The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal
Abstract
:1. Introduction
2. Results and Discussions
2.1. The Effect of Melanin on the Growth, Cocoon, and Silk Morphology of Silkworms
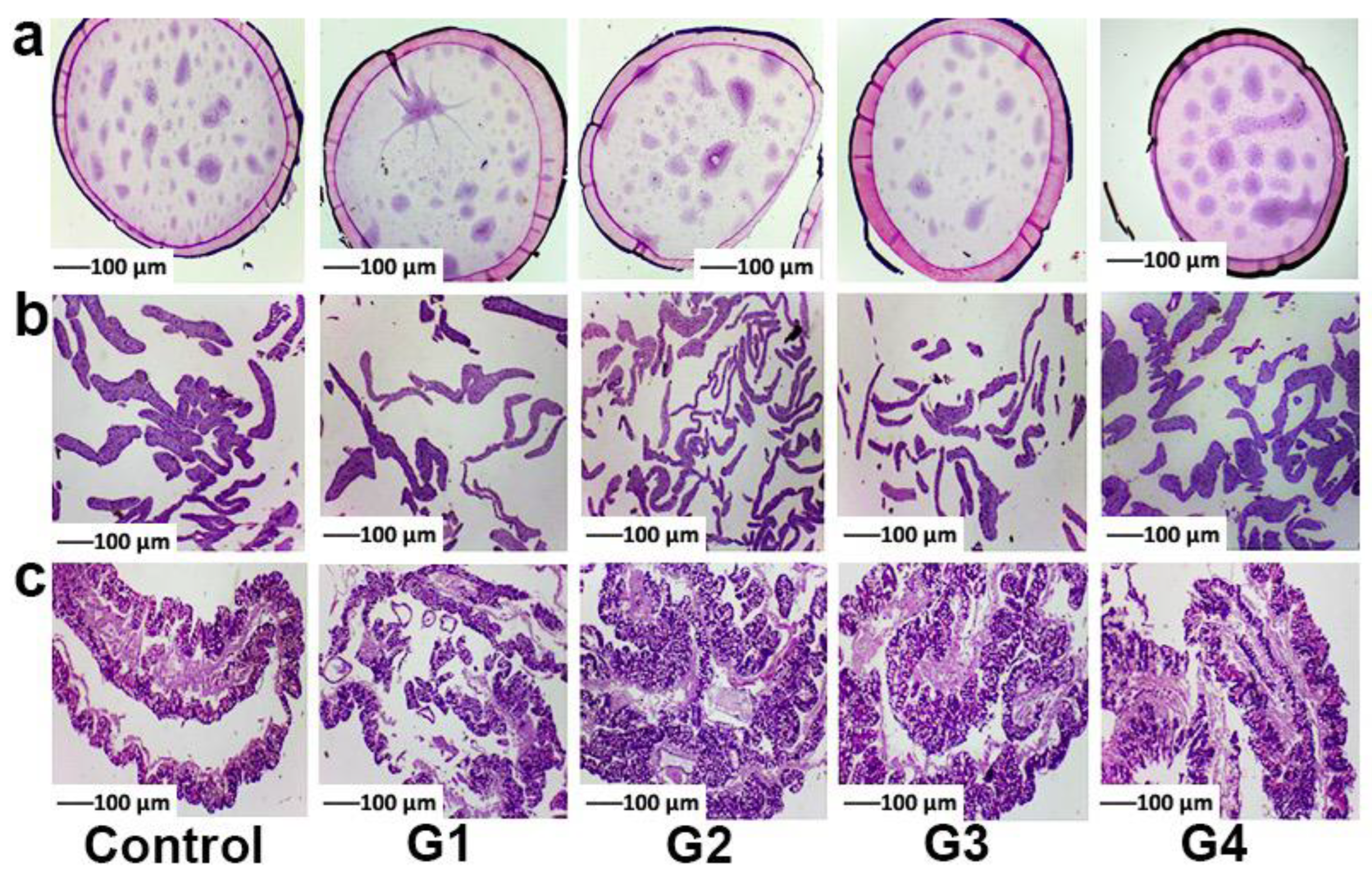
2.2. Histophysiological Study
2.3. The Effect of Melanin on the Structures of Silk
2.3.1. XRD Analysis
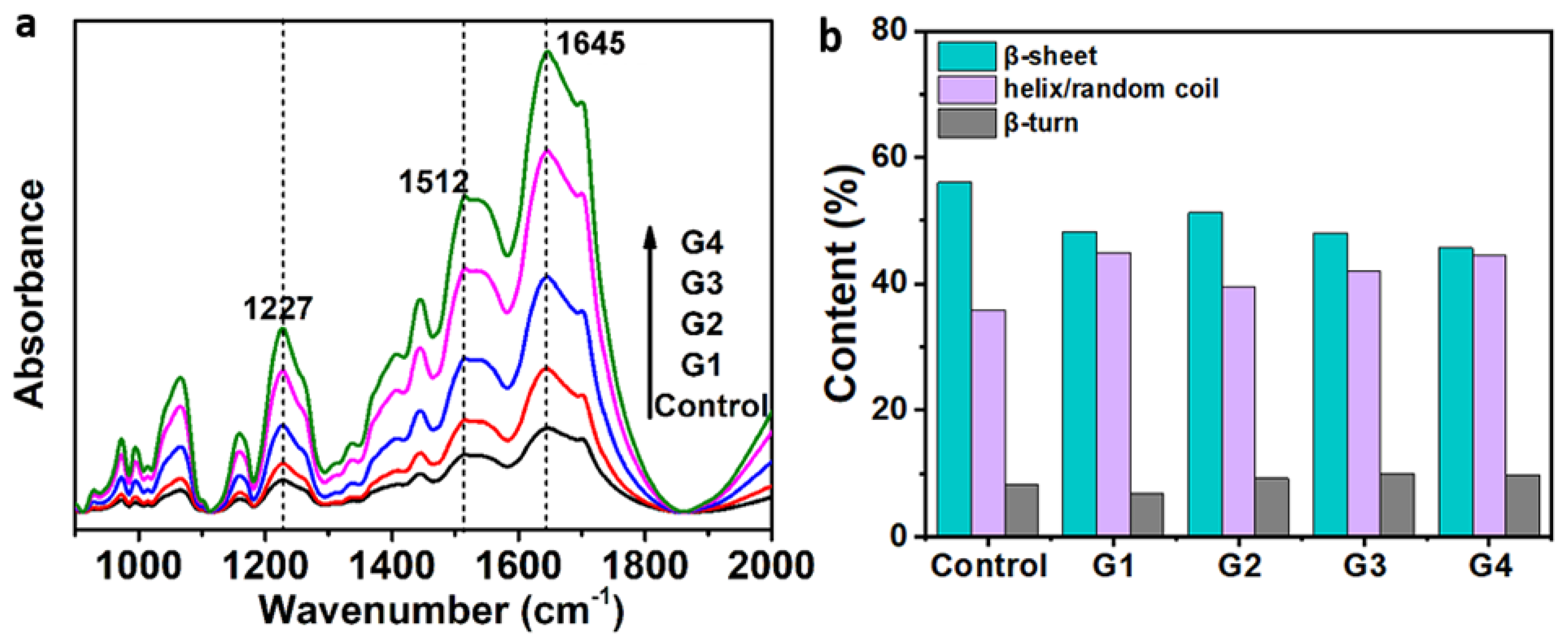
2.3.2. FTIR Analysis
2.4. The Effect of Melanin on the Properties of Silks
2.4.1. Thermal Stability
2.4.2. Mechanical Properties
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Characterizations
4.3. Silkworm Rearing and the Intake of Melanin
4.4. Silk Reeling
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goncalves, R.D.C.R.; Pombeiro-Sponchiado, S.R. Antioxidant activity of the melanin pigment extracted from aspergillus nidulans. Biol. Pharm. Bull. 2005, 28, 1129–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, A.P. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Aghajanyan, A.A.; Asaturian, A.R.; Hambardzumyan, A.A.; Sargsyan, L.B.; Hovsepyan, A.S.; Vardanyan, A.H.; Saghyan, A.S. Obtaining of water-soluble microbial melanin and study of its some properties. Appl. Biochem. Microbiol. 2011, 47, 500–506. [Google Scholar] [CrossRef]
- Cordero, B.J.R.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Siwicka, E.Z.; Son, A.F.; Battistella, C.; Moore, H.M.; Korpantry, J.; McCallum, C.N.; Wang, Z.; Johnson, J.B.; Farha, K.O.; Gianneschi, C.N. Synthetic porous melanin. J. Am. Chem. Soc. 2021, 143, 3094–3103. [Google Scholar] [CrossRef]
- Mauro, D.E.; Camaggi, M.; Vandooren, N.; Bayard, C.; Andlis, D.J.; Pezzella, A.; Baloukas, B.; Silverwood, R.; Ajji, A.; Pellerin, C.; et al. Eumelanin for nature-inspired UV-absorption enhancement of plastics. Polym. Int. 2019, 68, 984–991. [Google Scholar] [CrossRef]
- Cai, M.; Lin, Y.; Lou, Y.-L.; Liang, H.-H.; Sun, P. Extraction, Antimicrobial, and Antioxidant Activities of Crude Polysaccharides from the Wood Ear Medicinal Mushroom Auricularia auricula-judae (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 591–600. [Google Scholar] [CrossRef]
