P.C. Lipopolysaccharide modulation of dendritic cells is insufficient to mature dendritic cells to generate CTLs from naive polyclonal CD8+ T cells in vitro, whereas CD40 ligation is essential. J. Immunol. 2001, 167, 6247–6255. [Google Scholar] [CrossRef]
- Dadaglio, G.; Fayolle, C.; Zhang, X.; Ryffel, B.; Oberkampf, M.; Felix, T.; Hervas-Stubbs, S.; Osicka, R.; Sebo, P.; Ladant, D.; et al. Antigen targeting to CD11b+ dendritic cells in association with TLR4/TRIF signaling promotes strong CD8+ T cell responses. J. Immunol. 2014, 193, 1787–1798. [Google Scholar] [CrossRef]
- Ni, L.; Gayet, I.; Zurawski, S.; Duluc, D.; Flamar, A.L.; Li, X.H.; O’Bar, A.; Clayton, S.; Palucka, A.K.; Zurawski, G.; et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J. Immunol. 2010, 185, 3504–3513. [Google Scholar] [CrossRef]
- Cueto, F.J.; Del Fresno, C.; Sancho, D. DNGR-1, a Dendritic Cell-Specific Sensor of Tissue Damage That Dually Modulates Immunity and Inflammation. Front. Immunol. 2019, 10, 3146. [Google Scholar] [CrossRef]
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638. [Google Scholar] [CrossRef]
- Hawiger, D.; Inaba, K.; Dorsett, Y.; Guo, M.; Mahnke, K.; Rivera, M.; Ravetch, J.V.; Steinman, R.M.; Nussenzweig, M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001, 194, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Raper, A.; Sugita, N.; Hingorani, R.; Salio, M.; Palmowski, M.J.; Cerundolo, V.; Crocker, P.R. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood 2006, 107, 3600–3608. [Google Scholar] [CrossRef] [PubMed]
- Bruder, D.; Westendorf, A.M.; Hansen, W.; Prettin, S.; Gruber, A.D.; Qian, Y.; von Boehmer, H.; Mahnke, K.; Buer, J. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes 2005, 54, 3395–3401. [Google Scholar] [CrossRef] [PubMed]
- Spiering, R.; Margry, B.; Keijzer, C.; Petzold, C.; Hoek, A.; Wagenaar-Hilbers, J.; van der Zee, R.; van Eden, W.; Kretschmer, K.; Broere, F. DEC205+ Dendritic Cell-Targeted Tolerogenic Vaccination Promotes Immune Tolerance in Experimental Autoimmune Arthritis. J. Immunol. 2015, 194, 4804–4813. [Google Scholar] [CrossRef]
- Loschko, J.; Heink, S.; Hackl, D.; Dudziak, D.; Reindl, W.; Korn, T.; Krug, A.B. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J. Immunol. 2011, 187, 6346–6356. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Hsi, J.; Wang, H.; Tao, R.; Shao, Y. Cholera toxin B subunit acts as a potent systemic adjuvant for HIV-1 DNA vaccination intramuscularly in mice. Hum. Vaccines Immunother. 2014, 10, 1274–1283. [Google Scholar] [CrossRef]
- Leibundgut-Landmann, S.; Osorio, F.; Brown, G.D.; Reis e Sousa, C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 2008, 112, 4971–4980. [Google Scholar] [CrossRef]
- Ohmura-Hoshino, M.; Yamamoto, M.; Yuki, Y.; Takeda, Y.; Kiyono, H. Non-toxic Stx derivatives from Escherichia coli possess adjuvant activity for mucosal immunity. Vaccine 2004, 22, 3751–3761. [Google Scholar] [CrossRef]
- Ohmura, M.; Yamamoto, M.; Tomiyama-Miyaji, C.; Yuki, Y.; Takeda, Y.; Kiyono, H. Nontoxic Shiga toxin derivatives from Escherichia coli possess adjuvant activity for the augmentation of antigen-specific immune responses via dendritic cell activation. Infect. Immun. 2005, 73, 4088–4097. [Google Scholar] [CrossRef]
- Sakiri, R.; Ramegowda, B.; Tesh, V.L. Shiga toxin type 1 activates tumor necrosis factor-alpha gene transcription and nuclear translocation of the transcriptional activators nuclear factor-kappaB and activator protein-1. Blood 1998, 92, 558–566. [Google Scholar] [CrossRef]
