Abstract
The utilization of the invasive weed, Parthenium hysterophorus L. for producing value-added products is novel research for sustaining our environment. Therefore, the current study aims to document the phytotoxic compounds contained in the leaf of parthenium and to examine the phytotoxic effects of all those phytochemicals on the seed sprouting and growth of Crabgrass Digitaria sanguinalis (L.) Scop. and Goosegrass Eleusine indica (L.) Gaertn. The phytotoxic substances of the methanol extract of the P. hysterophorus leaf were analyzed by LC-ESI-QTOF-MS=MS. From the LC-MS study, many compounds, such as terpenoids, flavonoids, amino acids, pseudo guaianolides, and carbohydrate and phenolic acids, were identified. Among them, seven potential phytotoxic compounds (i.e., caffeic acid, vanillic acid, ferulic acid, chlorogenic acid, quinic acid, anisic acid, and parthenin) were documented, those are responsible for plant growth inhibition. The concentration needed to reach 50% growth inhibition in respect to germination (ECg50), root length (ECr50), and shoot length (ECs50) was estimated and the severity of phytotoxicity of the biochemicals was determined by the pooled values (rank value) of three inhibition parameters. The highest growth inhibition was demarcated by caffeic acid, which was confirmed and indicated by cluster analysis and principal component analysis (PCA). In the case of D. sanguinalis, the germination was reduced by 60.02%, root length was reduced by 76.49%, and shoot length was reduced by 71.14% when the chemical was applied at 800 μM concentration, but in the case of E. indica, 100% reduction of seed germination, root length, and shoot length reduction occurred at the same concentration. The lowest rank value was observed from caffeic acids in both E. indica (rank value 684.7) and D. sanguinalis (909.5) caused by parthenin. It means that caffeic acid showed the highest phytotoxicity. As a result, there is a significant chance that the parthenium weed will be used to create bioherbicides in the future.
Key Contribution:
The current study aimed to document the phytotoxic compounds existing in the leaf of Parthenium and their phytotoxicity in two types of grass such as Crabgrass (Digitaria sanguinalis (L.) Scop and Goosegrass (Eleusine indica (L.) Gaertn. Seven known phenolic derivatives were documented from the P. hysterophorus leaf methanol extract and among them, caffeic acid, chlorogenic acid, and parthenin were found the most phytotoxicity on germination and seedling growth on Crabgrass and Goosegrass, which might be the candidates for developing bio-herbicides.
1. Introduction
Crabgrass Digitaria sanguinalis (L.) Scop. and Goosegrass Eleusine indica (L.) Gaertn. are seasonal C4 plants [1] as well as tropical annual grass weeds that can be found in Africa, Asia, South America, and parts of North America, often causing problems in the production of highland crops. These weeds are one of the five most important destructive weeds in the world, hurting the yields of 46 different crop species in more than 60 countries [2,3]. It can withstand a wide range of salt concentrations, pH, and water stresses. Moreover, the seeds of goosegrass demonstrated a 79% viability at a depth of 20 cm after being buried for two years [4].
Agriculture faces a difficult problem when trying to manage weeds in crop fields. Because of their greater effectiveness, lower cost, and quicker payback, chemical herbicides are primarily favored by farmers to manage weeds. Another important issue for reliance in some countries is the transfer of labor away from agriculture to other industries or nations for jobs [5]. The impacts of climate change and health concerns are rising day by day due to the excessive use of synthetic herbicides, and we need effective alternatives to solve the weed management problem. Additionally, the primary requirements for the development of new selective herbicides are the ability to control the target plants at extremely low dosages that not harmful to the non-target organisms and to meet strict toxicological and environmental regulations. Understanding the mechanisms of herbicides’ selectivity would provide crucial knowledge for the development of novel herbicides [6].
The weed Parthenium hysterophorus L. is an invasive annual herbaceous weed which has global significance. Allelopathic chemicals can be released by this weed into the environment to suppress nearby competing plants. This weed causes allergic respiratory problems, contact dermatitis in human, cattle mutagenicity, and is a threat to crop production due to its potent allelopathic effects [7]. The management of this invasive and recalcitrant weed is an important issue in parthenium-infested countries, including Malaysia, through crop rotation, intercropping, cover cropping as living or dead mulches, green manuring, and use of allelochemical-based bioherbicides [8,9]. The utilization of this weed for extracting phytotoxic chemicals might be an option for parthenium management. So, the identification and separation of the allelopathic compounds from P. hysterophorus could be a technique for creating a bioherbicide. Terpenoids, steroids, phenols, coumarins, flavonoids, tannins, alkaloids, and cyanogenic glycosides, as well as their breakdown products, have been related to the allelopathic property of parthenium plants [10]. In terms of phytotoxicity, phenolic compounds have been the subject of the greatest investigation among these substances. These compounds are biologically active in suppressing weed seed germination and seedling growth [11]. The primary allelochemicals in parthenium were discovered in the phenolic compounds to be p-coumaric, p-hydroxybenzoic, ferulic acid, and vanillic acid [12]. At modest concentrations, the treatments with parthenin were also found to considerably delay germination but boost root growth [13]. The use of herbicides has expanded due to their budget-friendly solution to and labor-intensive technique of weed management. However, herbicide resistance in weeds has become more likely as a result of an over-reliance on them. For example, a good number of populations of crabgrass and goosegrass have evolved resistance to a variety of herbicides [14,15]. In the eight states of Malaysia, this grass has developed resistance to glyphosate, fluazifop, paraquat, and glufosinate [16]. Therefore, we need an alternative to chemical herbicides to solve the problems of weed resistance against herbicides. Bioherbicides are herbicides comprising phytotoxins, pathogens, and other microbes used as biological weed control [17]. Additionally, phytochemicals can be used as bioherbicides to boost crop output by biologically controlling weeds through allelopathy. By reducing the risk of mutagenic, genotoxic, and cytotoxic effects, these chemicals may also benefit human health [18]. Phytotoxicity, which can occur spontaneously or as a result of utilizing phytochemicals as bioherbicides, is one of the main effects of allelopathy [19]. Bioherbicides disintegrate quickly and do not leave residues in the soil after crops are harvested since they are based on natural chemicals and have short half-lives and process few halogen groups [20]. As a result, it is possible to control weeds using secondary metabolites derived from plants or other natural sources, helping to safeguard both people and the environment. On the other hand, bioassays are typically created to examine a plant species’ potential allelopathic effects. Because of the influence of several environmental circumstances, a plant that exhibits severe phytotoxicity toward the target plant species in laboratory conditions may not exhibit the same level of toxicity in the field context [21].
Some previous reports reveal the herbicidal potential of P. hysterophorus extracts in different plant species. As per our initial screening trials, parthenium leaf extracts have been examined to be a potential source of different allelochemicals with herbicidal and phytotoxic effects. However, inadequate evidence is available on the phytotoxicity of specific bioactive compounds which are identified in P. hysterophorus on the growth and development of crabgrass and goosegrass, which are the major weeds of rice and many of the field crops. Therefore, the main objective of this study is to evaluate the phytotoxicity of seven identified compounds from parthenium on the germination and growth of these weeds. The identification of its phytotoxic compounds was analyzed by using LC-ESI-QTOF-MS = MS (liquid chromatograph electrospray ionization quadrupole time of flight mass spectrometry).