- Ikura, K.; Otomo, C.; Natsuka, S.; Ichikawa, A.; Wakamatsu, K.; Ito, S.; Taoguchi, S. Inhition of tranglutamiase by synthetic tyrosine melanin. Biosci. Biotechnol. Biochem. 2002, 66, 1412–1414. [Google Scholar] [CrossRef]
- Jakubiak, P.; Lack, F.; Thun, J.; Urtti, A.; Alvarez-Sánchez, R. Influence of melanin characteristics on drug binding properties. Mol. Pharm. 2019, 16, 2549–2556. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R.; Schadendorf, D. Melanocytes as ‘sensory’ and regulatory cells in the epidermis. J. Theor. Biol. 1993, 164, 103–120. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhou, X.; McCallum, C.N.; Hu, Z.; Ni, Z.Q.; Kapoor, U.; Heil, M.C.; Cay, S.K.; Zand, T.; Mantanona, J.A.; et al. Unraveling the Structure and Function of Melanin through Synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef] [PubMed]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2021, 9, 1–27. [Google Scholar] [CrossRef]
- Ye, M.; Guo, G.Y.; Lu, Y.; Song, S.; Wang, L.-Y. Purification, structure and anti-radiation activity of melanin from LachnumYM404. Int. J. Biol. Macromol. 2014, 63, 170–176. [Google Scholar] [CrossRef]
- Hung, Y.C.; Sava, V.M.; Blagodarsky, V.A.; Hong, M.-Y.; Huang, G.S. Protection of tea melanin on hydrazine-induced liver injury. Life Sci. 2003, 72, 1061–1071. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Ling, X.; Wang, S.; Ding, J.; Li, Z.; Wang, L.; Jiang, Y. Preparation and characterization of epigallocatechin-3-gallate loaded melanin nanocomposite (EGCG @MNPs) for improved thermal stability, antioxidant and antibacterial activity. LWT 2022, 154, 112599. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.; Zeng, B.; James, A.A.; Tan, A.; Huang, Y. Bombyx mori P-element Somatic Inhibitor (BmPSI) is a key auxiliary factor for silkworm male sex determination. PLoS Genet. 2017, 13, e1006576. [Google Scholar] [CrossRef] [Green Version]
