FfCOX17 is Involved in Fumonisins Production, Growth, Asexual Reproduction, and Fungicide Sensitivity in Fusarium fujikuroi
Abstract
:1. Introduction
2. Results
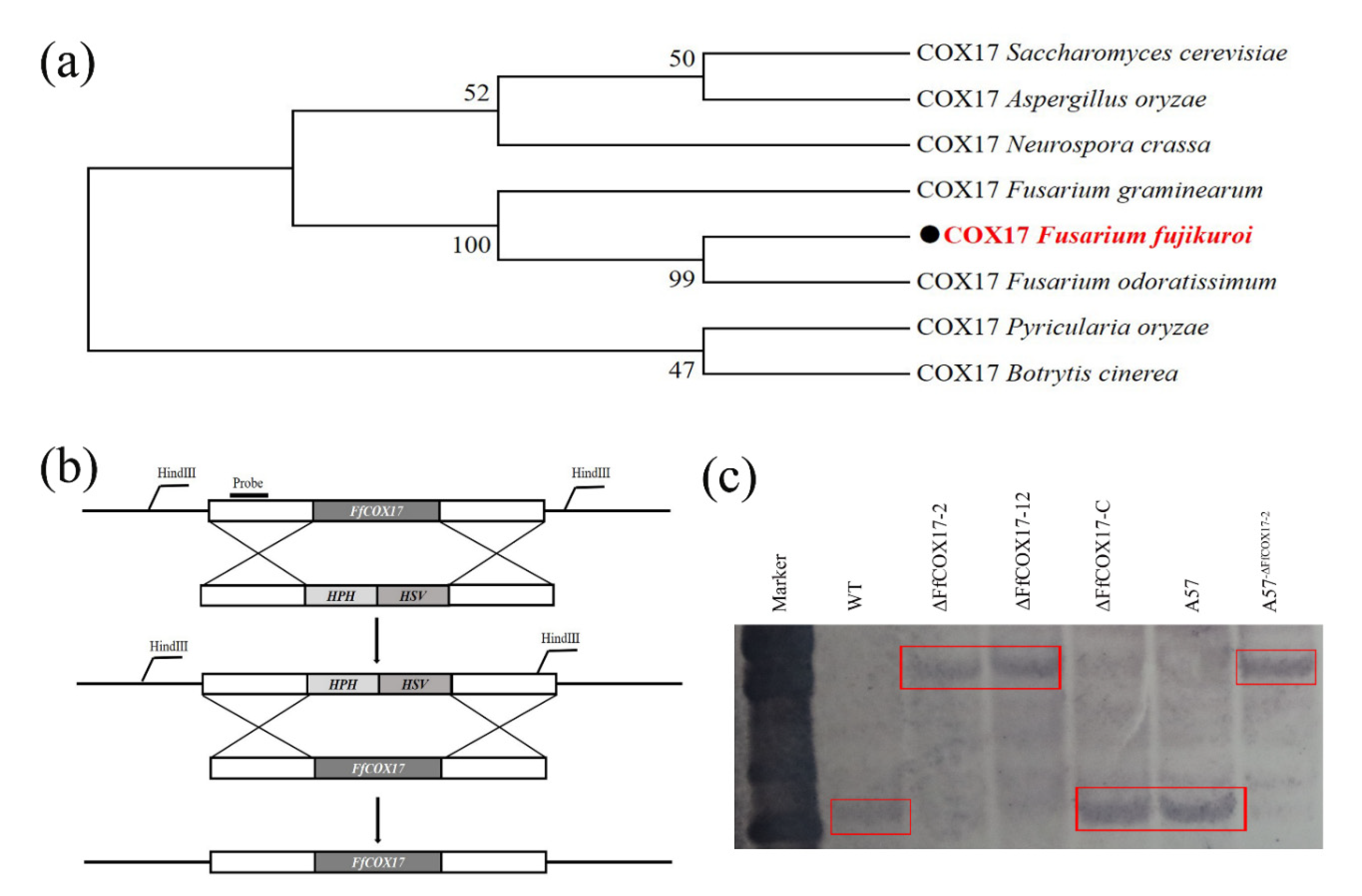
2.1. Identification, Deletion, and Complementation of FfCOX17
2.2. FfCOX17 Is Involved in Vegetative Growth and Asexual Reproduction