2. Results
2.1. Identified Compounds from P. hysterophorus Leaf Methanol Extract through LC-MS Analysis
The identified compounds from P. hysterophorus leaf methanol extract through LC-MS analysis and their relative proportions of P. hysterophorus leaf with methanolic extract from positive and negative polarity analyses are listed in Table 1 and Table 2. From positive [M-H]+ polarity analysis, 33 compounds were identified from the leaf between retention time of 0.742 to 12.466 with m/z ratio of 112.1123 to 310.3452 and molecules mass of 94.0784 to 308.1963 (Table 1 and Table 2). With the concentration of 50 g L−1, there were three known toxic compounds detected from the leaf extract. On the other hand, negative (M-H−) polarity analysis detected 148 compounds between the retention time of 0.666 to 15.661 with the m/z ratio of 121.02988 to 1089.5495 and the mass molecules of 112.02781 to 2181.11278.

Table 1.
Identified compounds in the methanol extract of leaf of P. hysterophorus from LC–MS positive polarity analysis.

Table 2.
Identified compounds in the methanol extract of leaf of Parthenium hysterophorus from LC–MS negative polarity analysis.
2.2. Documentation of Phytotoxic Compounds from P. hysterophorus Leaf Methanol Extract through LC–MS Analysis
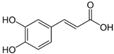
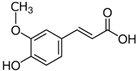
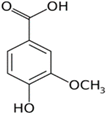
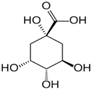
The LC–MS analyses of P. hysterophorus leaf methanol extract revealed the presence of many compounds, such as terpenoids, flavonoids, amino acids, pseudo guaianolides, carbohydrates, and phenolic acids. Among them, phenolic acids are responsible for plant growth inhibition. The list of proposed phytotoxic compounds (caffeic acid, ferulic acid, vanillic acid, quinic acid, parthenin, chlorogenic acid, and p anisic acid) with their retention time, molecular formula, polarity, and mass fragment (m/z) is presented in Table 3.

Table 3.
Phytotoxic compounds of P. hysterophorus leaf with methanolic extracts through LC–MS analysis.
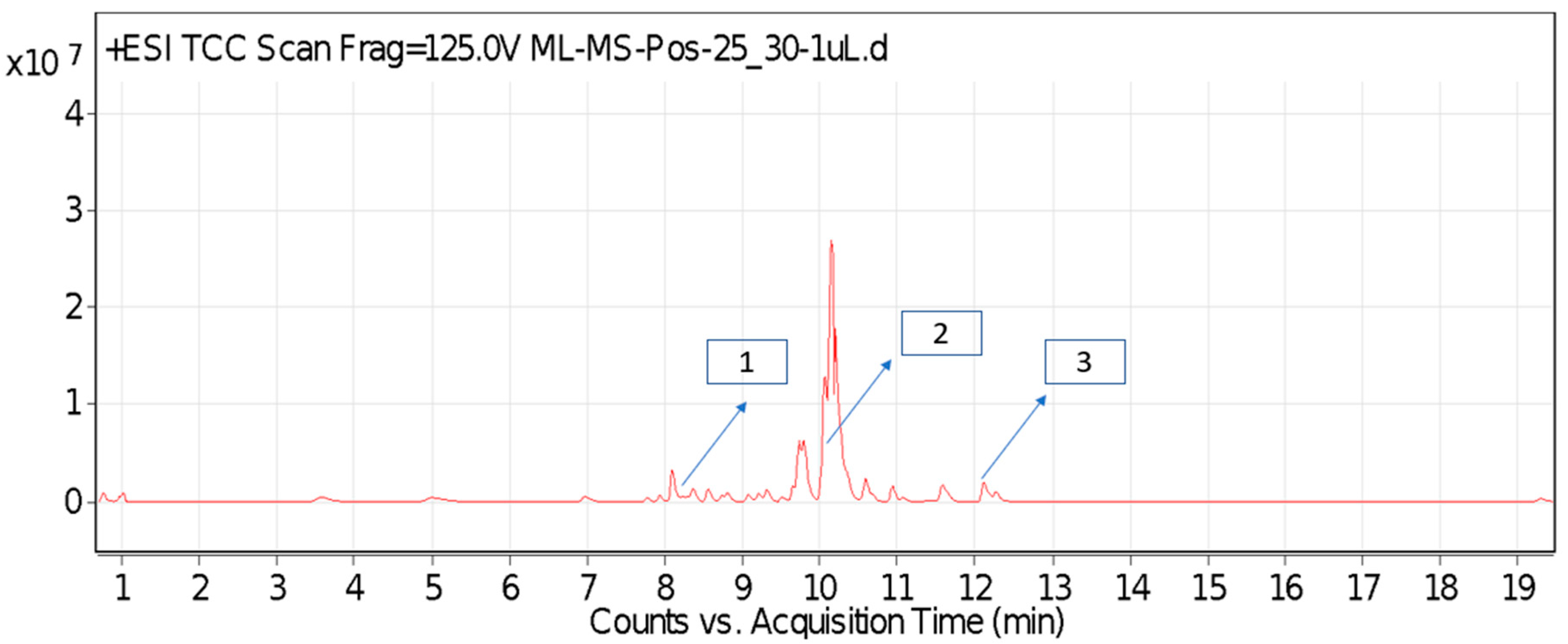
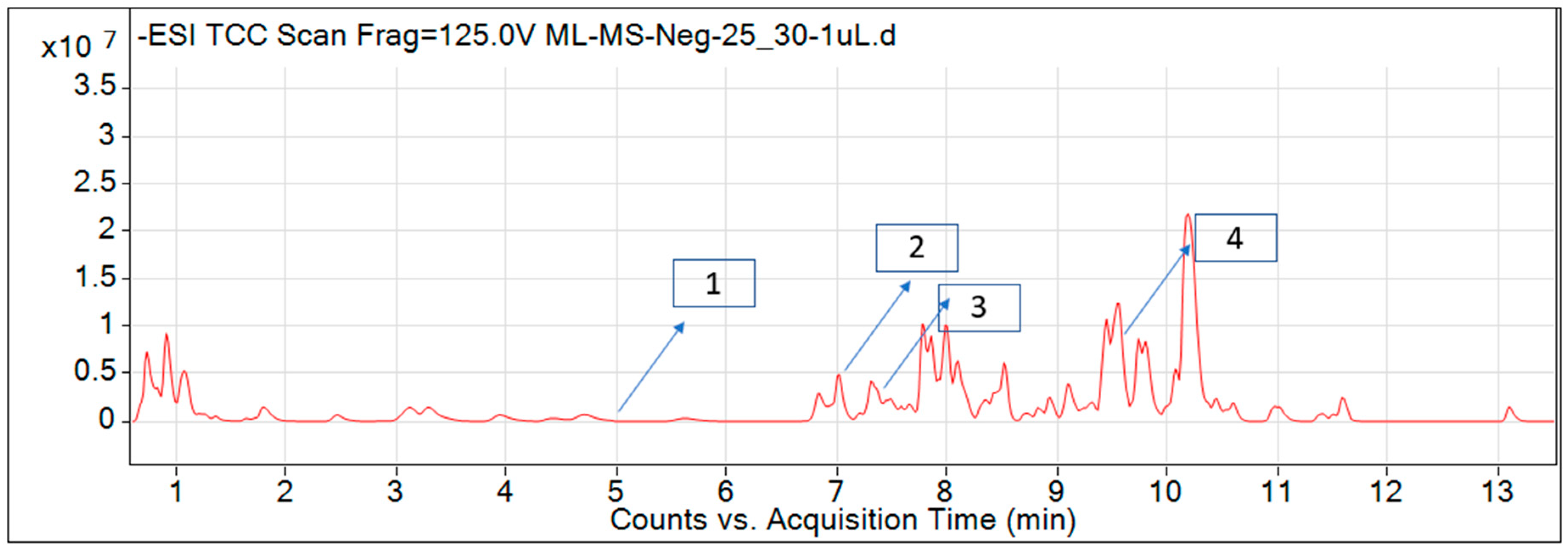
For most of the compounds, [M-H]+ and [M-H]− ions were observed. The total ion current chromatography in positive and negative ESI mode is shown in Figure 1 and Figure 2. Quinic acid, parthenin and chlorogenic acid were identified by positive ionization mode at 12.116, 10.004, and 8.09 min, with 181.12, 263.1267, and 300.183 m/z, respectively. Another four phenolics, namely caffeic acid, ferulic acid, vanillic acid and p-anisic acid, were documented from negative polarity analysis at 7.183, 9.84, 7.367, and 5.121 min with m/z 341.0894, 193.05129, 153.01983, and 151.04047.