- Katsuma, S.; Shoji, K.; Sugano, Y.; Suzuki, Y.; Kiuchi, T. Masc-induced dosage compensation in silkworm cultured cells. FEBS Open Bio. 2019, 9, 1573–1579. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, I.S.; Niu, B.L.; Ji, D.-F.; Liu, X.-J.; Li, M.-W.; Bai, H.; Palli, S.R.; Wang, C.-Z.; Tan, A.-J. A determining factor for insect feeding preference in the silkworm, Bombyx mori. PLoS Biol. 2019, 17, e3000162. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fan, Y.; Ge, Q.; Xu, J.; Taha, H.R.; Yuan, Y.; Chen, K. Time-Course Transcriptome Analysis Reveals Global Gene Expression Profiling and Dynamic Developmental Signatures across Complete Life Cycle of Bombyx mori. Processes 2021, 9, 1730. [Google Scholar] [CrossRef]
- Ding, X.-Y.; Wang, X.-Y.; Kong, Y.-H.; Zhao, C.-X.; Qin, S.; Sun, X.; Li, M.-W. Comparative Transcriptome Analysis of Bombyx mori (Lepidoptera) Larval Hemolymph in Response to Autographa californica Nucleopolyhedrovirus in Differentially Resistant Strains. Processes 2021, 9, 1401. [Google Scholar] [CrossRef]
- Liu, T.; Xing, R.; Zhou, Y.Z.; Zhang, J.; Su, Y.-Y.; Zhang, K.-Q.; He, Y.; Sima, Y.-H.; Xu, S.-Q. Hematopoiesis toxicity induced by CdTe quantum dots determined in an invertebrate model organism. Biomaterials 2014, 35, 2942–2951. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; He, N.J.; Xiang, Z.H. Advances in silkworm modeling research. Sci. Seric. 2010, 36, 0645–0649. [Google Scholar]
- Sekimizu, N.; Paudel, A.; Hamamoto, H. Animal welfare and use of silkworm as a model animal. Drug Discov. Ther. 2012, 6, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Panthee, S.; Paudel, A.; Hamamoto, H.; Sekimizu, K. Advantages of the silkworm as an animal model for developing novel antimicrobial agents. Front. Microbiol. 2017, 8, 373. [Google Scholar] [CrossRef] [Green Version]
- Nwibo, D.D.; Hamamoto, H.; Matsumoto, Y.; Kaito, C.; Sekimizu, K. Current use of silkworm larvae (Bombyx mori) as an animal model in pharmaco-medical research. Drug Discov. Ther. 2015, 9, 133–135. [Google Scholar]
- Song, J.; Zhang, J.; Dai, F. Advantages and limitations of silkworm as an invertebrate model in aging and lifespan research. OAJ Gerontol. Geriatr. Med. 2018, 4, 1–4. [Google Scholar]
