Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice
Abstract
:1. Introduction
2. Results and Discussions
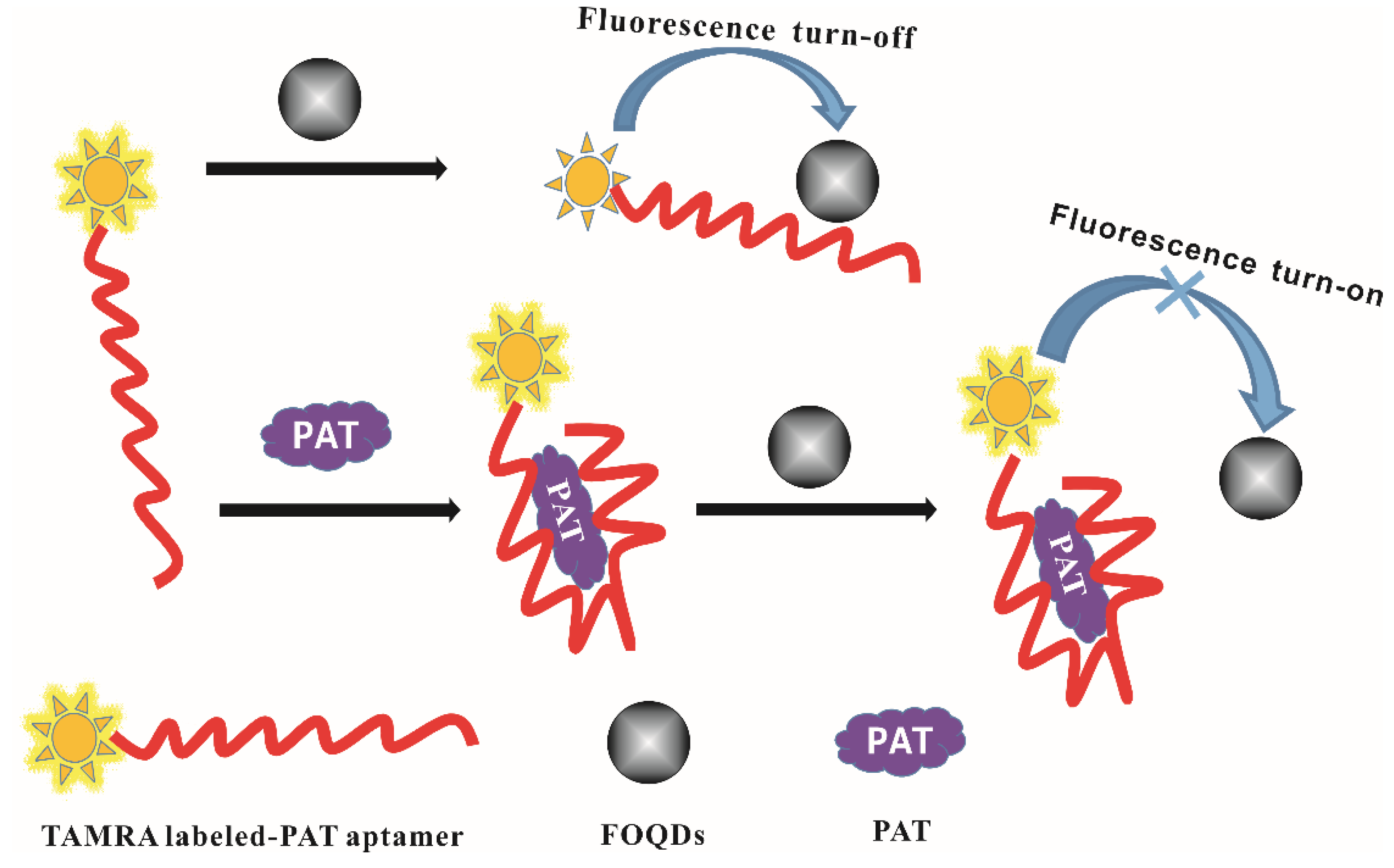
2.1. Fluorescence Aptasensor Development for PAT
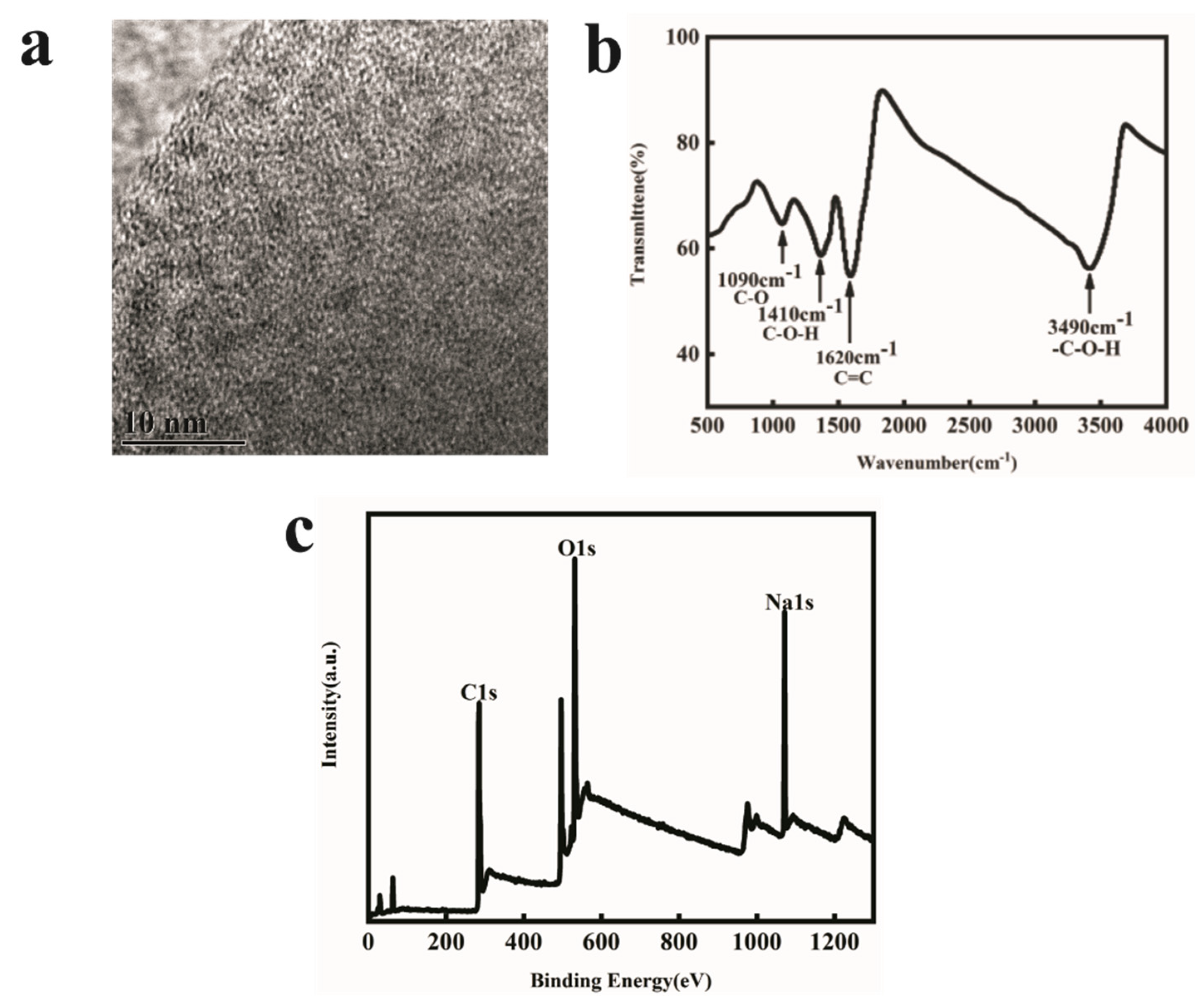
2.2. Properties Characterization of the FOQDs
2.3. Construction of the PAT Aptasensor
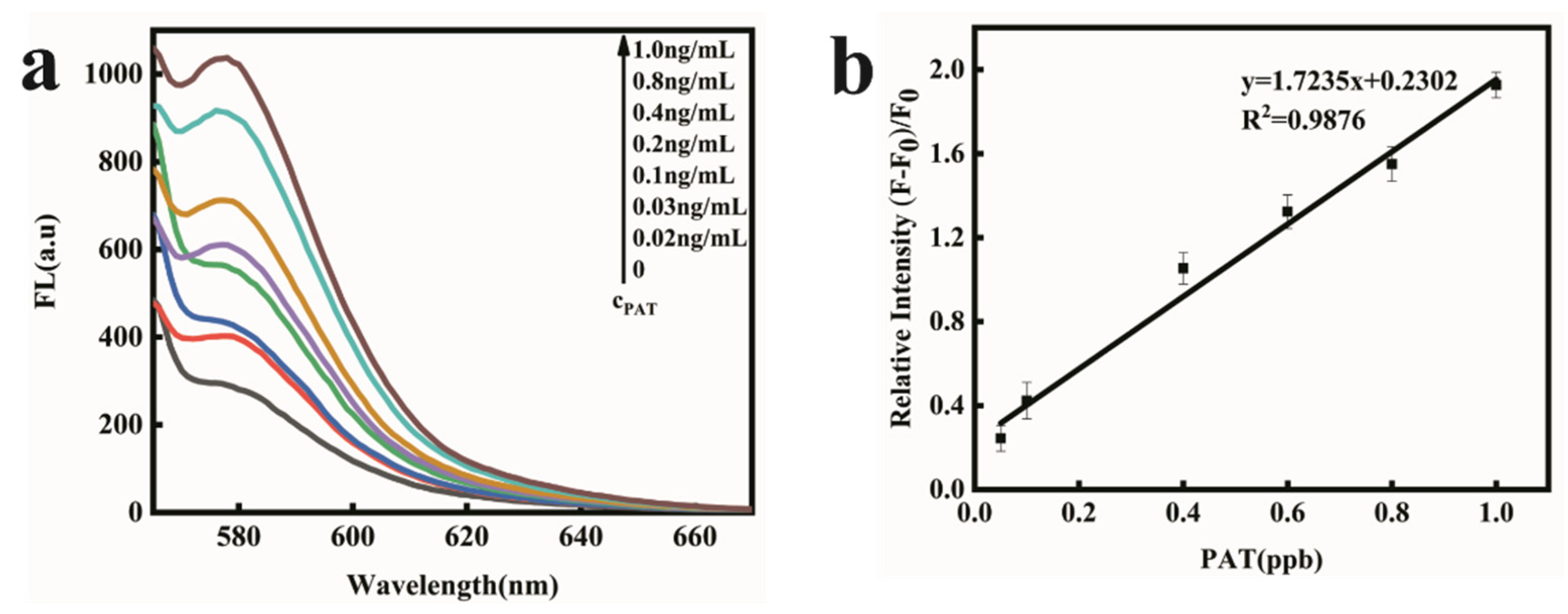
2.4. PAT Detection in Aqueous Buffer Solution
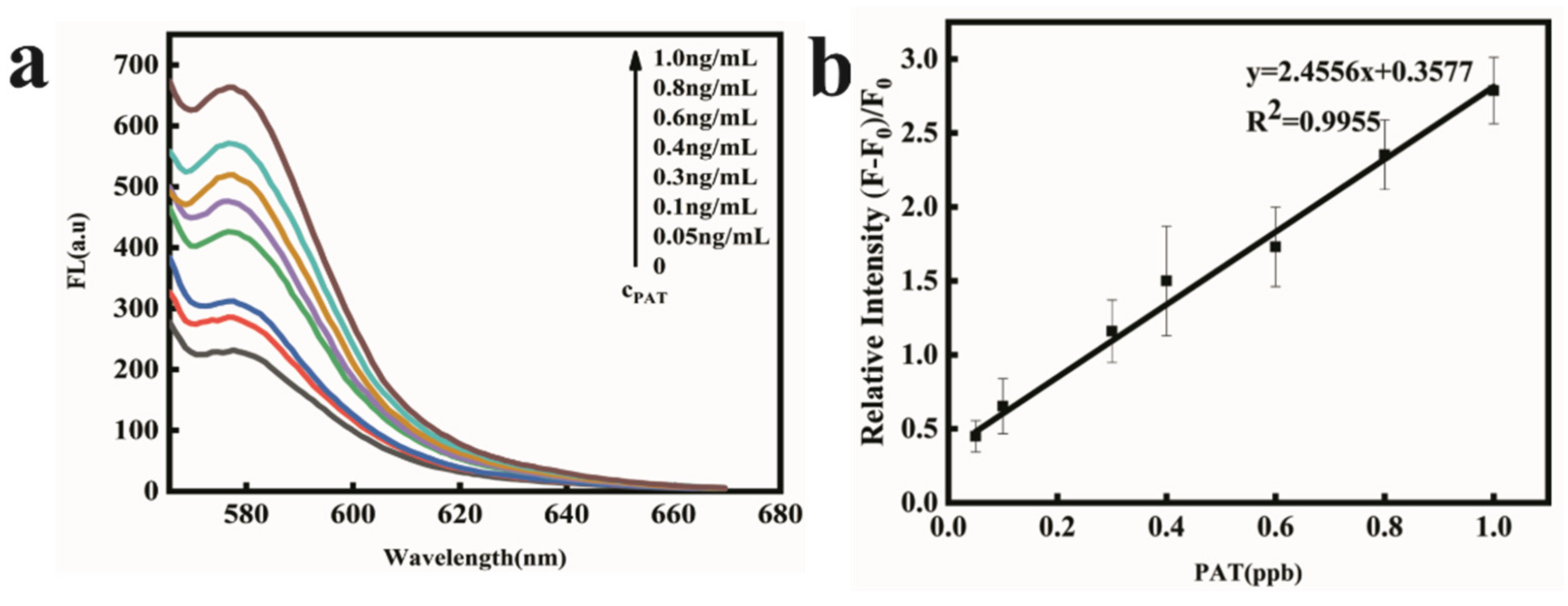
2.5. PAT Detection in Apple Juice
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Instrumentation
4.3. Quenching Measurements
4.4. PAT Detection in Buffer Solution
4.5. PAT Detection in Apple Juice Samples
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.L.; Zheng, X.F.; Yang, Q.Y.; Zhang, H.Y.; Apaliya, M.T.; Dhanasekaran, S.; Zhang, X.Y.; Zhao, L.N.; Jiang, Z.H. S-Adenosylmethionine-dependent methyltransferase helps pichia caribbica degrade patulin. J. Agric. Food Chem. 2019, 67, 11758–11768. [Google Scholar] [CrossRef] [PubMed]
- Fliege, R.; Metzler, M. Electrophilic properties of patulin. Adduct structures and reaction pathways with 4-bromothiophenol and other model nucleophiles. Chem. Res. Toxicol. 2000, 13, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Lu, X.T.; Zhang, E.X.; Yin, S.T.; Fan, L.H.; Hu, H.B. Methylseleninic acid prevents patulin-induced hepatotoxicity and nephrotoxicity via inhibition of oxidative stress and inactivation of p53 and MAPKs. J. Agric. Food Chem. 2017, 65, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation 2006/1881/EC of 19 December 2006 replacing Regulation (EC) 466/2001 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, L 364, 5–24.