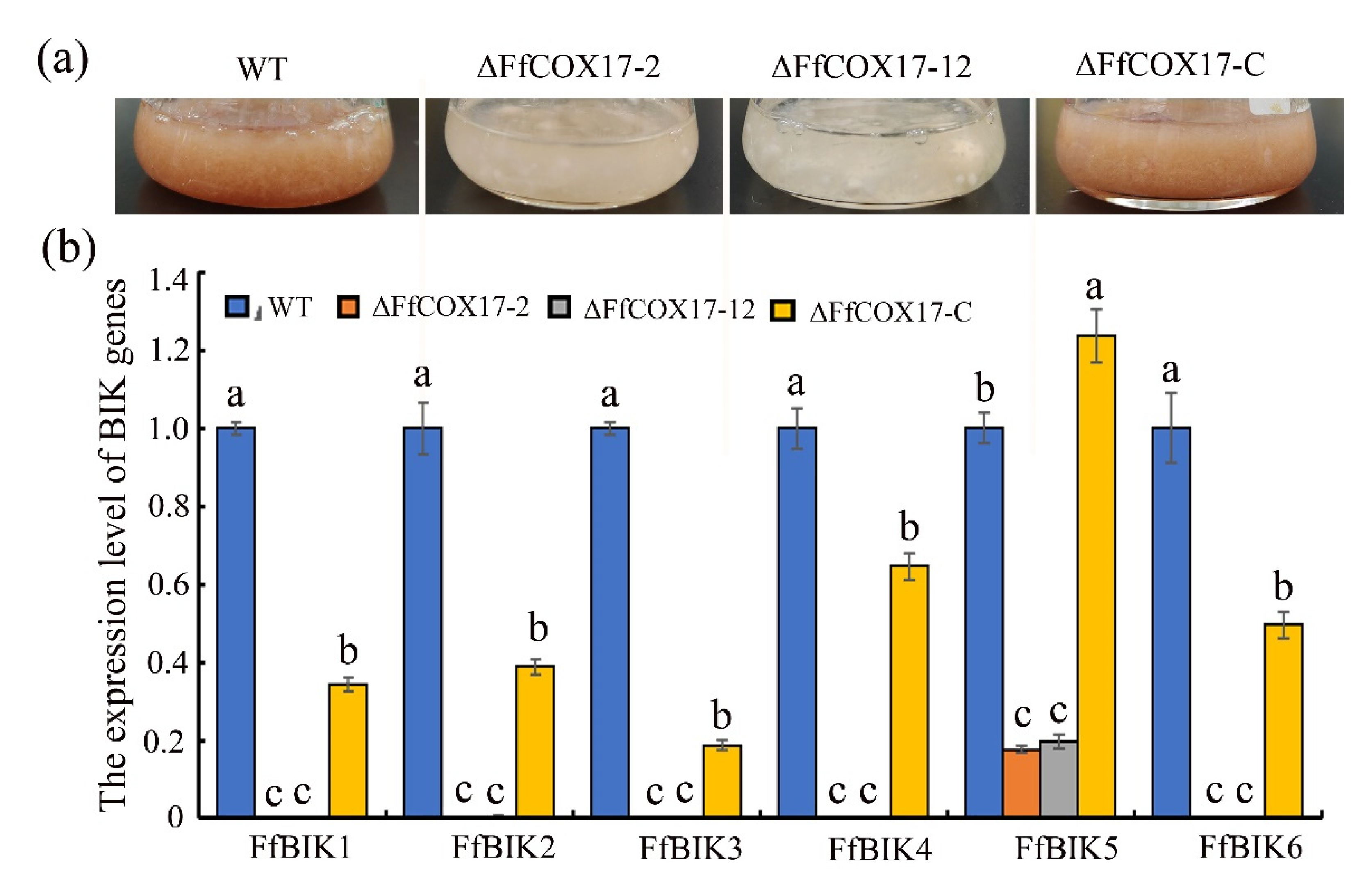
2.3. FfCOX17 Regulates the Expression Level of the BIK Synthesis-Related Genes
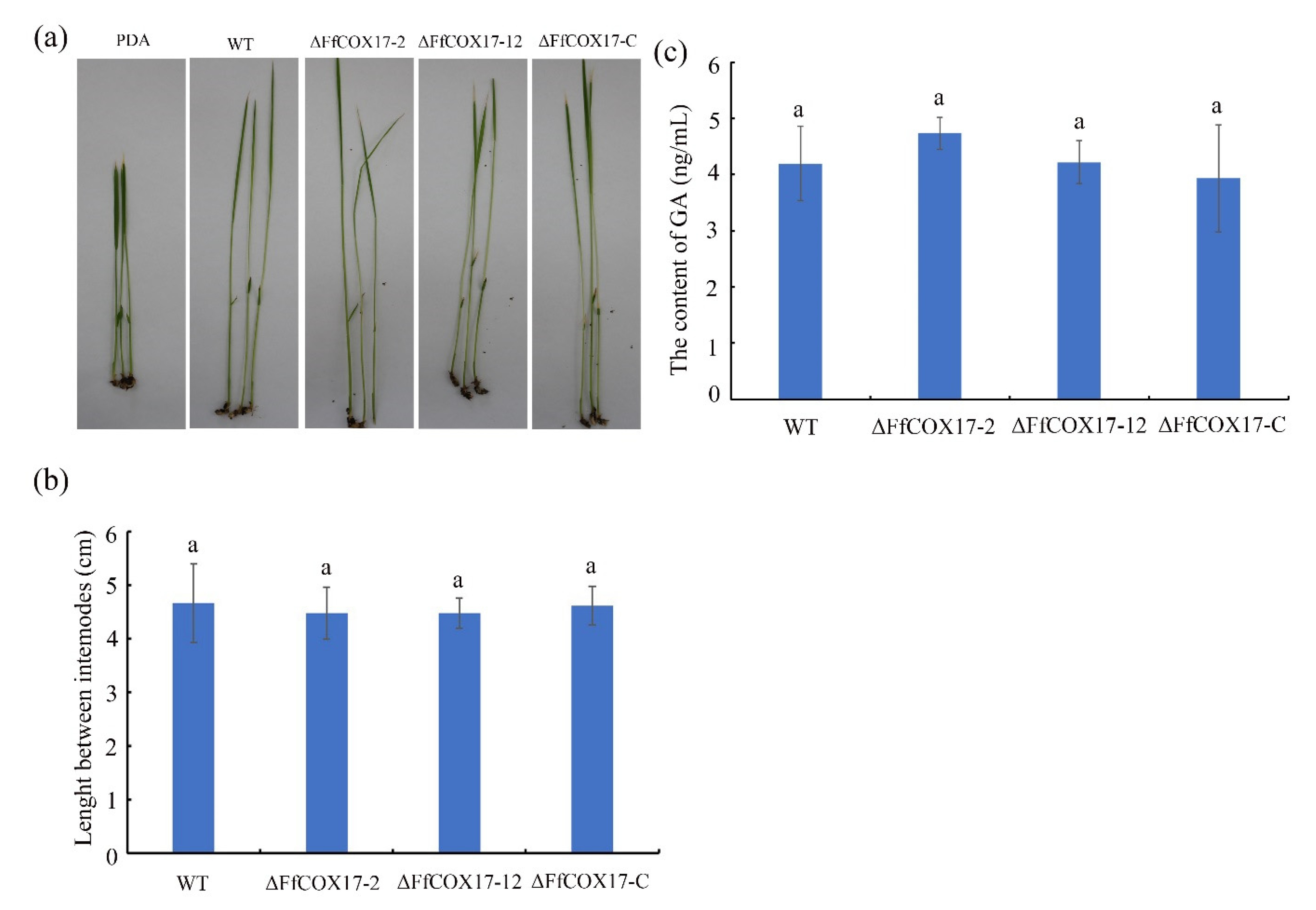
2.4. FfCOX17 Negatively Regulates