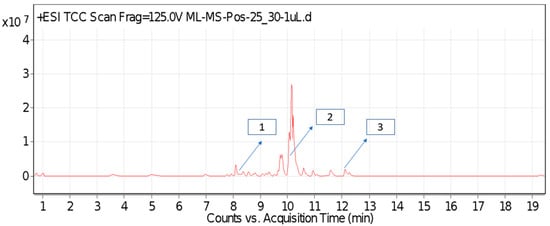
Figure 1.
LC-MS chromatograms phytotoxic compounds on P. hysterophorus leaf methanolic extract positive ion mode (1. Chlorogenic acid, 2. Parthenin and 3. Quinic acid).
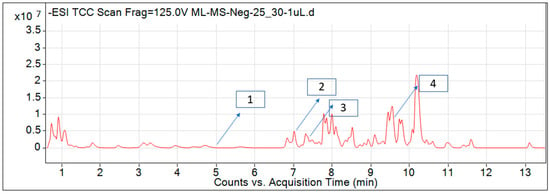
Figure 2.
LC–MS chromatograms phytotoxic compounds on P. hysterophorus leaf methanolic extract positive ion mode (1. p-Anisic acid, 2. Caffeic acid, 3. Vanillic acid, and 4. Ferulic acid).
P. hysterophorus leaf extracts have a range of chemical compounds. Among them, phenolic compounds cause dermatitis, autotoxicity, and the suppression of other plants. There was a correlation between the quantity and kind of compounds detected in each plant and herbicidal activity.
2.3. Allelopathic Effects of the Phytochemicals on D. sanguinalis and E. indica
Significant killing effects of the chemicals on the test weed species were observed. The chemicals and their mixtures produced varying degrees of inhibitory effects on the germination, root growth, and hypocotyl elongation of D. sanguinalis and E. indica. The doses required for a 50% growth inhibition (EC50) of the weeds, as indicated by ECg50 (germination), ECr50 (root), and ECs50 (shoot) growth, were computed and found different from the control.
2.3.1. Effects on Germination and Early Growth of D. sanguinalis
In the concentration–response bioassay, the inhibitory magnitude was increased for all compounds by increasing the concentration of chemicals from 100 μM to 1600 μM (Table 4). At the lowest concentrations (100 and 200 μM) of all tested compounds, less significant effect was found on the germination of D. sanguinalis, relative to the control except caffeic acid, which significantly suppressed the growth when applied with 200 μM; whereas for other chemicals, the germination percentage was significantly suppressed at rates higher than 400 μM. The germination of D. sanguinalis was severely decreased from 800 μM of chlorogenic acid, ferulic acid, parthenin, and vanillic acid. For quinic acid, anisic acid and a combination of compounds (mixture) inhibited growth when treated with 1600 μM. No germination was observed when treated with1600 μM of caffeic acid. Tested compounds did not exceed the doses to obtain EC50 employed in this study except caffeic acid, chlorogenic acid, and parthenin, which produced the highest growth inhibition at 100, 48, and 60%, respectively, and the lowest inhibition was caused by anisic acid (32%). Therefore, caffeic acid was the highest toxic in comparison to other chemicals in all the concentrations, followed by parthenin and chlorogenic acid.

Table 4.
Germination (%) of D. sanguinalis treated with selected phytochemicals.
Therefore, the phytochemicals have significant allelopathic effects on the root growth of the tested weeds. The root growth was significantly (p ≤ 0.05) reduced by caffeic acid, chlorogenic acid, quinic acid, parthenin, and a combination of their mixtures at all concentrations. Table 5 shows that caffeic acid, quinic acid, and parthenin were very toxic, reducing root development even at the lower doses (100 μM). An increase in the dose of these chemicals resulted in a higher degree of growth inhibition. The caffeic acid caused 76% inhibition at 800 μM, and from the 1600 μM concentration, no root was visible. Parthenin, quinic acid, chlorogenic acid, and a mixture of compounds, on the other hand, reduced the root growth by 60, 46, 47, and 47%, respectively, at a dose of 800 μM. The weakest inhibition (47%) was observed from ferulic acid even at the highest concentration.

Table 5.
Root length (cm) of D. sanguinalis treated with selected phytochemicals.
A more or less similar pattern of effects on shoot length also occurred due to the treatments (Table 6). However, the shoot elongation of D. sanguinalis was not significantly decreased by a lower (400 μM) concentration of all compounds except caffeic acid, quinic acid, parthenin, and their mixtures. Vanillic acid, ferulic acid, and chlorogenic acid exhibited an adverse effect on the shoot elongation at 800 μM and beyond. On the other hand, only anisic acid exhibited a 43% inhibition at the highest concentration.

Table 6.
Shoot length (cm) of D. sanguinalis treated with detected allelochemicals.
2.3.2. Comparison between Phytochemicals in Their Effects on Growth Parameters
Table 7 shows some remarkable differences among the allelochemicals in terms of D. sanguinalis growth inhibition. The differences were apparent from the rank values of composites. Caffeic acid (Re = 909.5) and parthenin (Re = 2569.4) exposed higher inhibitory influences on the germination and development of D. sanguinalis; in other words, these compounds showed the most phytotoxic impact, which indicates that less concentration is needed to suppress this plant. While anisic acid (Re = 14845.8), ferulic acid (Re = 8878.8), and quinic acid (Re = 8647.4) showed the weakest phytotoxicity compared to other chemicals. Consequently, it was apparent that the growth inhibitory effect of these compounds was the lowest. It means that anisic acid, ferulic acid, and quinic acid inhibit 50% of D. sanguinalis by more concentration than other tested compounds. According to Re value, the ranking of phytochemicals was caffeic acid < parthenin < chlorogenic acid < mixture < vanillic acid < quinic acid < ferulic acid < anisic acid. It can be mentioned here that the phytochemicals inhibited the growth of root length more than the growth of the shoot length and percent germination. The sum of ECr50 value for all compounds was 7811.2, whereas that of germination and shoot length were 32,597.9 and 54,700.8, respectively.

Table 7.
Inhibitory effect (EC50) of phytotoxic compounds, the sensitivity of examined initial growth parameters of D. sanguinalis.
2.3.3. Germination and Early Growth of E. indica Treated with Detected Allelochemicals
In the concentration–response bioassay, the inhibitory magnitude was increased for all compounds with increasing concentration from 100 μM to 1600 μM (Table 8). At the lowest concentrations (100 μM) of all tested compounds, less significant effect was found on the germination of E. indica, except by caffeic acid, chlorogenic acid, and the compound mixture. It significantly suppressed inhibition when applied at 200–1600 μM except for quinic acid and anisic acid. The germination of E. indica severely decreased from 800 μM of caffeic acid, chlorogenic acid, and parthenin.

Table 8.
Germination (%) of E. indica treated with the phytochemicals.