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef]
- WHO. World Health Organization, 44th Report of the Joint FAO/WHO Expert Committee on Food Additives. Tech. Rep. Ser. 1995, 859, 36. [Google Scholar]
- Jalali, A.; Khorasgani, Z.N.; Goudarzi, M.; Khoshlesan, N. HPLC determination of patulin in apple juice: A single center study of Southwest Area of Iran. J. Pharmacol. Toxicol. 2010, 5, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Rychlik, M.; Schieberle, P. Quantification of the mycotoxin patulin by a stable isotope dilution assay. J. Agric. Food Chem. 1999, 47, 3749–3755. [Google Scholar] [CrossRef]
- Xing, M.Y.; Li, B.Q.; Chen, Y.; Tian, S.P. Ribonucleoside diphosphate reductase plays an important role in patulin degradation by Enterobacter cloacae subsp. dissolvens. J. Agric. Food Chem. 2020, 68, 5232–5240. [Google Scholar] [CrossRef]
- Tan, S.Y.; Acquah, C.; Sidhu, A.; Ongkudon, C.M.; Yon, L.S.; Danquah, M.K. SELEX modifications and bioanalytical techniques for aptamer-target binding characterization. Crit. Rev. Anal. Chem. 2016, 46, 521–537. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.F.; Zhang, C.A.X.; Ma, T.T.; Liu, X.Y.; Chen, Z.; Li, S.; Deng, Y. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 2021, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.Q.; Wu, L.; Xu, X.; Wu, Q.Y.; Wang, Y.Z.; Shen, H.C.; Song, Y.L.; Wang, H.Y.; Zhu, Z.; Kang, D.Z.; et al. Aptamer generated by cell-SELEX for specific targeting of human glioma cells. ACS Appl. Mater. Inter. 2021, 13, 9306–9315. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Wieczerzak, M. Aptamer based tools for environmental and therapeutic monitoring: A review of developments, applications, future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 816–867. