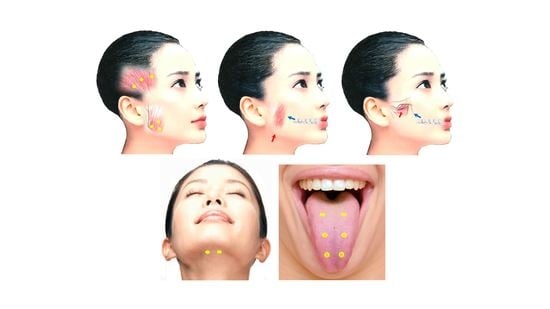
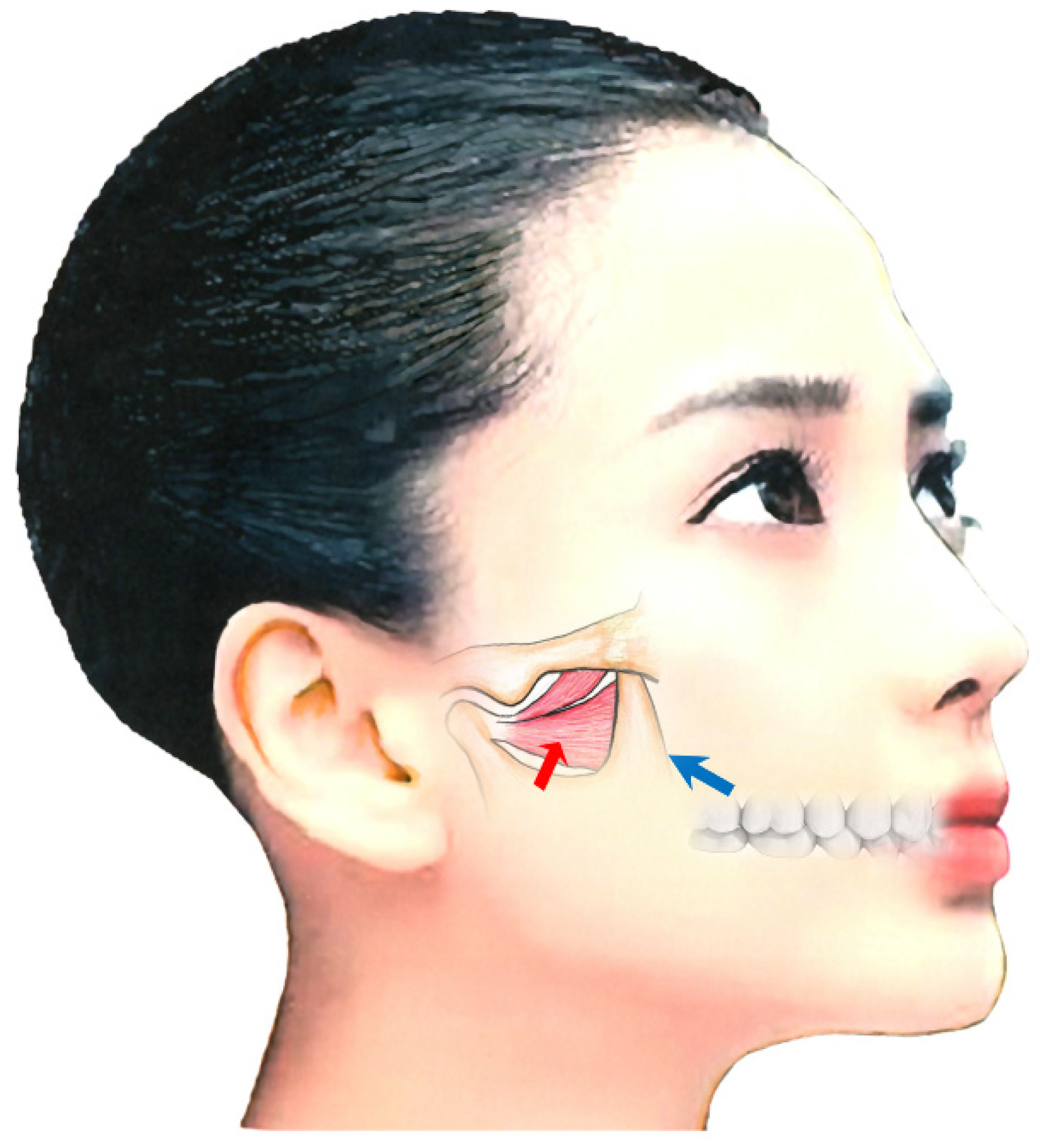
Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System
Abstract
:1. Introduction
2. The Clinical Problem: History, Presentation, and Epidemiology
2.1. OMD
2.1.1. Presentation
2.1.2. Epidemiology
2.2. Oral Dyskinesia
2.2.1. Presentation
2.2.2. Epidemiology
2.3. Bruxism
2.3.1. Presentation
2.3.2. Epidemiology
2.4. Functional Movement Disorder
2.4.1. Presentation
2.4.2. Epidemiology
2.5. Palatal Tremor
2.5.1. Presentation
2.5.2. Epidemiology
3. Treatment Challenges and Pitfalls
3.1. OMD
3.1.1. Jaw Closing Dystonia
Masseter Muscle
Temporalis Muscle
Medial Pterygoid Muscle
3.1.2. Jaw Opening, Deviation, and Protrusion Dystonia
Lateral Pterygoid Muscle
Digastric Muscle
Temporalis Muscle and Platysma
3.1.3. Lingual Dystonia
Genioglossus and Other Tongue Muscles
3.1.4. Lip Dystonia
3.1.5. Pitfalls
3.2. Oral Dyskinesia
Pitfalls
3.3. Bruxism
Pitfalls
3.4. Functional Movement Disorder
Pitfalls
3.5. Palatal Tremor
Pitfalls
4. Reported Trials—Evidence-Based Medicine
4.1. OMD
4.2. Oral Dyskinesia
4.3. Bruxism
4.4. Functional Movement Disorder
4.5. Palatal Tremor
5. Practical Guidelines for Treatment
5.1. OMD
5.2. Oral Dyskinesia
5.3. Bruxism
5.4. Functional Movement Disorder
5.5. Palatal Tremor
6. Proposals for Research and Future Studies
6.1. OMD
6.2. Oral Dyskinesia
6.3. Bruxism
6.4. Functional Movement Disorder
6.5. Palatal Tremor
7. Conclusions
8. Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT | botulinum neurotoxin |
| OMD | oromandibular dystonia |
| EMG | electromyography |
References
- Jankelson, B.; Hoffman, G.M.; Hendron, J.A., Jr. The physiology of the stomatognathic system. J. Am. Dent. Assoc. 1952, 46, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Krogh-Poulsen, W.G.; Olsson, A. Occlusal disharmonies and dysfunction of the stomatognathic system. Dent. Clin. N. Am. 1966, 10, 627–635. [Google Scholar]
- Yoshida, K. Development and validation of a disease-specific oromandibular dystonia rating scale (OMDRS). Front. Neurol. 2020, 11, 583177. [Google Scholar] [CrossRef]
- Yoshida, K. Behandlungsstrategien bei oromandibulärer Dystonie. Fortschr. Neurol. Psychiatr. 2021, 89, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J. Vergiftung durch verdorbene Wurste. Tübinger Blätter Nat. Arzneykunde 1817, 3, 1–25. [Google Scholar]
- Van Ermengem, E. Über eine neuen anaeroben Bacillus und seine Beziehungen zum Botulismus. Z. Hyg. Infektionskrankh. 1897, 26, 1–56. [Google Scholar]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Opthalmology 1980, 87, 1044–1049. [Google Scholar] [CrossRef]
- Jankovic, J.; Brin, M.F. Therapeutic uses of botulinum toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [Google Scholar]
- Truong, D.D.; Stenner, A.; Reichel, G. Current clinical applications of botulinum toxin. Curr. Pharm. Des. 2009, 15, 3671–3680. [Google Scholar] [CrossRef]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef]
- Persaud, R.; Garas, G.; Silva, S.; Stamatoglou, C.; Chatrath, P.; Patel, K. An evidence-based review of botulinum toxin (Botox) applications in non-cosmetic head and neck conditions. JRSM Short Rep. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. An update on new and unique uses of botulinum toxin in movement disorders. Toxicon 2018, 147, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Anandan, C.; Jankovic, J. Botulinum toxin in movement disorders: An update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Orman, J. Botulinum a toxin for cranial-cervical dystonia: A double-blind, placebo-controlled study. Neurology 1987, 37, 616–623. [Google Scholar] [CrossRef]
- Blitzer, A.; Brin, M.; Greene, P.E.; Fahn, S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann. Otol. Rhinol. Laryngol. 1989, 98, 93–97. [Google Scholar] [CrossRef]
- Jankovic, J.; Schwartz, K.; Donovan, D.T. Botulinum toxin treatment of cranial-cervical dystonia, spasmodic dysphonia, other focal dystonias and hemifacial spasm. J. Neurol. Neurosurg. Psychiatry 1990, 53, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Hermanowicz, N.; Truong, D.D. Treatment of oromandibular dystonia with botulinum toxin. Laryngoscope 1991, 101, 1216–1218. [Google Scholar] [CrossRef]
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia: Long-term follow-up. Neurology 1999, 53, 2102–2107. [Google Scholar] [CrossRef]
- Yoshida, K.; Iizuka, T. Botulinum toxin treatment for upper airway collapse resulting from temporomandibular joint dislocation due to jaw-opening dystonia. Cranio 2006, 24, 217–222. [Google Scholar] [CrossRef]
- Bakke, M.; Larsen, B.M.; Dalager, T.; Møller, E. Oromandibular dystonia–functional and clinical characteristics: A report on 21 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e21–e26. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Computer-aided design/computer-assisted manufacture-derived needle guide for injection of botulinum toxin into the lateral pterygoid muscle in patients with oromandibular dystonia. J. Oral Facial Pain Headache 2018, 32, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Scorr, L.M.; Silver, M.R.; Hanfelt, J.; Sperin, E.; Freeman, A.; Jinnah, H.A.; Factor, S.A. Pilot single-blind trial of AbobotulinumtoxinA in oromandibular dystonia. Neurotherapeutics 2018, 15, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skármeta, N.P.; Espinoza-Mellado, P.; Chana, P. Orofacial dystonia and other oromandibular movement disorders. In Dystonia; Rizk, T.M.G., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K. Botulinum neurotoxin therapy for lingual dystonia using an individualized injection method based on clinical features. Toxins 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, J.S. Botulinum toxin treatment of hemifacial spasm. J. Neurol. Neurosurg. Psychiatry 1986, 49, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, D.M.; Aminoff, M.J.; Tami, T.A.; Scott, A.B. Treatment of hemifacial spasm with botulinum toxin. Muscle Nerve 1992, 15, 1045–1049. [Google Scholar] [CrossRef]