- Haicheur, N.; Benchetrit, F.; Amessou, M.; Leclerc, C.; Falguieres, T.; Fayolle, C.; Bismuth, E.; Fridman, W.H.; Johannes, L.; Tartour, E. The B subunit of Shiga toxin coupled to full-size antigenic protein elicits humoral and cell-mediated immune responses associated with a Th1-dominant polarization. Int. Immunol. 2003, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adotevi, O.; Vingert, B.; Freyburger, L.; Shrikant, P.; Lone, Y.C.; Quintin-Colonna, F.; Haicheur, N.; Amessou, M.; Herbelin, A.; Langlade-Demoyen, P.; et al. B subunit of Shiga toxin-based vaccines synergize with alpha-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J. Immunol. 2007, 179, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Joffre, O.P.; Sancho, D.; Zelenay, S.; Keller, A.M.; Reis e Sousa, C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur. J. Immunol. 2010, 40, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Flamar, A.L.; Xue, Y.; Zurawski, S.M.; Montes, M.; King, B.; Sloan, L.; Oh, S.; Banchereau, J.; Levy, Y.; Zurawski, G. Targeting concatenated HIV antigens to human CD40 expands a broad repertoire of multifunctional CD4+ and CD8+ T cells. AIDS 2013, 27, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Caminschi, I.; Proietto, A.I.; Ahmet, F.; Kitsoulis, S.; Shin Teh, J.; Lo, J.C.; Rizzitelli, A.; Wu, L.; Vremec, D.; van Dommelen, S.L.; et al. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 2008, 112, 3264–3273. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Mourao-Sa, D.; Joffre, O.P.; Schulz, O.; Rogers, N.C.; Pennington, D.J.; Carlyle, J.R.; Reis e Sousa, C. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Investig. 2008, 118, 2098–2110. [Google Scholar] [CrossRef]
- Bonifaz, L.C.; Bonnyay, D.P.; Charalambous, A.; Darguste, D.I.; Fujii, S.; Soares, H.; Brimnes, M.K.; Moltedo, B.; Moran, T.M.; Steinman, R.M. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 2004, 199, 815–824. [Google Scholar] [CrossRef]
- Johnson, T.S.; Mahnke, K.; Storn, V.; Schonfeld, K.; Ring, S.; Nettelbeck, D.M.; Haisma, H.J.; Le Gall, F.; Kontermann, R.E.; Enk, A.H. Inhibition of melanoma growth by targeting of antigen to dendritic cells via an anti-DEC-205 single-chain fragment variable molecule. Clin. Cancer Res. 2008, 14, 8169–8177. [Google Scholar] [CrossRef]
- Mascarell, L.; Fayolle, C.; Bauche, C.; Ladant, D.; Leclerc, C. Induction of neutralizing antibodies and Th1-polarized and CD4-independent CD8+ T-cell responses following delivery of human immunodeficiency virus type 1 Tat protein by recombinant adenylate cyclase of Bordetella pertussis. J. Virol. 2005, 79, 9872–9884. [Google Scholar] [CrossRef]
- Duluc, D.; Joo, H.; Ni, L.; Yin, W.; Upchurch, K.; Li, D.; Xue, Y.; Klucar, P.; Zurawski, S.; Zurawski, G.; et al. Induction and activation of human Th17 by targeting antigens to dendritic cells via dectin-1. J. Immunol. 2014, 192, 5776–5788. [Google Scholar] [CrossRef]
- Tsuji, T.; Matsuzaki, J.; Kelly, M.P.; Ramakrishna, V.; Vitale, L.; He, L.Z.; Keler, T.; Odunsi, K.; Old, L.J.; Ritter, G.; et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J. Immunol. 2011, 186, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, G.; Shen, X.; Zurawski, S.; Tomaras, G.D.; Montefiori, D.C.; Roederer, M.; Ferrari, G.; Lacabaratz, C.; Klucar, P.; Wang, Z.; et al. Superiority in Rhesus Macaques of Targeting HIV-1 Env gp140 to CD40 versus LOX-1 in Combination with Replication-Competent NYVAC-KC for Induction of Env-Specific Antibody and T Cell Responses. J. Virol. 2017, 91, e01596-16. [Google Scholar] [CrossRef] [PubMed]
- Flamar, A.L.; Contreras, V.; Zurawski, S.; Montes, M.; Dereuddre-Bosquet, N.; Martinon, F.; Banchereau, J.; Le Grand, R.; Zurawski, G.; Levy, Y. Delivering HIV Gagp24 to DCIR Induces Strong Antibody Responses in vivo. PLoS ONE 2015, 10, e0135513. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ahmet, F.; Sullivan, L.C.; Brooks, A.G.; Kent, S.J.; De Rose, R.; Salazar, A.M.; Reis e Sousa, C.; Shortman, K.; Lahoud, M.H.; et al. Antibodies targeting Clec9A promote strong humoral immunity without adjuvant in mice and non-human primates. Eur. J. Immunol. 2015, 45, 854–864. [Google Scholar] [CrossRef]
- Tewari, K.; Flynn, B.J.; Boscardin, S.B.; Kastenmueller, K.; Salazar, A.M.; Anderson, C.A.; Soundarapandian, V.; Ahumada, A.; Keler, T.; Hoffman, S.L.; et al. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and alphaDEC-CSP in non human primates. Vaccine 2010, 28, 7256–7266. [Google Scholar] [CrossRef]