On the other hand, vanillic acid, ferulic acid, quinic acid, anisic acid, and a combination of compounds inhibited the weed growth when treated with 1600 μM. However, no germination of weed seeds was observed when treated with 800 μM of caffeic acid. Tested compounds did not produce a significantly lower value of EC50 with the investigational doses used in this study except by caffeic acid, chlorogenic acid, and parthenin. The maximum growth inhibition of these compounds was observed at the rate of 100, 64, and 77%, respectively, and the lowest inhibition was found from anisic acid (15%). Therefore, it is obvious from the analysis that caffeic acid inhibited the most in all the concentrations, followed by parthenin and chlorogenic acid.
Identified allelochemicals have significant allelopathic effects on the root growth of the tested plant at varying doses (Table 9). All chemicals except vanillic acid significantly inhibited root elongation (p ≤ 0.05) at doses from 100 to 400 μM. However, at doses of more than 400 μM concentration, it suppressed the weed growth heavily. Table 9 shows that caffeic acid, quinic acid, and parthenin were strongly active, reducing root development even at the lowest concentration (100 μM). The caffeic acid produced 100% inhibition at an 800 μM concentration and above, while no root was visible, but 75 and 79% inhibition were observed from quinic acid and parthenin, respectively. The weakest phytotoxic effect (48%) on root development was noticed from chlorogenic acid at the highest concentration, while the rest of the compounds caused slightly more than 50% inhibition at the highest concentration.

Table 9.
Root length (cm) of E. indica treated with the phytochemicals.
A similar pattern of effect on shoot length was noticed as was on germination and root length (Table 10). The hypocotyl elongation of E. indica was not significantly decreased by a lower concentration (400 μM) of all compounds except caffeic acid, and vanillic acid. These compounds exhibited an adverse effect on the shoot elongation at the 800 μM and beyond. On the other hand, only anisic acid exhibited a 45% inhibition at the highest concentration.

Table 10.
Shoot length (cm) of E. indica treated with the phytochemicals.
2.3.4. Comparison of Phytochemicals in Their Effects on Examined Initial Growth Parameters
Table 11 shows some remarkable differences among the identified allelochemicals in terms of the growth inhibition of E. indica. The differences were apparent from the rank values of composites. Caffeic acid (Re = 684.7) and parthenin (Re = 1637.66) showed the highest phytotoxicity on the germination and development of E. indica; in other words, these compounds showed the most phytotoxic impact, as indicated by the lower concentrations needed to suppress this plant. While anisic acid (Re = 19553.25), ferulic acid (Re = 7970.02), and a mixture (Re = 5613.8) showed the weakest phytotoxicity compared to the others. The anisic acid, ferulic acid, and mixture of these compounds inhibit 50% of E. indica at a higher concentration than other tested compounds. The overall ranking, according to Re value, is caffeic acid < parthenin < vanillic acid < quinic acid < chlorogenic acid < mixture < ferulic acid < anisic acid. It is clear from the findings that the growth of root length is more affected by the chemicals than the growth of shoot length and percent germination. The sum of ECr50 values for all compounds was 9557.51, whereas the values for germination and shoot length were 27,149.61 and 48,847.3, respectively.

Table 11.
Inhibitory effect of phytotoxic compounds, the sensitivity of examined initial growth parameters of E. indica.
2.3.5. Cluster Analysis and Assessment of Principal Component Analysis
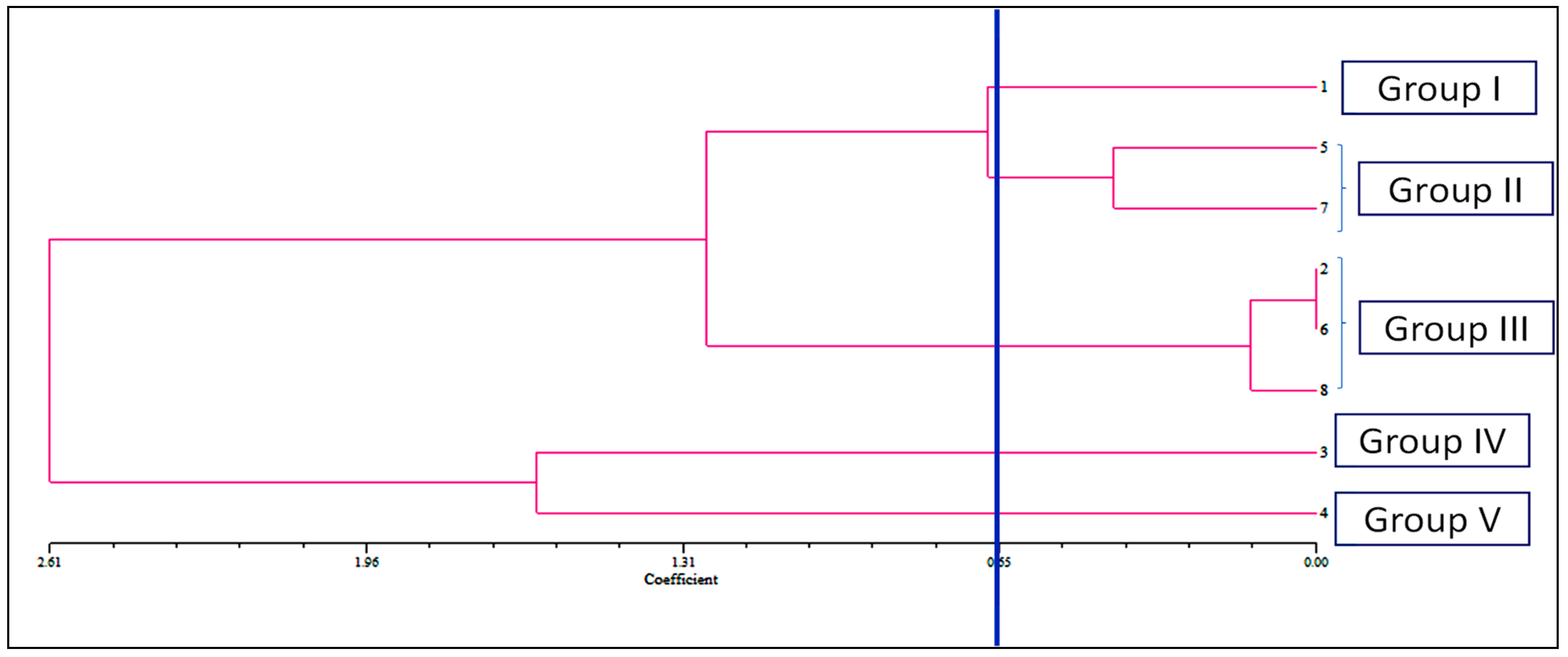
The allelopathic activities of examined compounds and their combination in bioassay were clustered into four interpretable groups, according to the dendrogram (group I–V) as indicated. In the dendrogram, there was a coefficient cut-off at 0.65 for ease of interpretation (Figure 3). Group I consisted of caffeic acid, which was characterized by the most inhibitory effects and with low-rank values. Parthenin and quinic acid are in group II with stronger inhibitory effects; Group III is comprised of vanillic acid, anisic acid, and mixture; group IV consists of ferulic acid; and chlorogenic acid is in group V, which had moderate inhibitory effects. The compounds under groups IV and V demonstrated a relatively weak phytotoxic effect in comparison with other groups.
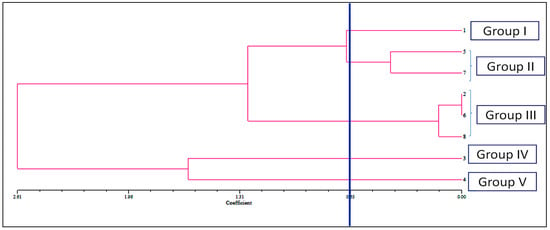
Figure 3.