- Toffola, E.D.; Furini, F.; Redaelli, C.; Prestifilippo, E.; Bejor, M. Evaluation and treatment of synkinesis with botulinum toxin following facial nerve palsy. Disabil. Rehabil. 2010, 32, 1414–1418. [Google Scholar] [CrossRef]
- Slotema, C.W.; van Harten, P.N.; Bruggeman, R.; Hoek, H.W. Botulinum toxin in the treatment of orofacial tardive dyskinesia: A single blind study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 507–509. [Google Scholar] [CrossRef]
- Yoshida, K. Clinical characteristics of functional movement disorders in the stomatognathic system. Front. Neurol. 2020, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Borodic, G.E.; Acquadro, M.A. The use of botulinum toxin for the treatment of chronic facial pain. J. Pain 2002, 3, 21–27. [Google Scholar] [CrossRef]
- Piovesan, E.J.; Teive, H.G.; Kowacs, P.A.; Della Coletta, M.V.; Werneck, L.C.; Silberstein, S.D. An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology 2005, 65, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, C.; Díaz, S.; Piedimonte, F.; Micheli, F. Beneficial effects of botulinum toxin type A in trigeminal neuralgia. Arq. Neuropsiquiatr. 2008, 66, 500–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.J.; Lian, Y.J.; Zheng, Y.K.; Zhang, H.F.; Chen, Y.; Xie, N.C.; Wang, L.J. Botulinum toxin type A for the treatment of trigeminal neuralgia: Results from a randomized, double-blind, placebo-controlled trial. Cephalalgia 2012, 32, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Shehata, H.S.; El-Tamawy, M.S.; Shalaby, N.M.; Ramzy, G. Botulinum toxin type A: Could it be an effective treatment option in intractable trigeminal neuralgia? J. Headache Pain 2013, 14, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lian, Y.; Ma, Y.; Chen, Y.; He, C.; Xie, N.; Wu, C. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: Observation of therapeutic effect from a randomized, double-blind, placebo-controlled trial. J. Headache Pain 2014, 15, 65. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K. Sphenopalatine ganglion block with botulinum neurotoxin for treating trigeminal neuralgia using CAD/CAM-derived injection guide. J. Oral Facial. Pain Headache 2020, 34, 135–140. [Google Scholar] [CrossRef]
- von Lindern, J.J. Type A botulinum toxin in the treatment of chronic facial pain associated with temporo-mandibular dysfunction. Acta Neurol. Belg. 2001, 101, 39–41. [Google Scholar]
- Nixdorf, D.R.; Heo, G.; Major, P.W. Randomized controlled trial of botulinum toxin for chronic myogenous orofacial pain. Pain 2002, 99, 465–473. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Manfredini, D.; Salamone, M.; Salmaso, S.; Tonello, S.; Ferronato, G. Efficacy of botulinum toxin in treating myofascial pain in bruxers: A controlled placebo pilot study. Cranio 2008, 26, 126–135. [Google Scholar] [CrossRef]
- Kurtoglu, C.; Gur, O.H.; Kurkcu, M.; Sertdemir, Y.; Guler-Uysal, F.; Uysal, H. Effect of botulinum toxin-A in myofascial pain patients with or without functional disc displacement. J. Oral Maxillofac. Surg. 2008, 66, 1644–1651. [Google Scholar] [CrossRef]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Canales, G.; Alvarez-Pinzon, N.; Muñoz-Lora, V.R.M.; Peroni, L.V.; Gomes, A.F.; Sánchez-Ayala, A.; Haiter-Neto, F.; Manfredini, D.; Rizzatti-Barbosa, C.M. Efficacy and safety of botulinum toxin type A on persistent myofascial pain: A randomized clinical trial. Toxins 2020, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Montes-Carmona, J.; Gonzalez-Perez, L.; Infante-Cossio, P. Treatment of localized and referred masticatory myofascial pain with botulinum toxin injection. Toxins 2021, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Effects of botulinum toxin type A on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins 2021, 13, 605. [Google Scholar] [CrossRef]
- Freund, B.; Schwartz, M. The use of botulinum toxin for the treatment of temporomandibular disorder. Oral Health 1998, 88, 32–37. [Google Scholar]
- Patel, A.A.; Lerner, M.Z.; Blitzer, A. IncobotulinumtoxinA injection for temporomandibular joint disorder. Ann. Otol. Rhinol. Laryngol. 2017, 126, 328–333. [Google Scholar] [CrossRef]
- Daelen, B.; Thorwirth, V.; Koch, A. Neurogene Kiefergelenkluxation Definition und Therapie mit Botulinumtoxin: Definition und Therapie mit Botulinumtoxin. Nervenarzt 1997, 68, 346–350. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Van Zandijcke, M.; Marchau, M.M.B. Treatment of bruxism with botulinum toxin injections. J. Neurol. Neurosurg. Psychiatry 1990, 53, 530. [Google Scholar] [CrossRef] [Green Version]
- Ivanhoe, C.B.; Lai, J.M.; Francisco, G.E. Bruxism after brain injury: Successful treatment with botulinum toxin-A. Arch. Phys. Med. Rehabil. 1997, 78, 1272–1273. [Google Scholar] [CrossRef]
- Lee, S.J.; McCall, W.D.; Kim, Y.K.; Chung, S.C.; Chung, J.W. Effect of botulinum toxin injection on nocturnal bruxism: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2010, 89, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Navarro, H.; Jiménez-Jiménez, F.J.; Plaza-Nieto, J.F.; Pilo-de-la-Fuente, B.; Navacerrada, F.; Arroyo-Solera, M.; Calleja, M. Tratamiento del bruxismo grave con toxina botulínica tipo A. Rev. Neurol. 2011, 53, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.J.; Lee, M.K.; Kato, T.; Park, H.U.; Heo, K.; Kim, S.T. Effects of botulinum toxin on jaw motor events during sleep in sleep bruxism patients: A polysomnographic evaluation. J. Clin. Sleep Med. 2014, 10, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhao, V.A.; Lokhande, N.; Habbu, S.G.; Sewane, S.; Dongare, S.; Goyal, N. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J. Dent. Res. 2017, 28, 493–497. [Google Scholar]