- Matsumoto, Y.; Isii, M.; Shimizu, K.; Kawamoto, S.; Sekimizu, K. A silkworm infection model to evaluate antifungal drugs for cryptococcosis. Med. Mycol. J. 2017, 58, E131–E137. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhu, F.; Chen, K. Silkworm: A promising model organism in life science. J. Insect Sci. 2017, 17, 97. [Google Scholar]
- Matsumoto, Y.; Miyazaki, S.; Fukunaga, D.H.; Shimizu, K.; Kawamoto, S.; Sekimizu, K. Quantitative evaluation of cryptococcal pathogenesis and antifungal drugs using a silkworm infection model with Cryptococcus neoformans. J. Appl. Microbiol. 2011, 112, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Zhang, Y.-H.; Xing, R.; Zhou, Y.-F.; Chen, X.-D.; Wang, H.; Song, B.; Sima, Y.-H.; He, Y.; Xu, S.-Q. Different toxicity of cadmium telluride, silicon, and carbon nanomaterials against hemocytes in silkworm, Bombyx mori. RSC Adv. 2017, 7, 50317–50327. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Zhuang, D.; Ilyin, E.A. Space flight experiment on Chinese silkworm on board the Russian 10th Biosatellite. Adv. Space Res. 1998, 21, 1145–1150. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sekimizu, K. Silkworm as an experimental animal for research on fungal infections. Microbiol. Immunol. 2019, 63, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Andoh, V.; Liu, H.; Song, J.; Wu, G.; Li, L. Biological effects of gold nanoclusters are evaluated by using silkworm as a model animal. J. Mater. Sci. 2019, 54, 4997–5007. [Google Scholar] [CrossRef]
- Ma, L.; Akurugu, M.A.; Andoh, V.; Liu, H.; Song, J.; Wu, G.; Li, L. Intrinsically reinforced silks obtained by incorporation of graphene quantum dots into silkworms. Sci. China Mater. 2019, 62, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Andoh, V.; Ajei, O.M.; Liu, H.; Shen, Z.; Li, L.; Song, J.; Zhao, W.; Wu, G. In vivo toxicity evaluation of boron nitride nanosheets in Bombyx mori silkworm model. Chemosphere 2020, 247, 125877. [Google Scholar] [CrossRef]