Dendrogram showing the mean EC50 values of seed sprouting, root, and hypocotyl length of D. sanguinalis and E. indica treated with the phytochemicals (1. caffeic acid, 2. vanillic acid, 3. ferulic acid, 4. chlorogenic acid, 5. quinic acid, 6. anisic acid, 7. Parthenin, and 8. Mixture) revealed by non-overlapping (SAHN) as produced by the UPGMA method.
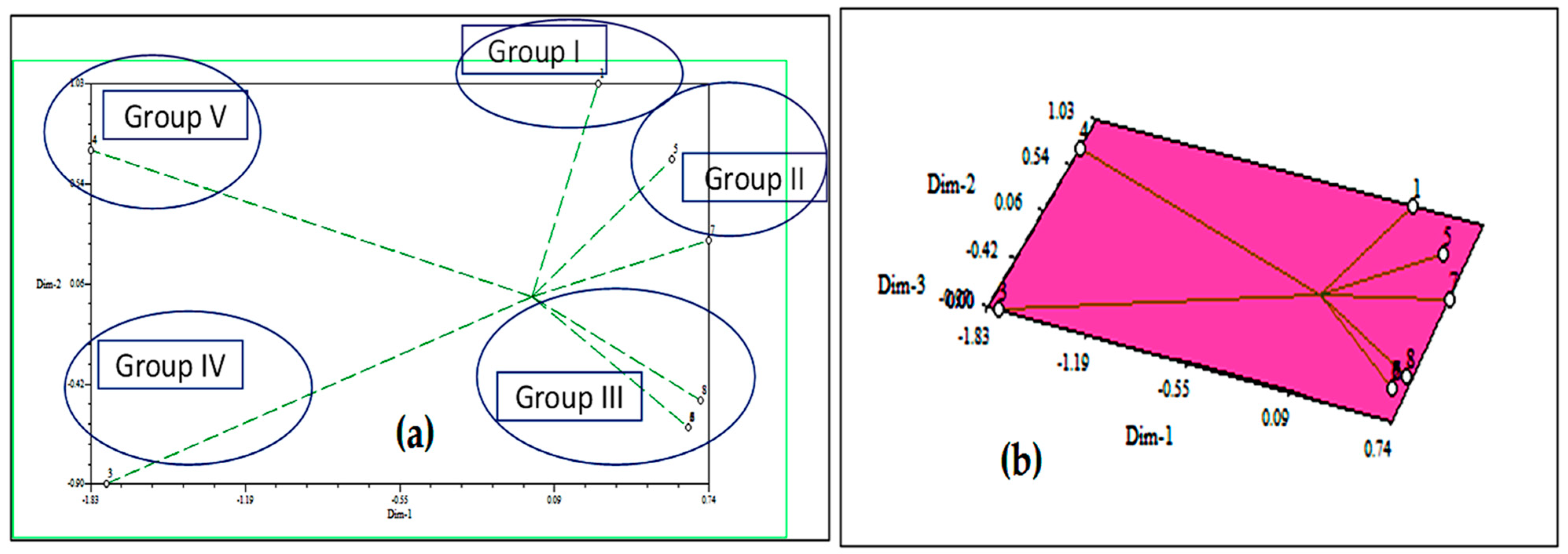
The effects of D. sanguinalis and E. indica were responsible for the majority of the differences observed in the cluster. The two-dimensional and three-dimensional (Figure 4) graphical elucidations confirmed that the maximum of the phytochemicals was discrete at low distances, the only two were discrete at long distances as represented by the eigenvector. The furthest accessions from the centroid were 3 and 4, whereas others were close to the centroid.
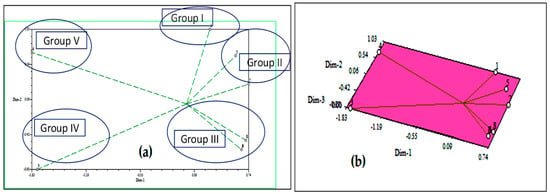
Figure 4.
Based on Euclidian distance, principal component analysis (PCA)-2D graphical relationship between the discovered allelochemicals; (a) eigenvectors and (b) eigenvalues.
3. Discussion
The P. hysterophorus extracts contained a large number of chemicals that were discovered using phytochemical screening, some of which had previously been recognized as toxins in other studies [28,29,30,31,32,33]. Furthermore, a variable number of chemicals were also present in different plant parts of P. hysterophorus. The leaf has a stronger inhibitory impact since it contains more harmful chemicals than the other plant parts. The suppressive influence of extracts, according to Verdeguer et al. [34] is determined by the extract’s chemical makeup as well as the plant sections to which it is applied. These findings are consistent with those of Javaid and Anjum [35] and Verma et al. [36] who discovered that the main causes of the inhibition of plant growth are parthenin and other phenolic acids including caffeic acid, vanillic acid, anisic acid, chlorogenic acid, and para-hydroxybenzoic acid.
In this investigation, tested the phytotoxicity of all previously identified allelopathic compounds. The pure compound bioassay (chemicals purchased from the market) demonstrated that all of the examined compounds and their mixtures were physiologically dynamic and toxic, reducing seed germination and development in crabgrass and goosegrass. These results confirmed that the compounds found in P. hysterophorus are potential allelochemicals and that they are most likely responsible for P. hysterophorus’ herbicidal behavior. Caffeic acid, chlorogenic acid, and parthenin were the most active of the compounds tested (Table 2 and Table 6). In fact, the plant’s allelochemicals have yet to be discovered.
Our results are also supported by the results of others [11,37,38,39,40,41,42] who discovered that caffeic acid, benzoic acid, p-anisic acid, chlorogenic acid, trans-ferulic acid, trans-cinnamic acid, and syringic acid had an allelopathic effect on the seed germination and early growth of Phaseolus vulgaris, Phaseolus aureus, Arabidopsis thaliana, Echinochloa crus-galli, Lactuca sativa, and Sagittaria montevidensis, respectively, despite clear dose–response differences.
According to Bajwa et al. [42] and Guo et al. [43] the extracts from allelopathic plant species produce much higher total phenolics than extracts from non-allelopathic plant species. The most vital and prevalent plant allelochemicals in the environment are phenolic derivatives [44]. Numerous papers have focused on the allelopathic and phytotoxic characteristics of phenolic and flavonoid chemicals [42,45]. Phenolic derivatives are an important class of allelopathic chemicals with a wide range of allelopathic actions. Regardless of dose, these components exhibited the most negative impact on seed germination and the development of barnyard grass [46]. Plant growth and development are inhibited by phenolic acids, which are one of the principal groups of metabolites implicated in allelopathic interactions in the soil atmosphere [47]. Amarowicz et al. [48] discovered that phenolics from the Jerusalem artichoke (Helianthus tuberosus L.) influenced lettuce development. According to Braga et al. [49], flavonoids inhibited the growth of standard target species (STS), such as Lactuca sativa (lettuce), Lycopersicon esculentum (tomato), and Allium cepa (Onion). Parthenin, chlorogenic acid, and ambrosian were also found to be favorably connected with germination inhibition and radicle elongation inhibition [42]. Caffeic acid, chlorogenic acid, ferulic acid, gallic acid, p-coumaric acid, 4-hydroxy-3-methoxybenzoic acid, m-coumaric acid, syringic acid, and vanillic acid were found as phytotoxins in parthenium, which cause allelopathic effects on crops [50]. Caffeic acid was shown to be the most effective inhibitor, as measured by thin-layer chromatography, melting point, infrared spectrum studies, and seedling emergence reduction [38,51].