- Ondo, W.G.; Simmons, J.H.; Shahid, M.H.; Hashem, V.; Hunter, C.; Jankovic, J. Onabotulinum toxin-A injections for sleep bruxism. Neurology 2018, 90, e559–e564. [Google Scholar] [CrossRef]
- Shim, Y.J.; Lee, H.J.; Park, K.J.; Kim, H.T.; Hong, I.L.; Kim, S.T. Botulinum toxin therapy for managing sleep bruxism: A randomized and placebo-controlled trial. Toxins 2020, 12, 168. [Google Scholar] [CrossRef] [Green Version]
- Penney, S.E.; Bruce, I.A.; Saeed, S.R. Botulinum toxin is effective and safe for palatal tremor: A report of five cases and a review of the literature. J. Neurol. 2006, 253, 857–860. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Palatal myoclonus: Algorithm for management with botulinum toxin based on clinical disease characteristics. Laryngoscope 2014, 124, 1164–1169. [Google Scholar] [CrossRef]
- Mancini, F.; Zangaglia, R.; Cristina, S.; Maria Grazia Sommaruga, M.G.; Martignoni, E.; Nappi, G.; Pacchetti, C. Double-blind, placebo-controlled study to evaluate the efficacy and safety of botulinum toxin type a in the treatment of drooling in parkinsonism. Mov. Disord. 2003, 18, 685–688. [Google Scholar] [CrossRef]
- Ondo, W.G.; Hunter, C.; Moore, W. A double-blind placebo-controlled trial of botulinum toxin b for sialorrhea in Parkinson’s disease. Neurology 2004, 62, 37–40. [Google Scholar] [CrossRef]
- Boutsen, F.; Cannito, M.P.; Taylor, M.; Bender, B. Botox treatment in adductor spasmodic dysphonia: A meta-analysis. J. Speech Lang. Hear. Res. 2002, 45, 469–481. [Google Scholar] [CrossRef]
- Cannito, M.P.; Woodson, G.E.; Murry, T.; Bender, B. Perceptual analyses of spasmodic dysphonia before and after treatment. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 1393–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler, C.H.; Bansberg, S.F.; Hentz, J.G.; Ramig, L.O.; Buder, E.H.; Witt, K.; Edwards, B.W.; Krein-Jones, K.; Caviness, J.N. Botulinum toxin type A for treating voice tremor. Arch. Neurol. 2004, 61, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.J.; Orloff, L.A.; Lustig, L.R.; Eisele, D.W. Botulinum toxin in the treatment of first bite syndrome. Otolaryngol. Head. Neck. Surg. 2008, 139, 742–743. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, J.C.; Lee, Y.O.; Wang, S.G.; Kim, H.J. Novel treatment of first bite syndrome using botulinum toxin type A. Head Neck 2009, 31, 989–993. [Google Scholar] [CrossRef]
- Beerens, A.J.; Snow, G.B. Botulinum toxin a in the treatment of patients with Frey syndrome. Br. J. Surg. 2002, 89, 116–119. [Google Scholar] [CrossRef]
- Cantarella, G.; Berlusconi, A.; Mele, V.; Cogiamanian, F.; Barbieri, S. Treatment of Frey’s syndrome with botulinum toxin type B. Otolaryngol. Head Neck Surg. 2010, 143, 214–218. [Google Scholar] [CrossRef]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; Delong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus up date. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Oppenheim, H. Über eine eigenartige Krampfkrankheit des kindlichen und jugendlichen Alters (Dysbasia lordotica progressiva, Dystonia musculorum deformans). Neurol. Cent. 1911, 30, 1090–1107. [Google Scholar]
- Romberg, M.H. Krampf im Muskelgebiete der Pars minor Quinti. Masticatorischer Gesichtskrampf. Trismus. In Lehrbuch der Nervenkaranheiten des Menschen; Alexander Duncker: Berlin, Germany, 1846; pp. 308–316. [Google Scholar]
- Marsden, C.D. The problem of adult-onset idiopathic torsion dystonia and other isolated dyskinesias in adult life (including blepharospasm, oromandibular dystonia, dystonic writer’s cramp, and torticollis, or axial dystonia). Adv. Neurol. 1976, 14, 259–276. [Google Scholar]
- Yoshida, K.; Kaji, R.; Kubori, T.; Kohara, N.; Iizuka, T.; Kimura, J. Muscle afferent block for the treatment of oromandibular dystonia. Mov. Disord. 1998, 13, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Dalager, T.; Møller, E. What clinical strategies are applied for botulinum toxin injection in the oromandibular region? In Botulinum Toxon Therapy Manual for Dystonia and Spasticity; Rosales, R.L., Dressler, D., Eds.; IntechOpen: London, UK, 2016; pp. 79–95. [Google Scholar] [CrossRef] [Green Version]
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Slaim, L.; Cohen, M.; Klap, P.; Vidailhet, M.; Perrin, A.; Brasnu, D.; Ayache, D.; Mailly, M. Oromandibular dystonia: Demographics and clinical data from 240 Patients. J. Mov. Disord. 2018, 11, 78–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadgardoust, P.D.; Rosales, R.L.; Asuncion, R.M.; Dressler, D. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: A meta-analysis. J. Neural Transm. 2019, 126, 141–148. [Google Scholar] [CrossRef]
- Yoshida, K. Oromandibular dystonia screening questionnaire for differential diagnosis. Clin. Oral Investig. 2019, 23, 405–411. [Google Scholar] [CrossRef]
- Scorr, L.M.; Factor, S.A.; Parra, S.P.; Kaye, R.; Paniello, R.C.; Norris, S.A.; Perlmutter, J.S.; Bäumer, T.; Usnich, T.; Berman, B.D.; et al. Oromandibular dystonia: A clinical examination of 2,020 cases. Front. Neurol. 2021, 12, 700714. [Google Scholar] [CrossRef]
- Yoshida, K. Prevalence and incidence of oromandibular dystonia: An oral and maxillofacial surgery service-based study. Clin. Oral Investig. 2021, 25, 5755–5764. [Google Scholar] [CrossRef]
- Defazio, G.; Albanese, A.; Pellicciari, R.; Scaglione, C.L.; Esposito, M.; Morgante, F.; Abbruzzese, G.; Bentivoglio, A.R.; Bono, F.; Moja, M.C.; et al. Expert recommendations for diagnosing cervical, oromandibular, and limb dystonia. Neurol. Sci. 2019, 40, 89–95. [Google Scholar] [CrossRef]
- Yoshida, K. Involuntary Movements of the Stomatognathic Region. Available online: https://sites.google.com/site/oromandibulardystoniaenglish (accessed on 1 March 2022).