- Tenbusch, M.; Ignatius, R.; Nchinda, G.; Trumpfheller, C.; Salazar, A.M.; Topfer, K.; Sauermann, U.; Wagner, R.; Hannaman, D.; Tenner-Racz, K.; et al. Immunogenicity of DNA vaccines encoding simian immunodeficiency virus antigen targeted to dendritic cells in rhesus macaques. PLoS ONE 2012, 7, e39038. [Google Scholar] [CrossRef]
- Tran, T.; Diniz, M.O.; Dransart, E.; Gey, A.; Merillon, N.; Lone, Y.C.; Godefroy, S.; Sibley, C.; Ferreira, L.C.; Medioni, J.; et al. A Therapeutic Her2/neu Vaccine Targeting Dendritic Cells Preferentially Inhibits the Growth of Low Her2/neu-Expressing Tumor in HLA-A2 Transgenic Mice. Clin. Cancer Res. 2016, 22, 4133–4144. [Google Scholar] [CrossRef]
- Hartung, E.; Becker, M.; Bachem, A.; Reeg, N.; Jakel, A.; Hutloff, A.; Weber, H.; Weise, C.; Giesecke, C.; Henn, V.; et al. Induction of potent CD8 T cell cytotoxicity by specific targeting of antigen to cross-presenting dendritic cells in vivo via murine or human XCR1. J. Immunol. 2015, 194, 1069–1079. [Google Scholar] [CrossRef]
- Fossum, E.; Grodeland, G.; Terhorst, D.; Tveita, A.A.; Vikse, E.; Mjaaland, S.; Henri, S.; Malissen, B.; Bogen, B. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur. J. Immunol. 2015, 45, 624–635. [Google Scholar] [CrossRef]
- Badillo-Godinez, O.; Pedroza-Saavedra, A.; Valverde-Garduno, V.; Bermudez-Morales, V.; Maldonado-Gama, M.; Leon-Letelier, R.; Bonifaz, L.C.; Esquivel-Guadarrama, F.; Gutierrez-Xicotencatl, L. Induction of Therapeutic Protection in an HPV16-Associated Mouse Tumor Model Through Targeting the Human Papillomavirus-16 E5 Protein to Dendritic Cells. Front. Immunol. 2021, 12, 593161. [Google Scholar] [CrossRef]
- Rivera, A.; Hohl, T.M.; Collins, N.; Leiner, I.; Gallegos, A.; Saijo, S.; Coward, J.W.; Iwakura, Y.; Pamer, E.G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. 2011, 208, 369–381. [Google Scholar] [CrossRef]
- Hernandez-Santos, N.; Gaffen, S.L. Th17 cells in immunity to Candida albicans. Cell Host Microbe 2012, 11, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.; Li, D.; Dullaers, M.; Kim, T.W.; Duluc, D.; Upchurch, K.; Xue, Y.; Zurawski, S.; Le Grand, R.; Liu, Y.J.; et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity 2014, 41, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Lahoud, M.H.; Ahmet, F.; Kitsoulis, S.; Wan, S.S.; Vremec, D.; Lee, C.N.; Phipson, B.; Shi, W.; Smyth, G.K.; Lew, A.M.; et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J. Immunol. 2011, 187, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Sulczewski, F.B.; Martino, L.A.; Almeida, B.D.S.; Zaneti, A.B.; Ferreira, N.S.; Amorim, K.; Yamamoto, M.M.; Apostolico, J.S.; Rosa, D.S.; Boscardin, S.B. Conventional type 1 dendritic cells induce TH 1, TH 1-like follicular helper T cells and regulatory T cells after antigen boost via DEC205 receptor. Eur. J. Immunol. 2020, 50, 1895–1911. [Google Scholar] [CrossRef]
- Sadraeian, M.; Khoshnood Mansoorkhani, M.J.; Mohkam, M.; Rasoul-Amini, S.; Hesaraki, M.; Ghasemi, Y. Prevention and Inhibition of TC-1 Cell Growth in Tumor Bearing Mice by HPV16 E7 Protein in Fusion with Shiga Toxin B-Subunit from shigella dysenteriae. Cell J. 2013, 15, 176–181. [Google Scholar]
- Xu, H.; Huang, J.; Liu, Z.; Li, X.; Wang, K.; Feng, E.; Wu, J.; Zhu, L.; Yao, K.; Pan, C.; et al. Expression of Bordetella pertussis Antigens Fused to Different Vectors and Their Effectiveness as Vaccines. Vaccines 2021, 9, 542. [Google Scholar] [CrossRef]
- Hosomi, K.; Hinenoya, A.; Suzuki, H.; Nagatake, T.; Nishino, T.; Tojima, Y.; Hirata, S.I.; Matsunaga, A.; Kondoh, M.; Yamasaki, S.; et al. Development of a bivalent food poisoning vaccine: Augmented antigenicity of the C-terminus of Clostridium perfringens enterotoxin by fusion with the B subunit of Escherichia coli Shiga toxin 2. Int. Immunol. 2019, 31, 91–100. [Google Scholar] [CrossRef]