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits-a case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.Y.; Chang, Y.Y.; Zhang, Q.; Chang, D.R.; Hui, C.Y.; Brennan, J.D.; Li, Y.F. In vitro selection of a DNA aptamer targeting degraded protein fragments for biosensing. Angew. Chem. Int. Ed. 2020, 59, 7706–7710. [Google Scholar] [CrossRef]
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Zhang, W.; Zhao, S.; Wang, Z. Screening and development of DNA aptamers as capture probes for colorimetric detection of patulin. Anal. Biochem. 2016, 508, 58–64. [Google Scholar] [CrossRef]
- Huang, Q.W.; Zhao, Z.H.; Nie, D.X.; Jiang, K.Q.; Guo, W.B.; Fan, K.; Zhang, Z.Q.; Meng, J.J.; Wu, Y.J.; Han, Z. Molecularly imprinted poly(thionine)-based electrochemical sensing platform for fast and selective ultratrace determination of patulin. Anal. Chem. 2019, 91, 4116–4123. [Google Scholar]
- Afzalinia, A.; Mirzaee, M. Ultrasensitive fluorescent miRNA biosensor based on a “sandwich” oligonucleotide hybridization and fluorescence resonance energy transfer process using an Ln(III)-MOF and Ag nanoparticles for early cancer diagnosis: Application of central composite design. ACS Appl. Mater. Inter. 2020, 12, 16076–16087. [Google Scholar] [CrossRef]
- Han, Y.; Yang, W.X.; Luo, X.L.; He, X.; Zhao, H.P.; Tang, W.Z.; Yue, T.L.; Li, Z.H. Carbon dots based ratiometric fluorescent sensing platform for food safety. Crit. Rev. Food Sci. Nutr. 2022, 62, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.H.; Sun, J.H.; Li, P.; Jing, L.Q. Current advances on g-C3N4-based fluorescence detection for environmental contaminants. J. Hazard. Mater. 2022, 425, 127990. [Google Scholar] [CrossRef] [PubMed]