- Frucht, S.J. Embouchure dystonia—Portrait of a task-specific cranial dystonia. Mov. Disord. 2009, 24, 1752–1762. [Google Scholar] [CrossRef]
- Saraf, U.; Chandarana, M.; Divya, K.P.; Krishnan, S. Oromandibular dystonia—A systematic review. Ann. Indian Acad. Neurol. 2022, 25, 26–34. [Google Scholar] [CrossRef]
- Meige, H. Les convulsions de la face, une forme clinique de convulsion faciale bilatérale et médiane. Revue Neurol. 1910, 20, 437–443. [Google Scholar]
- Tolosa, E.S.; Klawans, K.H. Meige’s Disease: A clinical form of facial convulsion, bilateral and medial. Arch. Neurol. 1979, 36, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.D.; Day, L.; Dykeman, J.; Jette, N.; Pringsheim, T. The prevalence of primary dystonia: A systematic review and meta-analysis. Mov. Disord. 2012, 27, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Muenter, M.D.; Aronson, A.; Kurland, L.T.; Melton, L.J. 3rd. Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov. Disord. 1988, 3, 188–194. [Google Scholar] [CrossRef]
- Duffey, P.O.; Butler, A.G.; Hawthorne, M.R.; Barnes, M.P. The epidemiology of the primary dystonias in the north of England. Ad. Neurol. 1998, 78, 121–125. [Google Scholar]
- Le, K.-D.; Nilsen, B.; Dietrichs, E. Prevalence of primary focal and segmental dystonia in Oslo. Neurology 2003, 61, 1294–1296. [Google Scholar] [CrossRef]
- Castelon Konkiewitz, E.; Trender-Gerhard, I.; Kamm, C.; Warner, T.; Ben-Shlomo, Y.; Gasser, T.; Conrad, B.; Ceballos-Baumann, A.O. Service-based survey of dystonia in Munich. Neuroepidemiology 2002, 21, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Nishimura, M.; Shibasaki, H.; Kaji, R. Epidemiology of primary dystonias in Japan: Comparison with Western countries. Mov. Disord. 2003, 18, 1196–1198. [Google Scholar] [CrossRef]
- Pekmezovic, T.; Ivanovic, N.; Svetel, M.; Nalić, D.; Smiljković, T.; Raicević, R.; Kostić, V.S. Prevalence of primary late-onset focal dystonia in the Belgrade population. Mov. Disord. 2003, 18, 1389–1392. [Google Scholar] [CrossRef]
- Asgeirsson, H.; Jakobsson, F.; Hjaltason, H.; Jonsdottir, H.; Sveinbjornsdottir, S. Prevalence study of primary dystonia in Iceland. Mov. Disord. 2006, 21, 293–298. [Google Scholar] [CrossRef]
- Joensen, P. High prevalence of primary focal dystonia in the Faroe Islands. Acta Neurol. Scand. 2016, 133, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; McGovern, E.; Kimmich, O.; Molloy, A.; Beiser, I.; Butler, J.S.; Molloy, F.; Logan, P.; Healy, D.G.; Lynch, T.T. Epidemiological, clinical and genetic aspects of adult onset isolated focal dystonia in Ireland. Eur. J. Neurol. 2017, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Multilingual website and cyberconsultations for oromandibular dystonia. Neurol. Int. 2018, 10, 7536. [Google Scholar] [CrossRef] [PubMed]
- Waln, O.; Jankovic, J. An update on tardive dyskinesia: From phenomenology to treatment. Tremor Other Hyperkinet. Mov. 2013, 3, tre-03-131-3076-1. Available online: http://tremorjournal.org/article/view/161 (accessed on 1 March 2022). [CrossRef]
- Blanchet, P.J.; Rompré, P.H.; Lavigne, G.J.; Lamarche, C. Oral dyskinejia: A clinical overview. Int. J. Prosthodont. 2005, 18, 10–19. [Google Scholar]
- Ortí-Pareja, M.; Jiménez-Jiménez, F.J.; Vázquez, A.; Catalán, M.J.; Zurdo, M.; Burguera, J.A.; Martínez-Martín, P.; Molina, J.A.; Grupo Centro de Trastornos del Movimiento. Drug-induced tardive syndromes. Parkinsonism Relat. Disord. 1999, 5, 59–65. [Google Scholar] [CrossRef]
- Faurbye, A.; Rasch, P.J.; Petersen, P.B.; Brandborg, G.; Pakkenberg, H. Neurological symptoms in pharmacotherapy of psychosis. Acta Psychiatr. Scand. 1964, 40, 10–27. [Google Scholar] [CrossRef]
- Hutny, M.; Jagoda Hofman, J.; Klimkowicz-Mrowiec, A.; Gorzkowska, A. Current Knowledge on the Background, Pathophysiology and Treatment of Levodopa-Induced Dyskinesia-Literature Review. J. Clin. Med. 2021, 10, 4377. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision (DSM-V-TR); American Psychiatric Association: Washington, DC, USA, 2013; pp. 803–805. [Google Scholar]
- Woerner, M.G.; Alvir, J.M.J.; Saltz, B.L.; Lieberman, J.A.; Kane, J.M. Prospective study of tardive dyskinesia in the elderly: Rates and risk factors. Am. J. Psychiatry 1988, 155, 1521–1528. [Google Scholar] [CrossRef] [Green Version]
- Jeste, D.V. Tardive dyskinesia in older patients. J. Clin. Psychiatry 2000, 61 (Suppl. S4), 27–32. [Google Scholar]
- Turner, T. Rich and mad in Victorian England. Psychol. Med. 1989, 19, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.E.; Hansen, T.E. Spontaneous dyskinesias. In Neuropsychiatric Movement Disorders; Jeste, D.V., Wyatt, R.J., Eds.; American Psychiatric Press: Washington, DC, USA, 1984; pp. 68–95. [Google Scholar]
- Kane, J.M.; Smith, J.M. Tardive dyskinesia: Prevalence and risk factors, 1959 to 1979. Arch. Gen. Psychiatry 1982, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Torrey, E.F. Studies of individuals with schizophrenia never treated with antipsychotic medications: A review. Schizophr. Res. 2002, 58, 101–115. [Google Scholar] [CrossRef]
- Gerlach, J.; Casey, D.E. Tardive dyskinesia. Acta Psychiatr. Scand. 1988, 77, 369–378. [Google Scholar] [CrossRef]
- Koller, W.C. Edentulous orodyskinesia. Ann. Neurol. 1983, 13, 97–99. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Abdillahi, O.; Beauvais, C.; Rompre, P.H.; Lavigne, G.J. Prevalence of spontaneous oral dyskinesia in the elderly: A reapraisal. Mov. Disord. 2004, 19, 892–896. [Google Scholar] [CrossRef]
- McCreadie, R.G.; Padmavati, R.; Thara, R.; Srivasan, T.N. Spontaneous dyskinesia and parkinsonism in never-medicated, chronically ill patients with schizophrenia: 18-month follow-up. Br. J. Psychiatry 2002, 181, 135–137. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, R.; Benassi, G.; Cristina, E.; Gallassi, R.; Manzaroli, D. The prevalence of lingual-facial-buccal dyskinesias in the elderly. Neurology 1986, 36, 1350–1351. [Google Scholar] [CrossRef]
- Blowers, A.J.; Borison, A.L.; Blowers, C.M.; Bicknell, D.J. Abnormal involuntary movements in the elderly. Br. J. Psychiatry 1981, 139, 363–364. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.C.; Firestone, J.M. Psychosomatic factors in the aetiology of periodontal diseases. Am. J. Orthodont. Oral Surg. 1947, 33, 675–680. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; de Laat, A.; de Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Melo, G.; Duarte, J.; Pauletto, P.; Porporatti, A.L.; Stunginski-Barbosa, J.; Winocur, E.; Flores-Mir, C.; De Luca Canto, G. Bruxism: An umbrella review of systematic reviews. J. Oral Rehabil. 2019, 46, 666–690. [Google Scholar] [CrossRef] [PubMed]