- Park, H.Y.; Tan, P.S.; Kavishna, R.; Ker, A.; Lu, J.; Chan, C.E.Z.; Hanson, B.J.; MacAry, P.A.; Caminschi, I.; Shortman, K.; et al. Enhancing vaccine antibody responses by targeting Clec9A on dendritic cells. NPJ Vaccines 2017, 2, 31. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, D.; Ng, W.Y.; Holz, L.E.; Ma, J.Z.; Zaid, A.; Wong, Y.C.; Lau, L.S.; Mollard, V.; Cozijnsen, A.; Collins, N.; et al. Liver-Resident Memory CD8(+) T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 2016, 45, 889–902. [Google Scholar] [CrossRef]
- Saron, M.F.; Fayolle, C.; Sebo, P.; Ladant, D.; Ullmann, A.; Leclerc, C. Anti-viral protection conferred by recombinant adenylate cyclase toxins from Bordetella pertussis carrying a CD8+ T cell epitope from lymphocytic choriomeningitis virus. Proc. Natl. Acad. Sci. USA 1997, 94, 3314–3319. [Google Scholar] [CrossRef] [PubMed]
- Hesse, C.; Ginter, W.; Forg, T.; Mayer, C.T.; Baru, A.M.; Arnold-Schrauf, C.; Unger, W.W.; Kalay, H.; van Kooyk, Y.; Berod, L.; et al. In vivo targeting of human DC-SIGN drastically enhances CD8(+) T-cell-mediated protective immunity. Eur. J. Immunol. 2013, 43, 2543–2553. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Q.; Li, G.; Banga, R.; Ma, J.; Yu, H.; Yasui, F.; Zhang, Z.; Pantaleo, G.; Perreau, M.; et al. TLR3 agonist and CD40-targeting vaccination induces immune responses and reduces HIV-1 reservoirs. J. Clin. Investig. 2018, 128, 4387–4396. [Google Scholar] [CrossRef] [PubMed]
- Preville, X.; Ladant, D.; Timmerman, B.; Leclerc, C. Eradication of established tumors by vaccination with recombinant Bordetella pertussis adenylate cyclase carrying the human papillomavirus 16 E7 oncoprotein. Cancer Res. 2005, 65, 641–649. [Google Scholar]
- Berraondo, P.; Nouze, C.; Preville, X.; Ladant, D.; Leclerc, C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007, 67, 8847–8855. [Google Scholar] [CrossRef]
- Mahnke, K.; Qian, Y.; Fondel, S.; Brueck, J.; Becker, C.; Enk, A.H. Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res. 2005, 65, 7007–7012. [Google Scholar] [CrossRef]
- Chen, K.; Wu, Z.; Zhao, H.; Wang, Y.; Ge, Y.; Wang, D.; Li, Z.; An, C.; Liu, Y.; Wang, F.; et al. XCL1/Glypican-3 Fusion Gene Immunization Generates Potent Antitumor Cellular Immunity and Enhances Anti-PD-1 Efficacy. Cancer Immunol. Res. 2020, 8, 81–93. [Google Scholar] [CrossRef]
- Kretz-Rommel, A.; Qin, F.; Dakappagari, N.; Torensma, R.; Faas, S.; Wu, D.; Bowdish, K.S. In vivo targeting of antigens to human dendritic cells through DC-SIGN elicits stimulatory immune responses and inhibits tumor growth in grafted mouse models. J. Immunother. 2007, 30, 715–726. [Google Scholar] [CrossRef]
- Chen, J.; Zurawski, G.; Zurawski, S.; Wang, Z.; Akagawa, K.; Oh, S.; Hideki, U.; Fay, J.; Banchereau, J.; Song, W.; et al. A novel vaccine for mantle cell lymphoma based on targeting cyclin D1 to dendritic cells via CD40. J. Hematol. Oncol. 2015, 8, 35. [Google Scholar] [CrossRef]
- Schjetne, K.W.; Fredriksen, A.B.; Bogen, B. Delivery of antigen to CD40 induces protective immune responses against tumors. J. Immunol. 2007, 178, 4169–4176. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kimura, S.; Hase, K. M cell-dependent antigen uptake on follicle-associated epithelium for mucosal immune surveillance. Inflamm. Regen. 2018, 38, 15. [Google Scholar] [CrossRef] [PubMed]
- Fujkuyama, Y.; Tokuhara, D.; Kataoka, K.; Gilbert, R.S.; McGhee, J.R.; Yuki, Y.; Kiyono, H.; Fujihashi, K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev. Vaccines 2012, 11, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Diniz, M.O.; Roussel, H.; Tran, T.; Ferreira, L.C.; Badoual, C.; Tartour, E. Mucosal vaccines: Novel strategies and applications for the control of pathogens and tumors at mucosal sites. Hum. Vaccines Immunother. 2014, 10, 2175–2187. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Mucosal immunity: Induction, dissemination, and effector functions. Scand. J. Immunol. 2009, 70, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ainai, A.; Hasegawa, H. Functional and structural characteristics of secretory IgA antibodies elicited by mucosal vaccines against influenza virus. Vaccine 2017, 35, 5297–5302. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Mami-Chouaib, F.; Tartour, E. Editorial: Tissue Resident Memory T Cells. Front. Immunol. 2019, 10, 1018. [Google Scholar] [CrossRef]