- Vinegoni, C.; Feruglio, P.F.; Gryczynski, I.; Mazitschek, R.; Weissleder, R. Fluorescence anisotropy imaging in drug discovery. Adv. Drug. Deliv. Rev. 2019, 151, 262–288. [Google Scholar] [CrossRef] [PubMed]
- Saladino, G.M.; Vogt, C.; Li, Y.Y.; Shaker, K.; Brodin, B.; Svenda, M.; Hertz, H.M.; Toprak, M.S. Optical and X-ray fluorescent nanoparticles for dual mode bioimaging. ACS Nano 2021, 15, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Wei, Q.Y.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pang, H.; Zhang, L.X.; Mao, J.; Zhang, W.; Jiang, J.; Li, P.W.; Zhang, Q. Ultrasensitive biosensing platform based on luminescence quenching ability of fullerenol quantum dots. RSC Adv. 2021, 11, 19690–19694. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Luo, Y.; Sun, X. Nano-C60: A novel, effective, fluorescent sensing platform for biomolecular detection. Small 2011, 7, 1562–1568. [Google Scholar] [CrossRef]
- Li, H.; Zhai, J.; Sun, X. Highly sensitive and selective detection of silver (I) ion using nano-C60 as an effective fluorscent sensing platform. Analyst 2011, 136, 2040–2043. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhou, P.; Wang, Q.G.; Huo, X.W.; Huang, X.; Ye, Q.; Xu, H.; Zhou, G.Y.; Liu, Y.H.; Zhang, J. Fullerol mediated enhancement of chloramphenicol degradation in Fe(III)/H2O2 system by accelerating Fe(III)/Fe(II) Cycle via a non-photochemical pathway. Chem. Eng. J. 2020, 402, 126176. [Google Scholar] [CrossRef]
- Zhang, M.F.; Xu, Z.Q.; Ge, Y.S.; Jiang, F.L.; Liu, Y. Binding of fullerol to human serum albumin: Spectroscopic and electrochemical approach. J. Photochem. Photobiol. B 2012, 108, 34–43. [Google Scholar] [CrossRef]