P. hysterophorus extracts were found to have a higher inhibitory effect than individual compounds and even a mixture of all identified components [52]. The extracts’ stronger inhibitory effects could be owing to unique chemical combinations that work in an additive or synergistic manner. This shows that undiscovered extract components may have a synergistic effect on phytotoxic action, if not direct activity [53]. It can be speculated that in addition to the established phenolic and flavonoid components, unknown chemicals are responsible for the overall allelopathic impact of extracts. Mixtures of phenolic compounds were less suppressive as compared to the allelopathic activity of individual phenolic compounds (Table 2 and Table 6), which might be due to the fact that the allelopathic effect is regulated by concentration interactions, chemical combinations, and test species sensitivity because growth inhibition in mixes is lower than in individual component chemicals [54].
4. Conclusions
From the LC–MS analysis, many compounds, such as terpenoids, flavonoids, amino acids, pseudo guaianolides, and carbohydrate and phenolic acids, were identified from positive and negative polarity analysis. Among them, seven known phenolic derivatives were documented from the P. hysterophorus leaf methanol extract, which was responsible for plant growth inhibition. Seed germination and the development of crabgrass and goosegrass was reduced by all of the compounds, indicating that all combinations of all compounds were physiologically active. Caffeic acid and parthenin had the maximum phytotoxicity on crabgrass and goosegrass during germination and seedling development; indicating that a lower dosage is required to inhibit this plant. In comparison to the others, anisic acid, ferulic acid, and combination demonstrated the least phytotoxicity. This means that these chemicals need to inhibit to a greater extent than other chemicals on crabgrass and goosegrass germination and seedling growth to achieve the same effect. Overall, the ranking values were caffeic acid < parthenin < vanillic acid < quinic acid < chlorogenic acid < mixture < ferulic acid < anisic acid. Among these tested compounds, caffeic acid, chlorogenic acid, and parthenin were found to be the most active, and thus might be appropriate candidates for developing bioherbicides.
5. Materials and Methods
5.1. Site Description
The experimentation was conducted in the weed science laboratory of the Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia. Liquid Chromatography–Mass Spectrophotometry (LC-MS) analysis was carried out at Monash Universiti, Malaysia.
5.2. Extract Preparation
Parthenium leaves were collected from the Ladang Infoternak farm in Sungai Siput, Perak, Malaysia. Plants leaf was collected randomly during the vegetative stage (15–20 days old plants), rinsed with tap water numerous times to remove dust particles, and air-dried for three weeks at room temperature (24–26 °C). In a laboratory blender, plant leaves were mashed into a fine powder and sieved through a 40-mesh sieve.
The extracts were made according to the procedure described by Ahn and Chung [55] and Aslani et al. [56]. An amount of 100 g leaf powder of parthenium was placed in a conical flask and allowed to soak in 1 L of 80% (v/v) methanol. The conical flask was wrapped in paraffin and shaken for 48 h at 24–26 °C room temperature in an orbital shaker at a 150 rpm agitation speed. To remove debris, cheesecloth in four layers were used to filter the mixtures. The supernatant was centrifuged for one hour at 3000 rpm in a centrifuge (5804/5804 R, Eppendorf, Germany). A single layer of Whatman No. 42 filter paper was used to filter the supernatant. A 0.2-mm Nalgene filter (Lincoln Park, NJ-based Becton Dickinson percent Labware) was used to filter the solutions one more time to exclude microbial development. Using a rotary evaporator (R 124, Buchi Rotary Evaporator, Germany), the solvents were evaporated from the extract to dryness (a thick mass of coagulated liquid) under vacuum at 40 °C and the sample was then collected. From a 100 g sample of P. hysterophorus powder, the average extracted sample was 17.56 g, which was estimated as per the following formula [57]:
[Extract weight (g)/powder weight (g)] × 100 = Extraction percentage
All extracts were stored at 4 °C in the dark until use. For LC–MS analysis, 100% HPLC GRADE methanol (20 mL) was diluted with the crude sample (20 mg) and filtered through 15-mm, 0.2-μm syringe filters (Phenex, Non-sterile, Luer/Slip, LT Resources Malaysia).
5.3. Identification of Phytotoxic Compounds from P. hysterophorus Leaf Methanol Extract
The analysis of the phytochemical compounds of the methanol extracts was performed using LC–MS followed by Schimanski et al. [58]. LC–MS analysis was carried out using Agilent spectrometry equipped with a binary pump. The LC–MS was interfaced with the Agilent 1290 Infinity LC system coupled to Agilent 6520 accurate-mass Q-TOF mass spectrometer with a dual ESI source. Full-scan mode from m/z 50 to 500 was set with a source temperature of 125 °C. The column of Agilent zorbax eclipse XDB-C18, narrow-bore 2.1 × 150 mm, 3.5 microns (P/N: 930990-902) was used at the temperature of 30 °C for the analysis. A—0.1% formic acid in water—and B—0.1% formic acid in methanol—were used as solvents. Isocratic elution was used to supply solvents at a total flow rate of 0.1 mL minutes−1. MS spectra were collected in both positive and negative ion modes. The drying gas was 300 °C, with a 10 mL min-1 gas flow rate and a 45-psi nebulizing pressure. Before analysis, sample extraction was diluted with methanol and filtered through a 0.22 m nylon filter. The extracts were injected into the analytical column in 1 μL volume for analysis. The mass fragmentations were discovered using an Agilent mass hunter qualitative analysis B.07.00 (Metabolom-ics-2019.m) tool and a spectrum database for organic chemicals.
5.4. Experimental Treatments and Layout
The treatments consisted of seven biochemicals e.g., caffeic acid, vanillic acid, ferulic acid, chlorogenic acid, quinic acid, anisic acid, and parthenin at different concentrations of 0 (distilled water), 100, 200, 400, 800, and 1600 μM., and two weed species, crabgrass, and goosegrass. Completely randomized designs (CRD) with four replications were used to arrange the experimental units (Petri dishes).
5.5. Plant Materials and Compounds
These detected seven phytotoxic compounds were purchased from Bio-solutions Sdn Bhd, Kuala Lumpur, Malaysia. The source of all chemicals is Sigma-Aldrich (St. Louis, MO, USA). The seeds of two weed species, crabgrass and goosegrass, were collected from UPM agricultural field and then kept in a refrigerator for 15 days at 4 °C for further use.
5.6. Bioassay
Individual chemicals and their mixtures were tested for their inhibitory effects on the germination and early growth of the weed species. Six different concentrations of the chemicals were achieved by dissolving the appropriate amount of chemicals in distilled water, i.e., 1600, 800, 400, 200, 100, and 0 μM (control), which were then sonicated at 60 kHz for one hour at 30 °C in an ultrasonic bath. The precise process for making various chemical concentrations includes dissolving the right amount of powder based on their molecular weight, such as the molecular weight of caffeic acid, i.e., 180.16 g. Thus, 1 mol equals 180.16 g. Therefore, a 1 molar solution will result from diluting 1 liter of distilled water by 180.16 g caffeic acid. Consequently, 1600 moles = (1600 × 180.16) = 540,480 g. In this manner, 540.48 mg of powder is required to create a 1000 mL solution in distilled water [59].
Healthy and uniform weed seeds were gathered and soaked for 24 h in 0.2% potassium nitrate (KNO3), then rinsed with distilled water and incubated at room temperature (24–26 °C) until the radicle emerged by about 1 mm. Thirty uniform pre-germinated seeds were inserted in disposable plastic 9.0-cm-diameter Petri dishes with two sheets of Whatman No. 1 filter paper. After that, the filter paper on the Petri dishes was wetted and soaked with 5 mL of six different chemical solutions. In the same way, 5 mL of pure water was treated as a control. The Petri dishes were then incubated under fluorescent light (8500 lux) in a growth chamber at 30/20 °C (day/night) with a 12 h/12 h (day/night cycle). The relative humidity ranged from 30% to 50%. To facilitate gas exchange and avoid anaerobic conditions, the lids of the Petri dishes were not sealed.