- Tran, T.; Blanc, C.; Granier, C.; Saldmann, A.; Tanchot, C.; Tartour, E. Therapeutic cancer vaccine: Building the future from lessons of the past. Semin. Immunopathol. 2018, 41, 69–85. [Google Scholar] [CrossRef]
- van Gisbergen, K.; Zens, K.D.; Munz, C. T-cell memory in tissues. Eur. J. Immunol. 2021, 51, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Blanc, C.; Hans, S.; Tran, T.; Granier, C.; Saldman, A.; Anson, M.; Oudard, S.; Tartour, E. Targeting Resident Memory T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Mami-Chouaib, F.; Blanc, C.; Corgnac, S.; Hans, S.; Malenica, I.; Granier, C.; Tihy, I.; Tartour, E. Resident memory T cells, critical components in tumor immunology. J. Immunother. Cancer 2018, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Nizard, M.; Roussel, H.; Tartour, E. Resident Memory T Cells as Surrogate Markers of the Efficacy of Cancer Vaccines. Clin. Cancer Res. 2016, 22, 530–532. [Google Scholar] [CrossRef]
- Sandoval, F.; Terme, M.; Nizard, M.; Badoual, C.; Bureau, M.F.; Freyburger, L.; Clement, O.; Marcheteau, E.; Gey, A.; Fraisse, G.; et al. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci. Transl. Med. 2013, 5, 172ra20. [Google Scholar] [CrossRef]
- Nizard, M.; Roussel, H.; Diniz, M.O.; Karaki, S.; Tran, T.; Voron, T.; Dransart, E.; Sandoval, F.; Riquet, M.; Rance, B.; et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 2017, 8, 15221. [Google Scholar] [CrossRef]
- Karaki, S.; Blanc, C.; Tran, T.; Galy-Fauroux, I.; Mougel, A.; Dransart, E.; Anson, M.; Tanchot, C.; Paolini, L.; Gruel, N.; et al. CXCR6 deficiency impairs cancer vaccine efficacy and CD8(+) resident memory T-cell recruitment in head and neck and lung tumors. J. Immunother. Cancer 2021, 9, e001948. [Google Scholar] [CrossRef]
- Choi, N.W.; Estes, M.K.; Langridge, W.H. Oral immunization with a shiga toxin B subunit: Rotavirus NSP4(90) fusion protein protects mice against gastroenteritis. Vaccine 2005, 23, 5168–5176. [Google Scholar] [CrossRef]
- Clarke, E.C.; Bradfute, S.B. Advances in Ebola virus vaccination. Lancet Infect. Dis. 2017, 17, 787–788. [Google Scholar] [CrossRef]
- Heppner, D.G., Jr.; Kemp, T.L.; Martin, B.K.; Ramsey, W.J.; Nichols, R.; Dasen, E.J.; Link, C.J.; Das, R.; Xu, Z.J.; Sheldon, E.A.; et al. Safety and immunogenicity of the rVSVG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: A phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect. Dis. 2017, 17, 854–866. [Google Scholar] [CrossRef]
- Zheng, X.; Oduro, J.D.; Boehme, J.D.; Borkner, L.; Ebensen, T.; Heise, U.; Gereke, M.; Pils, M.C.; Krmpotic, A.; Guzman, C.A.; et al. Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge. PLoS Pathog. 2019, 15, e1008036. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.X.; Wheatley, A.K.; Esterbauer, R.; Jegaskanda, S.; Glass, J.J.; Masopust, D.; De Rose, R.; Kent, S.J. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol. 2018, 11, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, H.J.; Chang, J. Superior immune responses induced by intranasal immunization with recombinant adenovirus-based vaccine expressing full-length Spike protein of Middle East respiratory syndrome coronavirus. PLoS ONE 2019, 14, e0220196. [Google Scholar] [CrossRef] [PubMed]
- Binjawadagi, B.; Ma, Y.; Binjawadagi, R.; Brakel, K.; Harder, O.; Peeples, M.; Li, J.; Niewiesk, S. Mucosal Delivery of Recombinant Vesicular Stomatitis Virus Vectors Expressing Envelope Proteins of Respiratory Syncytial Virus Induces Protective Immunity in Cotton Rats. J. Virol. 2021, 95, e02345-20. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184.e13. [Google Scholar] [CrossRef]