- Pei, Y.F.; Cui, F.A.; Du, X.J.; Shang, G.W.; Xiao, W.N.; Yang, X.L.; Cui, Q.J. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int. J. Nanomed. 2019, 14, 4145–4155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, G.S.; Vallejos, V.M.R.; Ladeira, M.S.; Reis, P.G.; Souza, D.M.; Machado, Y.A.; Ladeira, L.O.; Pinheiro, M.B.V.; Melo, M.N.; Fujiwara, R.T.; et al. Antileishmanial activity of fullerol and its liposomal formulation in experimental models of visceral leishmaniasis. Biomed. Pharmacother. 2021, 134, 111120. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, T.; Pan, S.; Zhang, Y. A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification. Microchim. Acta 2018, 185, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Wang, H.; Yang, Y.; Liu, G.; Wang, S. Development and application of a quartz crystal microbalance sensor based on molecularly imprinted sol-gel polymer for rapid detection of patulin in foods. Sens. Actuat. B Chem. 2016, 237, 239–246. [Google Scholar] [CrossRef]
- Zhang, W.; Han, Y.; Chen, X.; Luo, X.; Wang, J.; Yue, T.; Li, Z. Surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots as a phosphorescent nanosensor for detecting patulin in apple juice. Food Chem. 2017, 232, 145–154. [Google Scholar] [CrossRef]


| Method | LINEAR RANGE | Limit of Detection | Detection Time | References |
|---|---|---|---|---|
| Fluorometric aptasensor based on magnetized graphene oxide and DNase I-assisted target recycling amplification | 0.5–30 ng/mL | 0.28 ng/mL | ~2 h | [33] |