5.7. Data Measurement
Seed germination was counted, and root and shoot lengths of the weed species were measured after 1 week of seed placement with a ruler. The radicle and hypocotyl length was measured using Image J software (https://imagej.nih.gov/ij/docs/guide/user-guide.pdf; accessed on 10 July 2022) [60]. The inhibitory effect of P. hysterophorus extracts on germination, radicle length, and hypocotyl length was computed following the equation [25]:
where “I” is the percentage of inhibition, “C” is the control’s mean, and “A” is the treatment (extract) mean of germination, radicle length, and hypocotyl length.
I = 100 (C − A)/C
To find discrete groupings of allelochemicals with similar phytotoxicity, the most common application of NTSYSpc 2.02e (Numerical Taxonomy and Multivariate Analysis System) was used to perform various types of agglomerative cluster analyses and to estimate some type of similarity or dissimilarity matrix to further define the level of sensitivity to chemical compounds among the plants under investigation [59,60].
Effective dosages capable of suppressing 50% of germination, root length, and shoot length were calculated using ECg50, ECr50, and ECh50, respectively. The ECg50, ECr50, and ECh50 values were calculated using Probit analysis based on the percent of root and shoot length inhibition, respectively. The following equation was used to create an index (Re) for each of the most active extracts and the most sensitive plants for each plant tested:
where Re is the plant’s rank n and ECg50n, ECr50n, and ECh50n are the amounts of plant extract n that inhibit 50% of germination, root length, and shoot length, respectively. The lowest Re value had the most active chemical and the most sensitive plants, while the highest Re value had the least inhibition effect on the chemicals.
ECg50n (germination) + ECr50n (root) + ECh50n (shoot) = Rank (Re)
5.8. Identified Compounds from P. hysterophorus Leaf Extract
5.9. Details of the Phytotoxic Compounds
Details of the phytotoxic compounds (i.e., retention time, m/z, mass, polarity, synonyms, chemical formula and structure and biological activity with proper citations) of P. hysterophorus leaf with methanolic extracts through LC–MS analysis are available in Table 3.
5.10. Statistical Analysis
The data (germination percentage, root length, and shoot length) is transformed by the log transformation {log10 (x + 1)} system. The variance homogeneity was evaluated using Levene’s test. The data normality was analyzed using Shapiro–Wilk tests and after transformation, the data is assumed to be normally distributed. Two-way analysis of variance (ANOVA) was performed (two factors: concentrations and chemical compounds; fixed factor: weed species) using R-studio software to evaluate whether there was a significant difference between each treatment and the control, after that, the LSD test was used to separate the treatment and control means at 0.05 probability levels
Author Contributions
Conceptualization, A.S.J.; methodology, A.S.J., N.A., M.P.A. and M.S.A.-H.; validation, A.S.J., M.K.U., N.A., M.S.A.-H. and H.K.B.; formal analysis, H.K.B. and F.R.; investigation, H.K.B. and F.R.; resources, H.K.B. and A.S.J.; data curation, H.K.B.; writing—original draft preparation, H.K.B.; writing—review and editing, A.S.J., M.K.U., M.S.A.-H., M.P.A., M.A.H. and A.H.; visualization, H.K.B., S.R.K. and F.R.; supervision, A.S.J., M.S.A.-H., M.K.U. and N.A.; project administration, A.S.J. and H.K.B.; All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was given by the Ministry of Agriculture (MoA) of the People’s Republic of Bangladesh, Bangladesh Agricultural Research Council (NATP Phase-II Project, BARC), Bangladesh Agricultural Research Institute (BARI) (research grant: vote number 6282506), and the Universiti Putra Malaysia (UPM), Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
Many thanks to the Ministry of Agriculture (MoA) of the People’s Republic of Bangladesh, Bangladesh Agricultural Research Council (NATP Phase-II Project, BARC), Bangladesh Agricultural Research Institute (BARI) (research grant: vote number 6282506), for providing financial support and the Universiti Putra Malaysia (UPM) for logistical assistance. Many thanks also to Mohd Yunus Abd. Wahab and Azhar for their assistance in conducting the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kwinda, M. Weed Profiling Fields of Herbicide Tolerant Maize in the Mthatha Region, Eastern Cape Province. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2021; pp. 1–146. [Google Scholar]
- Chuah, T.S.; Lim, W.K. Combination Ratio Affects Synergistic Activity of Oil Palm Frond Residue and S-Metolachlor on Goosegrass (Eleusine indica). Pakistan J. Bot. 2021, 53, 1473–1477. [Google Scholar] [CrossRef]
- Chuah, T.S.; Lim, W.K. Assessment of Phytotoxic Potential of Oil Palm Leaflet, Rachis and Frond Extracts and Powders on Goosegrass (Eleusine indica (L.) Gaertn) Germination, Emergence and Seedling Growth. Malaysian Appl. Biol. 2015, 44, 75–84. [Google Scholar]
- Chauhan, B.S.; Johnson, D.E. Germination Ecology of Goosegrass (Eleusine indica): An Important Grass Weed of Rainfed Rice. Weed Sci. 2008, 56, 699–706. [Google Scholar] [CrossRef]
- Shrestha, A.; Anwar, M.P.; Islam, A.; Gurung, T.; Dhakal, S.; Tanveer, A.; Javaid, M.M.; Nadeem, M.; Ikram, N.A. Weed Science as a New Discipline and Its Status in Some South Asian Universities and Colleges: Examples from Bangladesh, Bhutan, Nepal and Pakistan. CAB Rev. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Sunohara, Y.; Shirai, S.; Wongkantrakorn, N.; Matsumoto, H. Sensitivity and Physiological Responses of Eleusine indica and Digitaria adscendens to Herbicide Quinclorac and 2,4-D. Environ. Exp. Bot. 2010, 68, 157–164. [Google Scholar] [CrossRef]
- Khaket, T.P.; Aggarwal, H.; Jodha, D.; Dhanda, S.; Singh, J. Parthenium hysterophorus in current scenario: A toxic weed with industrial, agricultural and medicinal applications. J. Plant Sci. 2015, 10, 42–53. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Bioherbicidal Properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata Extracts on Selected Crop and Weed Species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Ambika, S.R. Multifaceted Attributes of Allelochemicals and Mechanism of Allelopathy. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 389–405. [Google Scholar]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Hashemi, F.S.G.; Alam, M.A.; Hakim, M.A.; Uddin, M.K. Effects of Tinospora tuberculata Leaf Methanol Extract on Seedling Growth of Rice and Associated Weed Species in Hydroponic Culture. J. Integr. Agric. 2016, 15, 1521–1531. [Google Scholar] [CrossRef]
- Hao, W.; Ren, L.; Ran, W.; Shen, Q. Allelopathic Effects of Root Exudates from Watermelon and Rice Plants on Fusarium oxysporum f. Sp. Niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K.; Singh, I. Residue Allelopathy in Parthenium hysterophorus L.-Does Parthenin Play a Leading Role? Crop. Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Holt, J.S.; Welles, S.R.; Silvera, K.; Heap, I.M.; Heredia, S.M.; Martinez-Berdeja, A.; Palenscar, K.T.; Sweet, L.C.; Ellstrand, N.C. Taxonomic and Life History Bias in Herbicide Resistant Weeds: Implications for Deployment of Resistant Crops. PLoS ONE 2013, 8, 71916. [Google Scholar] [CrossRef] [PubMed]
- Dilipkumar, M.; Chuah, T.S.; Goh, S.S.; Sahid, I. Weed Management Issues, Challenges, and Opportunities in Malaysia. Crop. Prot. 2020, 134, 104347. [Google Scholar] [CrossRef]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Kaur, M.; Yadav, A. Utility Potential of Parthenium hysterophorus for Its Strategic Management. Adv. Agric. 2014, 381859, 381859. [Google Scholar] [CrossRef]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-noguchi, H. Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal Potential of the Essential Oils from Mediterranean lamiaceae for Weed Control in Organic Farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global Trends in Pesticides: A Looming Threat and Viable Alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Thien, B.N.; Ba, V.N.; Man, M.T.; Loan, T.T.H. Analysis of the Soil to Food Crops Transfer Factor and Risk Assessment of Multi-Elements at the Suburban Area of Ho Chi Minh City, Vietnam Using Instrumental Neutron Activation Analysis (INAA). J. Environ. Manag. 2021, 291, 112637. [Google Scholar] [CrossRef]
- Patel, S. Harmful and beneficial aspects of Parthenium hysterophorus: An update. 3 Biotech 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, J.; Yan, X. Encyclopedia of Traditional Chinese Medicines: Molecular Structures, Pharmacological Activities, Natural Sources and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 3642167381. [Google Scholar]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A Systematic Review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Khan, A.A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Phytochemical Investigation, Antioxidant Properties and In Vivo Evaluation of the Toxic Effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef] [PubMed]
- NTSYS-pc, N.T.; Taxonomy, N. Multivariate Analysis System; Version 2.2; Exet. Softw.: Setauket, NY, USA, 2005. [Google Scholar]
- Tarinezhad, A.; Sabouri, A.; Mohammadi, S.A. Statistical Software NTSYS PC Application in Plant Breeding. The 7th Conference of Iran Statistics. Allame Tabatabaei University, Tehran, Iran, September 2005. Available online: http://irstat.ir/files/site1/files/conference/7thconference_(English).pdf (accessed on 24 June 2022).