- Wu, H.Y.; Nikolova, E.B.; Beagley, K.W.; Eldridge, J.H.; Russell, M.W. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect. Immun. 1997, 65, 227–235. [Google Scholar] [CrossRef]
- Hervouet, C.; Luci, C.; Cuburu, N.; Cremel, M.; Bekri, S.; Vimeux, L.; Maranon, C.; Czerkinsky, C.; Hosmalin, A.; Anjuere, F. Sublingual immunization with an HIV subunit vaccine induces antibodies and cytotoxic T cells in the mouse female genital tract. Vaccine 2010, 28, 5582–5590. [Google Scholar] [CrossRef]
- Lakhrif, Z.; Moreau, A.; Herault, B.; Di-Tommaso, A.; Juste, M.; Moire, N.; Dimier-Poisson, I.; Mevelec, M.N.; Aubrey, N. Targeted Delivery of Toxoplasma gondii Antigens to Dendritic Cells Promote Immunogenicity and Protective Efficiency against Toxoplasmosis. Front. Immunol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Daudel, D.; Weidinger, G.; Spreng, S. Use of attenuated bacteria as delivery vectors for DNA vaccines. Expert Rev. Vaccines 2007, 6, 97–110. [Google Scholar] [CrossRef]
- Grillot-Courvalin, C.; Goussard, S.; Huetz, F.; Ojcius, D.M.; Courvalin, P. Functional gene transfer from intracellular bacteria to mammalian cells. Nat. Biotechnol. 1998, 16, 862–866. [Google Scholar] [CrossRef]
- Kozlowski, P.A.; Aldovini, A. Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission. Curr. Immunol. Rev. 2019, 15, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Vilander, A.C.; Dean, G.A. Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines. Vaccines 2019, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Z.; Zhang, Y.; Pan, L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016, 100, 5691–5701. [Google Scholar] [CrossRef]
- Yao, X.Y.; Yuan, M.M.; Li, D.J. Molecular adjuvant C3d3 improved the anti-hCGbeta humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCGbeta-C3d3 fusion protein. Vaccine 2007, 25, 6129–6139. [Google Scholar] [CrossRef]
- Michon, C.; Kuczkowska, K.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Surface display of an anti-DEC-205 single chain Fv fragment in Lactobacillus plantarum increases internalization and plasmid transfer to dendritic cells in vitro and in vivo. Microb. Cell Fact. 2015, 14, 95. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Wang, Q.; Huang, H.B.; Jiang, Y.L.; Shi, C.W.; Wang, J.Z.; Huang, K.Y.; Jin, Y.B.; Wang, C.F. Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antiviral Res. 2017, 138, 9–21. [Google Scholar] [CrossRef]
- Liu, J.; Yang, G.; Huang, H.; Shi, C.; Gao, X.; Yang, W.; Zhang, Z.; Liu, Y.; Xu, K.; Wang, J.; et al. Dendritic Cells Targeting Lactobacillus plantarum Strain NC8 with a Surface-Displayed Single-Chain Variable Fragment of CD11c Induce an Antigen-Specific Protective Cellular Immune Response. Infect. Immun. 2020, 88, e00759-19. [Google Scholar] [CrossRef]
- van Broekhoven, C.L.; Parish, C.R.; Demangel, C.; Britton, W.J.; Altin, J.G. Targeting dendritic cells with antigen-containing liposomes: A highly effective procedure for induction of antitumor immunity and for tumor immunotherapy. Cancer Res. 2004, 64, 4357–4365. [Google Scholar] [CrossRef]
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Lowik, C.; Oostendorp, J.; van der Burg, S.H.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88–97. [Google Scholar] [CrossRef]
- Pichon, C.; Midoux, P. Mannosylated and histidylated LPR technology for vaccination with tumor antigen mRNA. Methods Mol. Biol. 2013, 969, 247–274. [Google Scholar] [PubMed]
- Matsuo, H.; Somiya, M.; Iijima, M.; Arakawa, T.; Kuroda, S. CD11c-specific bio-nanocapsule enhances vaccine immunogenicity by targeting immune cells. J. Nanobiotechnol. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Phua, K.K.; Staats, H.F.; Leong, K.W.; Nair, S.K. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci. Rep. 2014, 4, 5128. [Google Scholar] [CrossRef]