| Quartz crystal microbalance sensor based on molecularly imprinted sol-gel polymer | 7.5 × 10−3 μg mL−1–6 × 10−2 μg mL−1 | 3.1 × 10−3 μg mL−1 | ~4 h | [34] |
| colorimetric method based on aptamer and gold nanoparticles | 50–2500 pg mL−1 | 48 pg mL−1 | ~2 h | [18] |
| Phosphorescent nanosensor based on surface molecularly imprinted polymer capped Mn-doped ZnS quantum dots | 0.43–6.50 μmol L−1 | 0.32 μmol L−1 | 2 h | [35] |
| Fluorescence aptasensor based on FOQDs | 0.02–1 ng/mL | 0.01 ng/mL | 1.5 h | This work |
| Apple Juice Sample | PAT Added (ng/mL) | PAT Founded (ng/mL) | Recovery(%) | RSD(%) n = 3 |
|---|---|---|---|---|
| 1 | 0.40 | 0.38 | 95.0 | 5.3 |
| 2 | 0.80 | 0.85 | 106.3 | 3.1 |
| 3 | 1.00 | 1.03 | 103.0 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, H.; Li, H.; Zhang, W.; Mao, J.; Zhang, L.; Zhang, Z.; Zhang, Q.; Wang, D.; Jiang, J.; Li, P. Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice. Toxins 2022, 14, 272. https://doi.org/10.3390/toxins14040272
Pang H, Li H, Zhang W, Mao J, Zhang L, Zhang Z, Zhang Q, Wang D, Jiang J, Li P. Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice. Toxins. 2022; 14(4):272. https://doi.org/10.3390/toxins14040272
Chicago/Turabian StylePang, Hua, Hui Li, Wen Zhang, Jin Mao, Liangxiao Zhang, Zhaowei Zhang, Qi Zhang, Du Wang, Jun Jiang, and Peiwu Li. 2022. "Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice" Toxins 14, no. 4: 272. https://doi.org/10.3390/toxins14040272
APA StylePang, H., Li, H., Zhang, W., Mao, J., Zhang, L., Zhang, Z., Zhang, Q., Wang, D., Jiang, J., & Li, P. (2022). Fullerenol Quantum Dots-Based Highly Sensitive Fluorescence Aptasensor for Patulin in Apple Juice. Toxins, 14(4), 272. https://doi.org/10.3390/toxins14040272