- Polmann, H.; Réus, J.C.; Massignan, C.; Serra-Negra, J.M.; Dick, B.D.; Flores-Mir, C.; Lavigne, G.L.; De Luca Canto, G. Association between sleep bruxism and stress symptoms in adults: A systematic review and meta-analysis. J. Oral Rehabil. 2021, 48, 621–631. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual; American Academy of Sleep Medicine: Chicago, IL, USA, 2001. [Google Scholar]
- Thymi, M.; Lobbezoo, F.; Aarab, G.; Ahlberg, J.; Baba, K.; Carra, M.C.; Gallo, L.M.; De Laat, A.; Manfredini, D.; Lavigne, G.; et al. Signal acquisition and analysis of ambulatory electromyographic recordings for the assessment of sleep bruxism: A scoping review. J. Oral Rehabil. 2021, 48, 846–871. [Google Scholar] [CrossRef]
- Wetselaar, P.; Vermaire, E.J.H.; Lobbezoo, F.; Schuller, A.A. The prevalence of awake bruxism and sleep bruxism in the Dutch adult population. J. Oral Rehabil. 2019, 46, 617–623. [Google Scholar] [CrossRef]
- Khoury, S.; Carra, M.C.; Huynh, N.; Montplaisir, J.; Lavigne, G.J. Sleep bruxism-tooth grinding prevalence, characteristics and familial aggregation: A large cross-sectional survey and polysomnographic validation. Sleep 2016, 39, 2049–2056. [Google Scholar] [CrossRef] [Green Version]
- Carson, A.; Lehn, A. Epidemiology. In Functional Neurologic Disorders; Hallett, M., Stone, J., Carson, A., Eds.; Handbook of Clinical Neurology, 3rd Series; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 47–60. [Google Scholar]
- Edwards, M.J.; Stone, J.; Lang, A.E. From psychogenic movement disorder to functional movement disorder: It’s time to change the name. Mov. Disord. 2014, 29, 849–852. [Google Scholar] [CrossRef]
- Espay, A.J.; Aybek, S.; Carson, A.; Edwards, M.J.; Goldstein, L.H.; Hallett, M.; LaFaver, K.; LaFrance Jr, W.C.; Lang, A.E.; Nicholson, T.; et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. 2018, 75, 1132–1141. [Google Scholar] [CrossRef]
- Charcot, J.M. Leçons sur les Maladies du Système Nerveux Faites à la Salpêtrière; A. Delahaye: Paris, France, 1887. [Google Scholar]
- Fasano, A.; Valadas, A.; Bhatia, K.P.; Prashanth, L.K.; Lang, A.E.; Munhoz, R.P.; Morgante, F.; Tarsy, D.; Duker, A.P.; Girlanda, P.; et al. Psychogenic facial movement disorders: Clinical features and associated conditions. Mov. Disord. 2012, 27, 1544–1551. [Google Scholar] [CrossRef] [Green Version]
- Morgante, F.; Edwards, M.J.; Espay, A.J. Psychogenic movement disorders. Continuum 2013, 19, 1983–1996. [Google Scholar] [CrossRef] [Green Version]
- Espay, A.J.; Lang, A.E. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Curr. Neurol. Neurosci. Rep. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Salas, C.; Lang, A.E. Neurogenic diagnostic criteria for functional neurogenic disorders. In Functional Neurologic Disorders; Hallett, M., Stone, J., Carson, A., Eds.; Handbook of Clinical Neurology, 3rd Series; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 193–212. [Google Scholar]
- Kaski, D.; Bronstein, A.M.; Edwards, M.J.; Stone, J. Cranial functional (psychogenic) movement disorders. Lancet Neurol. 2015, 14, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Fasano, F.; Tinazzi, M. Functional facial and tongue movement disorders. In Functional Neurologic Disorders; Hallett, M., Stone, J., Carson, A., Eds.; Handbook of Clinical Neurology, 3rd Series; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 353–365. [Google Scholar]
- Stone, J.; Hoeritzauer, I.; Tesolin, L.; Carson, A. Functional movement disorders of the face: A historical review and case series. J. Neurolog. Sci. 2018, 395, 35–40. [Google Scholar] [CrossRef]
- Miyasaki, J.M.; Sa, D.S.; Galvez-Jimenez, N.; Lang, A.E. Psychogenic movement disorders. Can. J. Neurol. Sci. 2003, 30 (Suppl. S1), S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Boeck, O. Rhinoskopischer Befund bei einem knackenden Geräusch im Ohr. Arch. Ohrenheilkd. 1867, 2, 203–206. [Google Scholar] [CrossRef]
- Deuschl, G.; Mische, G.; Schenk, E.; Schulte-Mönting, J.; Lücking, C.H. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990, 113, 1645–1672. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Toro, C.; Valls-Solé, J.; Zeffiro, T.; Zee, D.S.; Hallett, M. Symptomatic and essential palatal tremor. Clinical, physiological and MRI analysis. Brain 1994, 117, 775–788. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Riley, D.E.; Gunzler, S.A. Teaching video neuroimages: Essential palatal tremor: Is it a peripherally triggered central movement disorder? Neurology 2012, 79, e142. [Google Scholar] [CrossRef]
- Zadikoff, C.; Lang, A.E.; Klein, C. The ‘essentials’ of essential palatal tremor: A reappraisal of the nosology. Brain 2006, 129, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, S. Palatal tremor—Pathophysiology, clinical features, investigations, management and future challenges. Tremor Other Hyperkinet. Mov. 2020, 10, 40. [Google Scholar] [CrossRef]
- Ross, S.; Jankovic, J. Palatal myoclonus: An unusual presentation. Mov. Disord. 2005, 20, 1200–1203. [Google Scholar] [CrossRef]
- Stamelou, M.; Saifee, T.A.; Edwards, M.J.; Bhatia, K.P. Psychogenic palatal tremor may be underrecognized: Reappraisal of a large series of cases. Mov. Disord. 2012, 27, 1164–1168. [Google Scholar] [CrossRef]
- Van den Bergh, P.; Francart, J.; Mourin, S.; Kollmann, P.; Laterre, E.C. Five-year experience in the treatment of focal movement disorders with low-dose Dysport botulinum toxin. Muscle Nerve 1995, 18, 720–729. [Google Scholar] [CrossRef]
- Bhattacharyya, N.; Tarsy, D. Impact on quality of life of botulinum toxin treatments for spasmodic dysphonia and oromandibular dystonia. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 389–392. [Google Scholar] [CrossRef] [Green Version]
- Singer, C.; Papapetropoulos, S. A comparison of jaw-closing and jaw-opening idiopathic oromandibular dystonia. Parkinsonism Relat. Disord. 2006, 12, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.L.; Ng, A.R.; Santos, M.M.D.-D.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (Part II): An open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121 (Suppl. S1), 44–56. [Google Scholar] [CrossRef] [PubMed]
- Teive, H.A.; Kluppel, L.E.; Munhoz, R.P.; Becker, N.; Muller, P.R.; Werneck, L.C. Jaw-opening oromandibular dystonia secondary to Wilson’s disease treated with botulinum toxin type A. Arq. Neuropsiquiatr. 2012, 70, 407–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jost, W. Pictorial Atlas of Botulinum Toxin Injection: Dosage, Localization, Application, 2nd ed.; Quintessence: Berlin, Germany, 2013. [Google Scholar]