- Mai, Y.; Guo, J.; Zhao, Y.; Ma, S.; Hou, Y.; Yang, J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell. Immunol. 2020, 354, 104143. [Google Scholar] [CrossRef]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Estanbouli, H.; Bouteau, A.; Igyarto, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. Iscience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts 2011, 1, 23–30. [Google Scholar]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J.L. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef]
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Seguier, S.; Tran, T.; Paolini, L.; Lebbe, C.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 2020, 147, 1509–1518. [Google Scholar] [CrossRef]
- Munari, E.; Mariotti, F.R.; Quatrini, L.; Bertoglio, P.; Tumino, N.; Vacca, P.; Eccher, A.; Ciompi, F.; Brunelli, M.; Martignoni, G.; et al. PD-1/PD-L1 in Cancer: Pathophysiological, Diagnostic and Therapeutic Aspects. Int. J. Mol. Sci. 2021, 22, 5123. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.D.; Zhang, B. Cancer Immunotherapy Update: FDA-Approved Checkpoint Inhibitors and Companion Diagnostics. AAPS J. 2021, 23, 39. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Bagley, S.J.; Wen, P.Y.; Lim, M.; Platten, M.; Colman, H.; Ashley, D.M.; Wick, W.; Chang, S.M.; Galanis, E.; et al. Systematic review of combinations of targeted or immunotherapy in advanced solid tumors. J Immunother. Cancer 2021, 9, e002459. [Google Scholar] [CrossRef] [PubMed]
- Badoual, C.; Hans, S.; Merillon, N.; Van Ryswick, C.; Ravel, P.; Benhamouda, N.; Levionnois, E.; Nizard, M.; Si-Mohamed, A.; Besnier, N.; et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013, 73, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.V.; Regnier, F.; Thoreau, M.; Vimeux, L.; Benard, M.; Dransart, E.; Penny, H.L.; Johannes, L.; Trautmann, A.; Bercovici, N. Local IFNalpha enhances the anti-tumoral efficacy of systemic anti-PD1 to prevent tumor relapse. J. Immunother. Cancer 2020, 8, e000996. [Google Scholar] [CrossRef]
- Thoreau, M.; Penny, H.L.; Tan, K.; Regnier, F.; Weiss, J.M.; Lee, B.; Johannes, L.; Dransart, E.; Le Bon, A.; Abastado, J.P.; et al. Vaccine-induced tumor regression requires a dynamic cooperation between T cells and myeloid cells at the tumor site. Oncotarget 2015, 6, 27832–27846. [Google Scholar] [CrossRef]
- Pere, H.; Montier, Y.; Bayry, J.; Quintin-Colonna, F.; Merillon, N.; Dransart, E.; Badoual, C.; Gey, A.; Ravel, P.; Marcheteau, E.; et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011, 118, 4853–4862. [Google Scholar] [CrossRef]
- Beziaud, L.; Boullerot, L.; Tran, T.; Mansi, L.; Marie-Joseph, E.L.; Ravel, P.; Johannes, L.; Bayry, J.; Tartour, E.; Adotevi, O. Rapalog combined with CCR4 antagonist improves anticancer vaccines efficacy. Int. J. Cancer 2018, 143, 3008–3018. [Google Scholar] [CrossRef]
- Mondini, M.; Nizard, M.; Tran, T.; Mauge, L.; Loi, M.; Clemenson, C.; Dugue, D.; Maroun, P.; Louvet, E.; Adam, J.; et al. Synergy of Radiotherapy and a Cancer Vaccine for the Treatment of HPV-Associated Head and Neck Cancer. Mol. Cancer Ther. 2015, 14, 1336–1345. [Google Scholar] [CrossRef]
- Daillere, R.; Vetizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Daher, C.; Vimeux, L.; Stoeva, R.; Peranzoni, E.; Bismuth, G.; Wieduwild, E.; Lucas, B.; Donnadieu, E.; Bercovici, N.; Trautmann, A.; et al. Blockade of beta-Adrenergic Receptors Improves CD8(+) T-cell Priming and Cancer Vaccine Efficacy. Cancer Immunol. Res. 2019, 7, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Chart, H.; Law, D.; Rowe, B.; Acheson, D.W. Patients with haemolytic uraemic syndrome caused by Escherichia coli O157: Absence of antibodies to Vero cytotoxin 1 (VT1) or VT2. J. Clin. Pathol. 1993, 46, 1053–1054. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; McEwen, J.; Losonsky, G.; Reymann, M.; Harari, I.; Brown, J.E.; Taylor, D.N.; Donohue-Rolfe, A.; Cohen, D.; Bennish, M.; et al. Antibodies to shiga holotoxin and to two synthetic peptides of the B subunit in sera of patients with Shigella dysenteriae 1 dysentery. J. Clin. Microbiol. 1992, 30, 1636–1641. [Google Scholar] [CrossRef]