- Koehler, A.; Heidrich, D.; Pagani, M.D.; Corbellini, A.V.; Scroferneker, L.M. Melanin and chromoblastomycosis agents: Characterization, functions, and relation with antifungals. J. Basic Microbial. 2021, 61, 203–211. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Zheng, M.; Zhang, Y.A.; Han, Z. Melanin Nanoparticles as an Alternative to Natural Melanin in Retinal Pigment Epithelium Cells and Their Therapeutic Effects against Age-Related Macular Degeneration. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sampath, S.; Isdebski, T.; Jenkins, E.J.; Ayon, J.V.; Henning, R.W.; Orgel, J.P.R.O.; Antipoa, O.; Yarger, J.L. X-ray diffraction study of nanocrystalline and amorphous structure within major and minor ampullate dragline spider silks. Soft Matter 2012, 8, 6713–6722. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Long, H.; Chen, L.; Liu, Y.; Li, Q. Ultrasonics induced variations in molecular structure and tensile properties of silk fibers in a chemical free environment. Nano Select 2021, 2, 1962–1967. [Google Scholar] [CrossRef]
- Shao, J.; Zheng, J.; Liu, J.; Carr, C.M. Fourier transform raman and fourier transform infrared spectroscopy studies of silk fibroin. Inc. J. Appl. Polym. Sci. 2005, 96, 1999–2004. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Matsumoto, A.; Meinel, L.; Li, C.; Kaplan, D.L. Silk microspheres for encapsulation and controlled release. J. Control. Release 2007, 117, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Rajkhowa, R.; Levin, B.; Redmond, L.S.; Li, L.H.; Wang, L.; Kanwar, J.R.; Atlas, M.D.; Wang, X. Structure and properties of biomedical films prepared from aqueous and acidic silk fibroin solutions. Inc. J. Biomed. Mater. Res. 2010, 97, 37–45. [Google Scholar] [CrossRef]
- De Meutter, J.; Goormaghtigh, E. Evaluation of protein secondary structure from FTIR spectra improved after partial deuteration. Eur. Biophys. J. 2021, 50, 613–628. [Google Scholar] [CrossRef]
- Manesa, K.C.; Kebede, T.G.; Dube, S.; Nindi, M.M. Profiling of Silk Sericin from Cocoons of Three Southern African Wild Silk Moths with a Focus on Their Antimicrobial and Antioxidant Properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef]