- Moscovich, M.; Chen, Z.P.; Rodriguez, R. Successful treatment of open jaw and jaw deviation dystonia with botulinum toxin using a simple intraoral approach. J. Clin. Neurosci. 2015, 22, 594–596. [Google Scholar] [CrossRef]
- Teemul, T.A.; Patel, R.; Kanatas, A.; Carter, L.M. Management of oromandibular dystonia with botulinum A toxin: A series of cases. Br. J. Oral Maxillofac. Surg. 2016, 54, 1080–1084. [Google Scholar] [CrossRef]
- Page, A.D.; Siegel, L.; Jog, M. Self-Rated communication-related quality of life of individuals with oromandibular dystonia receiving botulinum toxin injections. Am. J. Speech Lang. Pathol. 2017, 26, 674–681. [Google Scholar] [CrossRef]
- Bakke, M.; Henriksen, T.; Biernat, H.B.; Dalager, T.; Møller, E. Interdisciplinary recognizing and managing of drug-induced tardive oromandibular dystonia: Two case reports. Clin. Case Rep. 2018, 6, 2150–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakke, M.; Baram, S.; Dalager, T.; Biernat, H.B.; Møller, E. Oromandibular dystonia, mental distress and oro-facial dysfunction— A follow-up 8–10 years after start of treatment with botulinum toxin. J. Oral Rehabil. 2019, 46, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Altavista, M.C.; Altenmueller, E.; Bhidayasiri, R.; Bohlega, S.; Chana, P.; Chung, T.M.; Colosimo, C.; Fheodoroff, K.; Garcia-Ruiz, P.J.; et al. Consensus guidelines for botulinum toxin therapy: General algorithms and dosing tables for dystonia and spasticity. J. Neural. Transm. 2021, 128, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Shibasaki, H.; Iizuka, T. Factors influencing the therapeutic effect of muscle afferent block for oromandibular dystonia and dyskinesia: Implications for their distinct pathophysiology. Int. J. Oral Maxillofac. Surg. 2002, 31, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kaji, R.; Takagi, A.; Iizuka, T. Customized EMG needle insertion guide for the muscle afferent block of jaw-deviation and jaw-opening dystonias. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 664–669. [Google Scholar] [CrossRef]
- Yoshida, K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J. Prosthodont. Res. 2018, 62, 239–244. [Google Scholar] [CrossRef]
- De Meyer, M.; Vereecke, L.; Bottenberg, P.; Jacquet, W.; Sims, A.B.; Santens, P. Oral appliances in the treatment of oromandibular dystonia: A systematic review. Acta Neurol. Belg. 2020, 120, 831–836. [Google Scholar] [CrossRef]
- Yoshida, K. Coronoidotomy as treatment for trismus due to jaw-closing oromandibular dystonia. Mov. Disord. 2006, 21, 1028–1031. [Google Scholar] [CrossRef]
- Yoshida, K. Surgical intervention for oromandibular dystonia-related limited mouth opening: Long-term follow-up. J. Cranio-Maxillofac. Surg. 2017, 45, 56–62. [Google Scholar] [CrossRef]
- Yoshida, K. Mouth opening retaining appliance after coronoidotomy for the treatment of trismus: Effects on pain during postoperative training and maximal extent of mouth opening. Clin. Surg. 2020, 5, 2737. [Google Scholar] [CrossRef]
- Spiegel, L.L.; Ostrem, J.L.; Bledsoe, I.O. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Schwartz, K. Response and immunoresistance to botulinum toxin injections. Neurology 1995, 45, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Brin, M.F.; Comella, C.L.; Jankovic, J.; Lai, F.; Naumann, M.; Ahmed, F.; Brashear, A.; Chehrenama, M.; Erjanti, H.; Evatt, M.; et al. Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov. Disord. 2008, 23, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Boyle, M.H.; McGwin Jr, G.; Flanagan, C.E.; Vicinanzo, M.G.; Long, J.A. High versus low concentration botulinum toxin A for benign essential blepharospasm: Does dilution make a difference? Ophthalmic Plast. Reconstr. Surg. 2009, 25, 81–84. [Google Scholar] [CrossRef]
- Jost, W.H. How Do I treat cervical dystonia with botulinum toxin by using ultrasound? Mov. Disord. Clin. Pract. 2017, 4, 647. [Google Scholar] [CrossRef]
- Fietzek, U.M.; Nene, D.; Schramm, A.; Appel-Cresswell, S.; Košutzká, Z.; Walter, U.; Wissel, J.; Berweck, S.; Chouinard, S.; Bäumer, T. The role of ultrasound for the personalized botulinum toxin treatment of cervical dystonia. Toxins 2021, 13, 365. [Google Scholar] [CrossRef]
- Yoshida, K. Is botulinum toxin therapy effective for bruxism? Anti-Aging Med. 2017, 13, 394–398. [Google Scholar]
- Yoshida, K. Clinical application of botulinum neurotoxin for diseases in the stomatognathic system. J. Jpn. Dent. Soc. Anesthesiol. 2020, 48, 33–40. [Google Scholar]
- Yoshida, K. How do I inject botulinum toxin into the lateral and medial pterygoid muscles? Mov. Disord. Clin. Pract. 2016, 4, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K. An electromyographic study on the superior head of the lateral pterygoid muscle during mastication from the standpoint of condylar movement. J. Jpn. Prosthodont. Soc. 1992, 36, 110–120. [Google Scholar]
- Yoshida, K. Masticatory muscle responses associated with unloading of biting force during food crushing. J. Oral Rehabil. 1998, 25, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Clinical and phenomelogical characteristics of patients with task-specific lingual dystonia: Possible association with occupation. Front. Neurol. 2017, 8, 649. [Google Scholar] [CrossRef] [Green Version]
- Berkovitz, B.K.B. Tongue. In Gray’s Anatomy, 41st ed.; Standring, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 511–517. [Google Scholar]
- Blitzer, A.; Brin, M.F.; Fahn, S. Botulinum toxin injections for lingual dystonia. Laryngoscope 1991, 101, 799. [Google Scholar] [CrossRef]
- Schneider, S.A.; Aggarwal, A.; Bhatt, M.; Dupont, E.; Tisch, S.; Limousin, P.; Lee, P.; Quinn, N.; Bhatia, K.P. Severe tongue protrusion dystonia Clinical syndromes and possible treatment. Neurology 2006, 67, 940–943. [Google Scholar] [CrossRef]
- Esper, C.D.; Freeman, A.; Factor, S.A. Lingual protrusion dystonia: Frequency, etiology and botulinum toxin therapy. Park. Relat. Disord. 2010, 16, 438–441. [Google Scholar] [CrossRef]
- Nastasi, L.; Mostile, G.; Nicoletti, A.; Zappia, M.; Reggio, E.; Catania, S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J. Neurol. 2016, 263, 1702–1708. [Google Scholar] [CrossRef]
- Charles, P.D.; Davis, T.L.; Shannon, K.M.; Hook, M.A.; Warner, J.S. Tongue protrusion dystonia: Treatment with botulinum toxin. South Med. J. 1997, 90, 522–525. [Google Scholar] [CrossRef]