- Parsons, W.T.; Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Canberra, Australia; Inkata Press: Melbourne, Australia, 2001; ISBN 0643065148. [Google Scholar]
- Petersen, J.; Belz, R.; Walker, F.; Hurle, K. Weed Suppression by Release of Isothiocyanates from Turnip-rape Mulch. Agron. J. 2001, 93, 37–43. [Google Scholar] [CrossRef]
- Bezuneh, T.T. Phytochemistry and antimicrobial activity of Parthenium hysterophorus L.: A review. Sci. J. Anal. Chem. 2015, 3, 30–38. [Google Scholar] [CrossRef]
- Pandey, R.A.; Gole, A.R.; Sankpal, R.V.; Jadav, P.V.; Waghmode, M.S.; Patil, N.N. Bioactive Potential of Parthenium hysterophorus and Cytotoxicity Assay of Parthenin. Int. J. Pharm. Biol. Sci. 2019, 9, 296–313. [Google Scholar]
- Marwat, S.K.; Fazal-ur-Rehman; Khan, I.U. Ethnobotanical Importance and Phytochemical Constituents of Parthenium Weed (Parthenium hysterophorus L.)—A Review. Plant Sci. Today 2015, 2, 77–81. [Google Scholar] [CrossRef]
- Roy, D.C.; Shaik, M. Journal of Medicinal Plants Studies Toxicology, Phytochemistry, Bioactive Compounds and Pharmacology of Parthenium hysterophorus. J. Med. Plants Stud. 2013, 1, 126–141. [Google Scholar]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic Effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus Essential Oils in Weeds of Mediterranean Summer Crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Javaid, A.; Anjum, T. Control of Parthenium hysterophorus L., by Aqueous Extracts of Allelopathic Grasses. Pakistan J. Bot. 2006, 38, 139. [Google Scholar]
- Verma, A.K.; Maurya, S.K.; Kumar, A.; Barik, M.; Yadav, V.; Umar, B.; Lawal, M.; Usman, Z.A.; Adam, M.A.; Awal, B. Inhibition of Multidrug Resistance Property of Candida Albicans by Natural Compounds of Parthenium hysterophorus L. An In-Silico Approach. J. Pharmacogn. Phytochem. 2020, 9, 55–64. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Asao, T. Autotoxicity in Strawberry under Recycled Hydroponics and Its Mitigation Methods. Hortic. J. 2020, 89, 124–137. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic Acid Affects Early Growth, and Morphogenetic Response of Hypocotyl Cuttings of Mung Bean (Phaseolus aureus). J. Plant Physiol. 2008, 165, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic Effects of Volatile Oil from Artemisia scoparia against Weeds and Its Possible Use as a Bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical Profiling, Cytotoxicity and Phytotoxicity of Foliar Volatiles of Hyptis suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic Effects of 21 Plant Secondary Metabolites on Arabidopsis thaliana Germination and Root Growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. [Google Scholar] [CrossRef]
- Guo, Y.; Kim, K.-U.; Yoder, J.I.; Shin, D. Parasitic Plants as a New Target Plant for Screening Rice Allelopathic Potential. J. Life Sci. 2011, 5, 201. [Google Scholar]
- Rasouli, H.; Farzaei, M.H.; Mansouri, K.; Mohammadzadeh, S.; Khodarahmi, R. Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules 2016, 21, 1104. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence. In How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. ISBN 0065-2296. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z.A. Allelopathy in Agro-Ecosystems: A Critical Review of Wheat Allelopathy-Concepts and Implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N Fertilization on the Content of Phenolic Compounds in Jerusalem Artichoke (Helianthus tuberosus L.) Tubers and Their Antioxidant Capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin—A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Tanveer, A.; Khaliq, A.; Safdar, M.E.; Nadeem, M.A. Allelopathic Effects of Aquatic Weeds on Germination and Seedling Growth of Wheat. Herbologia 2014, 14, 11–25. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Esposito, A.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; Tsafantakis, N.; Monaco, P.; Fiorentino, A. Isolation, Distribution and Allelopathic Effect of Caffeic Acid Derivatives from Bellis perennis L. Biochem. Syst. Ecol. 2012, 43, 108–113. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Pacifico, S.; Monaco, P.; Fiorentino, A. Plant Growth Inhibitors: Allelopathic Role or Phytotoxic Effects? Focus on Mediterranean Biomes. Phytochem. Rev. 2013, 12, 803–830. [Google Scholar] [CrossRef]
- Safdar, M.E.; Aslam, A.; Qamar, R.; Ali, A.; Javaid, M.M.; Hayyat, M.S.; Raza, A. Allelopathic Effect of Prickly Chaff Flower (Achyranthes aspera L.) Used as a Tool for Managing Noxious Weeds. Asian J. Agric. Biol. 2021, 2021. [Google Scholar] [CrossRef]
- Ahn, J.K.; Chung, I.M. Allelopathic Potential of Rice Hulls on Germination and Seedling Growth of Barnyardgrass. Agron. J. 2000, 92, 1162–1167. [Google Scholar] [CrossRef]
- Salam, M.A.; Kato-Noguchi, H. Allelopathic potential of methanol extract of Bangladesh rice seedlings. Asian J. Crop Sci. 2010, 2, 70–77. [Google Scholar] [CrossRef]
- Mao, R.; Shabbir, A.; Adkins, S. Parthenium hysterophorus: A Tale of Global Invasion over Two Centuries, Spread and Prevention Measures. J. Environ. Manag. 2021, 279, 111751. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Omar, D.; Alam, M.A.; Hashemi, F.S.G.; Hakim, M.A.; Uddin, M.K. Allelopathic Effect of Methanol Extracts from Tinospora Tuberculata on Selected Crops and Rice Weeds. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 165–177. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).