- Zhao, M.; Qi, Z.; Tao, X.; Newkirk, C.; Hu, X.; Lu, S. Chemical, Thermal, Time, and Enzymatic Stability of Silk Materials with Silk I Structure. Int. J. Mol. Sci. 2021, 22, 4136. [Google Scholar] [CrossRef]
- Sterenzon, E.; Vadivel, K.V.; Gerchman, Y.; Luxbacher, T.; Narayanan, R.; Mamane, H. Effective Removal of Acid Dye in Synthetic and Silk Dyeing Effluent: Isotherm and Kinetic Studies. ACS Omega 2022, 7, 118–128. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Huang, C.; Xiang, S.; Ma, P.; Ni, Z.; Chen, M. Enhanced thermal stability of poly (vinyl alcohol) in presence of melanin. J. Therm. Anal. Calorim. 2014, 115, 1661–1668. [Google Scholar] [CrossRef]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M. Nanoconfinement Controls Stiffness, Strength and Mechanical Toughness of β-sheet Crystals in Silk. Nat. Matr. 2010, 9, 359–367. [Google Scholar] [CrossRef]




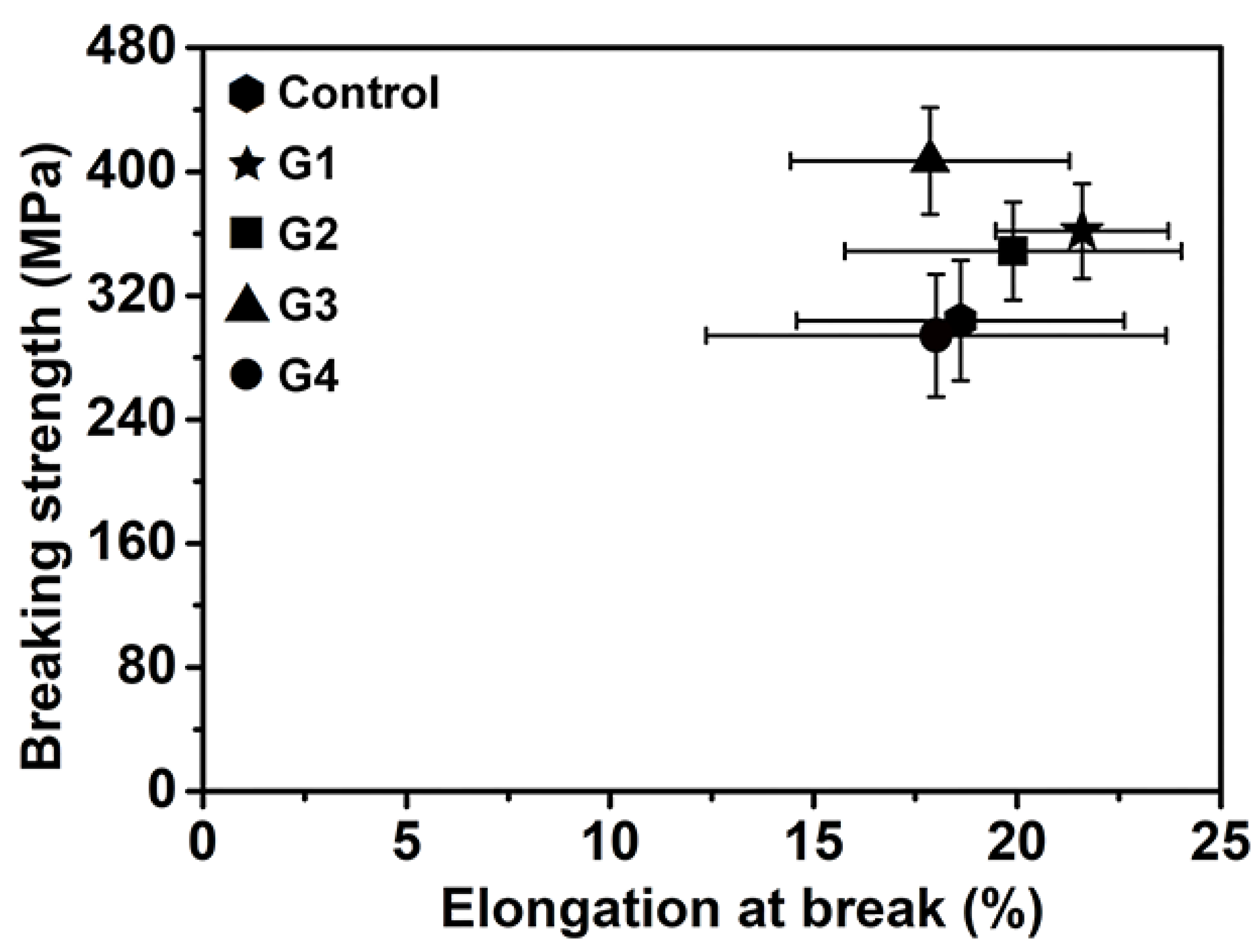
| Sample | Elongation at Break (%) | Breaking Strength (MPa) | Toughness Modulus (MPa) |
|---|---|---|---|
| Control | 18.61 | 303.92 | 42.73 |
| G1 | 21.59 | 361.60 | 56.98 |
| G2 | 19.90 | 348.76 | 51.56 |
| G3 | 17.87 | 406.99 | 55.28 |
| G4 | 18.01 | 294.12 | 40.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andoh, V.; Chen, L.; Zhu, F.; Ge, Q.; Ma, L.; Wang, Q.; Chen, K. The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal. Toxins 2022, 14, 421. https://doi.org/10.3390/toxins14070421
Andoh V, Chen L, Zhu F, Ge Q, Ma L, Wang Q, Chen K. The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal. Toxins. 2022; 14(7):421. https://doi.org/10.3390/toxins14070421
Chicago/Turabian StyleAndoh, Vivian, Liang Chen, Feifei Zhu, Qi Ge, Lin Ma, Qiang Wang, and Keping Chen. 2022. "The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal" Toxins 14, no. 7: 421. https://doi.org/10.3390/toxins14070421
APA StyleAndoh, V., Chen, L., Zhu, F., Ge, Q., Ma, L., Wang, Q., & Chen, K. (2022). The Evaluation of the Biological Effects of Melanin by Using Silkworm as a Model Animal. Toxins, 14(7), 421. https://doi.org/10.3390/toxins14070421