- Ondo, W. Lingual dystonia following electrical injury. Mov. Disord. 1997, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Hennings, J.M.H.; Krause, E.; Bötzel, K.; Wetter, T.C. Successful treatment of tardive lingual dystonia with botulinum toxin: Case report and review of the literature. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Kasravi, N.; Jog, M.S. Botulinum toxin in the treatment of lingual movement disorders. Mov. Disord. 2009, 24, 2199–2202. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, C.; Lai, E.C.; Jankovic, J. Peripherally induced oromandibular dystonia. J. Neurol. Neurosurg. Psychiatry 1998, 65, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphael, K.G.; Tadinada, A.; Bradshaw, J.M.; Janal, M.N.; Sirois, D.A.; Chan, K.C.; Lurie, A.G. Osteopenic consequences of botulinum toxin injections in the masticatory muscles: A pilot study. J. Oral Rehabil. 2014, 41, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, S.J.; Lee, K.J.; Yu, H.S.; Baik, H.S. Repeated injections of botulinum toxin into the masseter muscle induce bony changes in human adults: A longitudinal study. Korean J. Orthod. 2017, 47, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.S.; Bergeron, L.; Yu, C.C.; Chen, P.K.; Chen, Y.R. Mandible changes evaluated by computed tomography following Botulinum Toxin A injections in square-faced patients. Aesthetic Plast. Surg. 2011, 35, 452–455. [Google Scholar] [CrossRef]
- Merz, R.I.; Deakin, J.; Hawthorne, M.R. Oromandibular dystonia questionnaire (OMDQ-25): A valid and reliable instrument for measuring health-related quality of life. Clin. Otolaryngol. 2010, 35, 390–396. [Google Scholar] [CrossRef]
- Ricciardi, L.; Pringsheim, T.; Barnes, T.R.E.; Martino, D.; Gardner, D.; Remington, G.; Addington, D.; Morgante, F.; Poole, N.; Carson, A.; et al. Treatment Recommendations for Tardive Dyskinesia. Can. J. Psychiatry 2019, 64, 388–399. [Google Scholar] [CrossRef]
- Sutcher, H.D.; Underwood, R.B.; Beaty, R.A.; Sugar, O. Orofacial dyskinesia—A dental dimension. J. Am. Med. Assoc. 1971, 216, 1459–1463. [Google Scholar] [CrossRef]
- Lauciello, F.; Appelbaum, M. Pristhodontic implications of tardive dyskinesia. N. Y. State Dent. J. 1977, 43, 214–217. [Google Scholar] [PubMed]
- Weijs, W.A.; Hillen, B. Cross-sectional areas and estimated intrinsic strength of the human jaw muscles. Acta Morphol. Neerl. Scand. 1985, 23, 267–274. [Google Scholar] [PubMed]
- Ahn, K.Y.; Kim, S.T. The change of maximum bite force after botulinum toxin type A injection for treating masseteric hypertrophy. Plast. Reconstr. Surg. 2007, 120, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Sendra, L.A.; Montez, C.; Vianna, K.C.; Barboza, E.P. Clinical outcomes of botulinum toxin type a injections in the management of primary bruxism in adults: A systematic review. J. Prosthet. Dent. 2020, 126, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.P.; Peng, J.H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J. Cosmet. Dermatol. 2018, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Schmerler, D.A.; Espay, A.J. Functional dystonia. In Functional Neurologic Disorders; Hallett, M., Stone, J., Carson, A., Eds.; Handbook of Clinical Neurology, 3rd Series; Elsevier: Amsterdam, The Netherlands, 2016; Volume 139, pp. 235–245. [Google Scholar]
- LaFaver, K.; Lang, A.E.; Stone, J.; Morgante, F.; Edwards, M.; Lidstone, S.; Maurer, C.W.; Hallett, M.; Dwivedi, A.K.; Espay, A.J. Opinions and clinical practices related to diagnosing and managing functional (psychogenic) movement disorders: Changes in the last decade. Eur. J. Neurol. 2020, 27, 975–984. [Google Scholar] [CrossRef]
- Hallett, M. Functional movement disorders: Is the crisis resolved? Mov. Disord. 2019, 34, 971–974. [Google Scholar] [CrossRef]
- Tolchin, B.; Baslet, G.; Carson, A.; Dworetzky, B.A.; Goldstein, L.H.; LaFrance, W.C., Jr.; Martino, S.; Perez, D.L.; Reuber, M.; Stone, J.; et al. The role of evidence-based guidelines in the diagnosis and treatment of functional neurological disorder. Epilepsy Behav. Rep. 2021, 16, 100494. [Google Scholar] [CrossRef]
- Saeed, S.R.; Brookes, G.B. The use of Clostridium botulinum toxin in palatal myoclonus. A preliminary report. J. Laryngol. Otol. 1993, 107, 208–210. [Google Scholar] [CrossRef] [Green Version]
- Krause, E.; Leunig, A.; Klopstock, T.; Gürkov, R. Treatment of essential palatal myoclonus in a 10-year-old girl with botulinum neurotoxin. Otol. Neurotol. 2006, 27, 672–675. [Google Scholar]
- Bryce, G.E.; Morrison, M.D. Botulinum toxin treatment of essential palatal myoclonus tinnitus. J. Otolaryngol. 1998, 27, 213–216. [Google Scholar] [PubMed]
- Tan, E.K.; Jankovic, J. Treating severe bruxism with botulinum toxin. J. Am. Dent. Assoc. 2000, 131, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ågren, M.; Sahin, C.; Pettersson, M. The effect of botulinum toxin injections on bruxism: A systematic review. J. Oral Rehabil. 2020, 47, 395–402. [Google Scholar] [PubMed]
- Al-Wayli, H. Treatment of chronic pain associated with nocturnal bruxism with botulinum toxin. A prospective and randomized clinical study. J. Clin. Exp. Dent. 2017, 9, e112–e117. [Google Scholar] [CrossRef] [PubMed]
- Bolayir, G.; Bolayir, E.; Coskun, A.; Özdemir, A.K. Botulinum toxin type-A practice in bruxism cases. Neurol. Psychiatry Brain Res. 2005, 12, 43–45. [Google Scholar]
- Redaelli, A. Botulinum toxin A in bruxers: One year experience. Saudi Med. J. 2011, 32, 156–158. [Google Scholar]
- Finiels, P.J.; Batifol, D. The use of botulinum toxin in the treatment of the consequences of bruxism on cervical spine musculature. Toxicon 2014, 80, 58–63. [Google Scholar] [CrossRef]
- Asutay, F.; Atalay, Y.; Asutay, H.; Acar, A.H. The evaluation of the clinical effects of botulinum toxin on nocturnal bruxism. Pain Res. Manag. 2017, 2017, 6264146. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, L.H.; Chalder, T.; Chigwedere, C.; Khondoker, M.R.; Moriarty, J.; Toone, B.K.; Mellers, J.D.C. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: A pilot RCT. Neurology 2010, 74, 1986–1994. [Google Scholar] [CrossRef]