- Fernandez-Brando, R.J.; Bentancor, L.V.; Mejias, M.P.; Ramos, M.V.; Exeni, A.; Exeni, C.; Laso Mdel, C.; Exeni, R.; Isturiz, M.A.; Palermo, M.S. Antibody response to Shiga toxins in Argentinean children with enteropathic hemolytic uremic syndrome at acute and long-term follow-up periods. PLoS ONE 2011, 6, e19136. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Karmali, M.A.; Sarkim, V.; Bobrowski, C.; Petric, M.; Karch, H.; Muller-Wiefel, D.E.; Arbeitsgemeinschaft fur Padiatrische, N. Antibody response to Shiga toxins Stx2 and Stx1 in children with enteropathic hemolytic-uremic syndrome. J. Clin. Microbiol. 2001, 39, 2272–2279. [Google Scholar] [CrossRef][Green Version]
- Ohmura, M.; Yamasaki, S.; Kurazono, H.; Kashiwagi, K.; Igarashi, K.; Takeda, Y. Characterization of non-toxic mutant toxins of Vero toxin 1 that were constructed by replacing amino acids in the A subunit. Microb. Pathog. 1993, 15, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.X.; Teel, L.D.; Judge, N.A.; O’Brien, A.D. Genetic toxoids of Shiga toxin types 1 and 2 protect mice against homologous but not heterologous toxin challenge. Vaccine 2006, 24, 1142–1148. [Google Scholar] [CrossRef]
- Kovbasnjuk, O.; Mourtazina, R.; Baibakov, B.; Wang, T.; Elowsky, C.; Choti, M.A.; Kane, A.; Donowitz, M. The glycosphingolipid globotriaosylceramide in the metastatic transformation of colon cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 19087–19092. [Google Scholar] [CrossRef]
- Fulcher, J.M.; Petersen, M.E.; Giesler, R.J.; Cruz, Z.S.; Eckert, D.M.; Francis, J.N.; Kawamoto, E.M.; Jacobsen, M.T.; Kay, M.S. Chemical synthesis of Shiga toxin subunit B using a next-generation traceless “helping hand” solubilizing tag. Org. Biomol. Chem. 2019, 17, 10237–10244. [Google Scholar] [CrossRef]
- Viel, T.; Dransart, E.; Nemati, F.; Henry, E.; Theze, B.; Decaudin, D.; Lewandowski, D.; Boisgard, R.; Johannes, L.; Tavitian, B. In vivo tumor targeting by the B-subunit of shiga toxin. Mol. Imaging 2008, 7, 239–247. [Google Scholar] [CrossRef]
- Janssen, K.P.; Vignjevic, D.; Boisgard, R.; Falguieres, T.; Bousquet, G.; Decaudin, D.; Dolle, F.; Louvard, D.; Tavitian, B.; Robine, S.; et al. In vivo tumor targeting using a novel intestinal pathogen-based delivery approach. Cancer Res. 2006, 66, 7230–7236. [Google Scholar] [CrossRef] [PubMed]
- Hervouet, K.; Thedrez, P.; Lesieur, J.; Sai-Maurel, C.; Louvard, D.; Robine, S.; Amessou, M.; Barbet, J.; Johannes, L.; Decaudin, D. Biodistribution and tumor targeting of indium-111 and iodine-125-labeled Shiga toxin B-subunit. Curr. Radiopharm. 2009, 2, 184–190. [Google Scholar] [CrossRef]
- Lamblet, M.; Delord, B.; Johannes, L.; van Effenterre, D.; Bassereau, P. Key role of receptor density in colloid/cell specific interaction: A quantitative biomimetic study on giant vesicles. Eur. Phys. J. E Soft Matter 2008, 26, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chenal, A.; Ladant, D. Bioengineering of Bordetella pertussis Adenylate Cyclase Toxin for Antigen-Delivery and Immunotherapy. Toxins 2018, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.J.; James, J.; Ryan, F.J.; Caparros-Martin, J.; Eden, G.L.; Tee, Y.C.; Salamon, J.R.; Benson, S.C.; Tumes, D.J.; Sribnaia, A.; et al. The immunotoxicity, but not anti-tumor efficacy, of anti-CD40 and anti-CD137 immunotherapies is dependent on the gut microbiota. Cell Rep. Med. 2021, 2, 100464. [Google Scholar] [CrossRef]
- Knorr, D.A.; Dahan, R.; Ravetch, J.V. Toxicity of an Fc-engineered anti-CD40 antibody is abrogated by intratumoral injection and results in durable antitumor immunity. Proc. Natl. Acad. Sci. USA 2018, 115, 11048–11053. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Peng, K.; Wang, Y.; Gong, T.; Zhang, Z.; He, Q.; Sun, X. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017, 64, 237–248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).