- Sharpe, M.; Walker, J.; Williams, C.; Stone, J.; Cavanagh, J.; Murray, G.; Butcher, I.; Duncan, R.; Smith, S.; Carson, A. Guided self-help for functional (psychogenic) symptoms: A randomized controlled efficacy trial. Neurology 2011, 77, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Jordbru, A.; Smedstad, L.; Klungsøyr, O.; Martinsen, E. Psychogenic gait disorder: A randomized controlled trial of physical rehabilitation with one-year follow-up. J. Rehabil. Med. 2014, 46, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFrance, W.C.; Baird, G.L.; Barry, J.J.; Blum, A.S.; Webb, A.F.; Keitner, G.I.; Machan, J.T.; Miller, I.; Szaflarski, J.P.; NES Treatment Trial (NEST-T) Consortium. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: A randomized clinical trial. JAMA Psychiatry 2014, 71, 997. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.; Buszewicz, M.; Stevenson, F.; Hunter, R.; Holt, K.; Dudziec, M.; Ricciardi, L.; Marsden, J.; Joyce, E.; Edwards, M.J. Randomised feasibility study of physiotherapy for patients with functional motor symptoms. J. Neurol. Neurosurg. Psychiatry 2017, 88, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Le Pajolec, C.; Marion, M.H.; Bobin, S. Acouphène objective et myoclonies vélaires. Nouvelle approche thérapeutique. Ann. Otolaryngol. Chir. Cervico-Fac. 1990, 107, 363–365. [Google Scholar]
- Varney, S.M.; Demetroulakos, J.L.; Fletcher, M.H.; McQueen, W.J.; Hamilton, M.K. Palatal myoclonus: Treatment with Clostridium botulinum toxin injection. Otolaryngol. Head Neck Surg. 1996, 114, 317–320. [Google Scholar] [CrossRef]
- Jamieson, D.R.; Mann, C.; O’Reilly, B.; Thomas, A.M. Ear clicks in palatal tremor caused by activity of the levator veli palatini. Neurology 1996, 46, 1168–1169. [Google Scholar] [CrossRef]
- Jero, J.; Salmi, T. Palatal myoclonus and clicking tinnitus in a 12-year-old girl-case report. Acta Otolaryngol. Suppl. 2000, 543, 61–62. [Google Scholar] [CrossRef]
- Cho, J.W.; Chu, K.; Jeon, B.S. Case of essential palatal tremor: Atypical features and remarkable benefit from botulinum toxin injection. Mov. Disord. 2001, 16, 779–782. [Google Scholar] [CrossRef]
- Kutukcu, Y.; Imirzalioglu, N.; Odabasi, Z.; Gokcil, Z.; Vural, O. Essential palatal myoclonus in monozygotic male twins. J. Neurol. 2003, 250, 885–886. [Google Scholar] [CrossRef]
- Simpson, D.M.; Blitzer, A.; Brashear, A.; Comella, C.; Dubinsky, R.; Hallett, M.; Jankovic, J.; Karp, B.; Ludlow, C.L.; Miyasaki, J.M.; et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 1699–1706. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Asmus, F.; Bhatia, K.P.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 2011, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- France, K.; Stoopler, E.T. The American Academy of Oral Medicine Clinical Practice Statement: Oromandibular dystonia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 283–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K.; Kaji, R.; Hamano, T.; Kohara, N.; Kimura, J.; Iizuka, T. Cortical distribution of Bereitschaftspotential and negative slope potential preceding mouth opening movements in humans. Arch. Oral Biol. 1999, 44, 183–190. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Hamano, T.; Kohara, N.; Kimura, J.; Shibasaki, H.; Iizuka, T. Cortical potentials associated wi1h volun1ary mandibular movements. J. Dent. Res. 2000, 79, 1514–1518. [Google Scholar] [CrossRef]
- Fève, A.; Bathien, N.; Rondot, P. Abnormal movement related potentials in patients wi1h lesions of basal ganglia and anterior thalamus. J. Ncurol. Neurosurg. Psychiatry 1994, 57, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Deuschl, G.; Toro, C.; Matsumoto, J.; Hallett, M. Movement-related cortical potentials in writer’s cramp. Ann. Neurol. 1995, 38, 862–868. [Google Scholar] [CrossRef]
- Van der Kamp, W.; Rothwell, J.C.; Thompson, P.D.; Day, B.L.; Marsden, C.D. The movement-related cortical potential is abnormal in patients with idiopathic torsion dystonia. Mov. Disord. 1995, 10, 630–633. [Google Scholar] [CrossRef]
- Yoshida, K.; Kaji, R.; Kohara, N.; Murase, N.; Ikeda, A.; Shibasaki, H.; Iizuka, T. Movement-related cortical potentials jaw excursions in patients with oromandibular dystonia. Mov. Disord. 2003, 18, 94–100. [Google Scholar] [CrossRef]


| Main Muscles (Doses (Units)) | Additional Muscles (Doses (Units)) | |
|---|---|---|
| Bilateral masseter (10–50) | Bilateral medial pterygoid (10–30) | Insufficient cases only for masseter and temporalis, or cases in which the effect was diminished by repeated injections |
| Bilateral temporalis (10–50) | Contralateral or bilateral lateral pterygoid (10–30) | With mandibular deviation, grinding, and myalgia of the lateral pterygoid muscle |
| Subtypes | Main Muscles (Doses (Units)) | Additional Muscles (Doses (Units)) | |
|---|---|---|---|
| Jaw opening dystonia | Bilateral lateral pterygoid (10–50) | Anterior digastric (5–10) | Insufficient cases only for lateral pterygoid |
| Platysma (10–20) | With anterior neck tension | ||
| Genioglossus (10–20) | With tongue protrusion | ||
| Jaw deviation dystonia | Contralateral lateral pterygoid (10–50) | Ipsilateral posterior temporalis (10–20) | Insufficient cases only for lateral pterygoid |
| Platysma (10–20) | With anterior neck tension | ||
| Jaw protrusion dystonia | Bilateral lateral pterygoid (10–40) | Anterior digastric (5–10) | Insufficient cases only for lateral pterygoid |
| Platysma (10–20) | With anterior neck tension | ||
| Subtypes | Doses (Units) | Main Muscles (Doses (Units)) | Additional Muscles | |
|---|---|---|---|---|
| Protrusion type | 15–60 | Bilateral genioglossus (50–100% of total dose) | Ipsilateral superior and inferior longitudinal (0–50%) | With laterotrusion |
| Bilateral superior longitudinal (0–50%) | With curling | |||
| Bilateral vertical (0–50%) | With flattening | |||
| Bilateral transverse (0–50%) | With elongation | |||
| Bilateral lateral pterygoid (0–50%) | With jaw opening | |||
| Retraction type | 15–50 | Bilateral genioglossus (30–70% of total dose) | Intrinsic and geniohyoid (30–70%) | Insufficient cases only for genioglossus |
| Laterotrusion type | 10–40 | Ipsilateral superior and inferior longitudinal (70–100% of total dose) | Contralateral genioglossus (0–30%) | Insufficient cases only for superior and inferior longitudinal |
| Curling type | 10–40 | Bilateral superior longitudinal (80–100% of total dose) | Bilateral genioglossus (0–20%) | With protrusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins 2022, 14, 282. https://doi.org/10.3390/toxins14040282
Yoshida K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins. 2022; 14(4):282. https://doi.org/10.3390/toxins14040282
Chicago/Turabian StyleYoshida, Kazuya. 2022. "Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System" Toxins 14, no. 4: 282. https://doi.org/10.3390/toxins14040282
APA StyleYoshida, K. (2022). Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins, 14(4), 282. https://doi.org/10.3390/toxins14040282