FUM Biosynthesis in F. fujikuroi
2.5. FfCOX17 Is Not Required for Pathogenicity
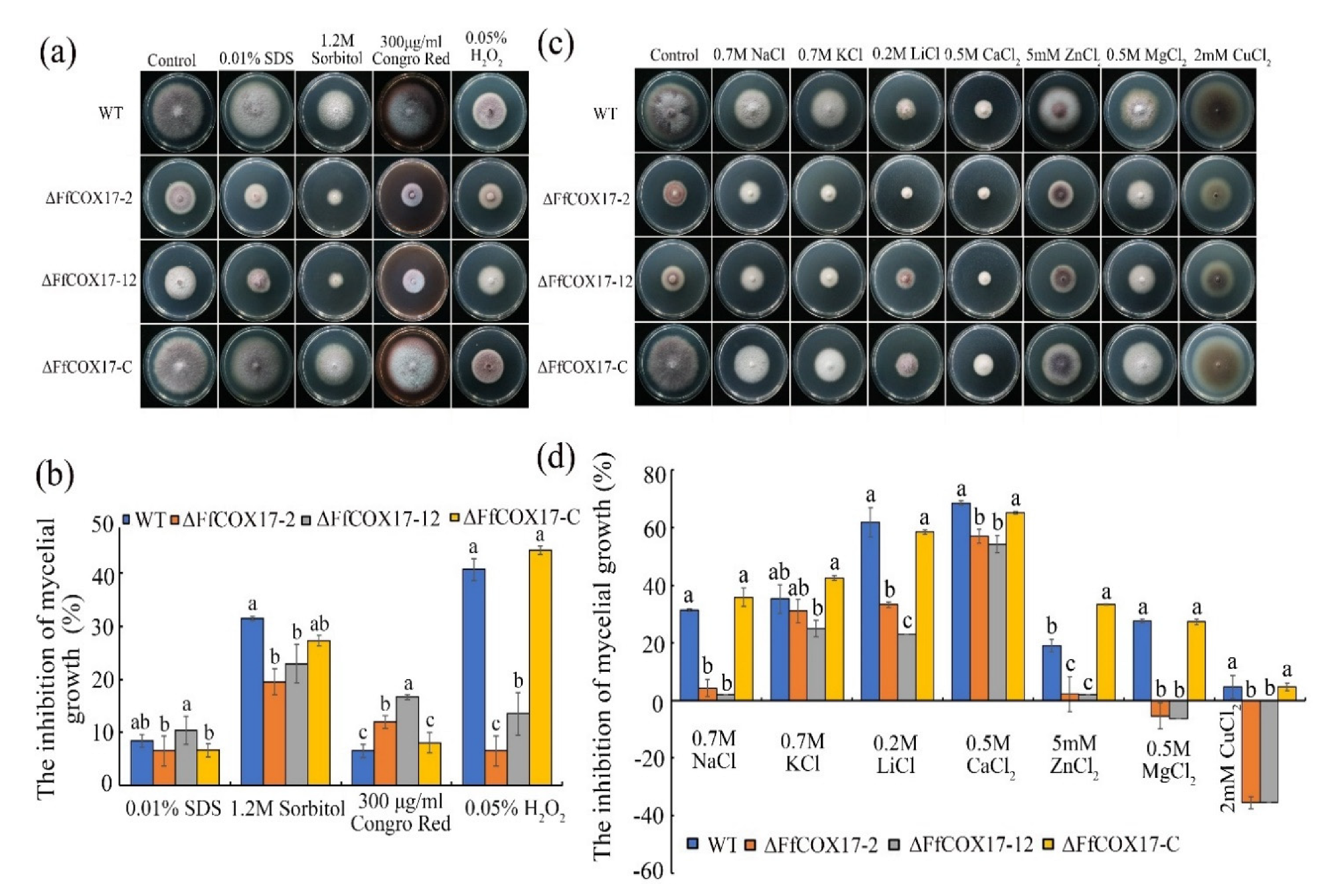
2.6. Sensitivity of the ∆FfCOX17 to Different Stresses
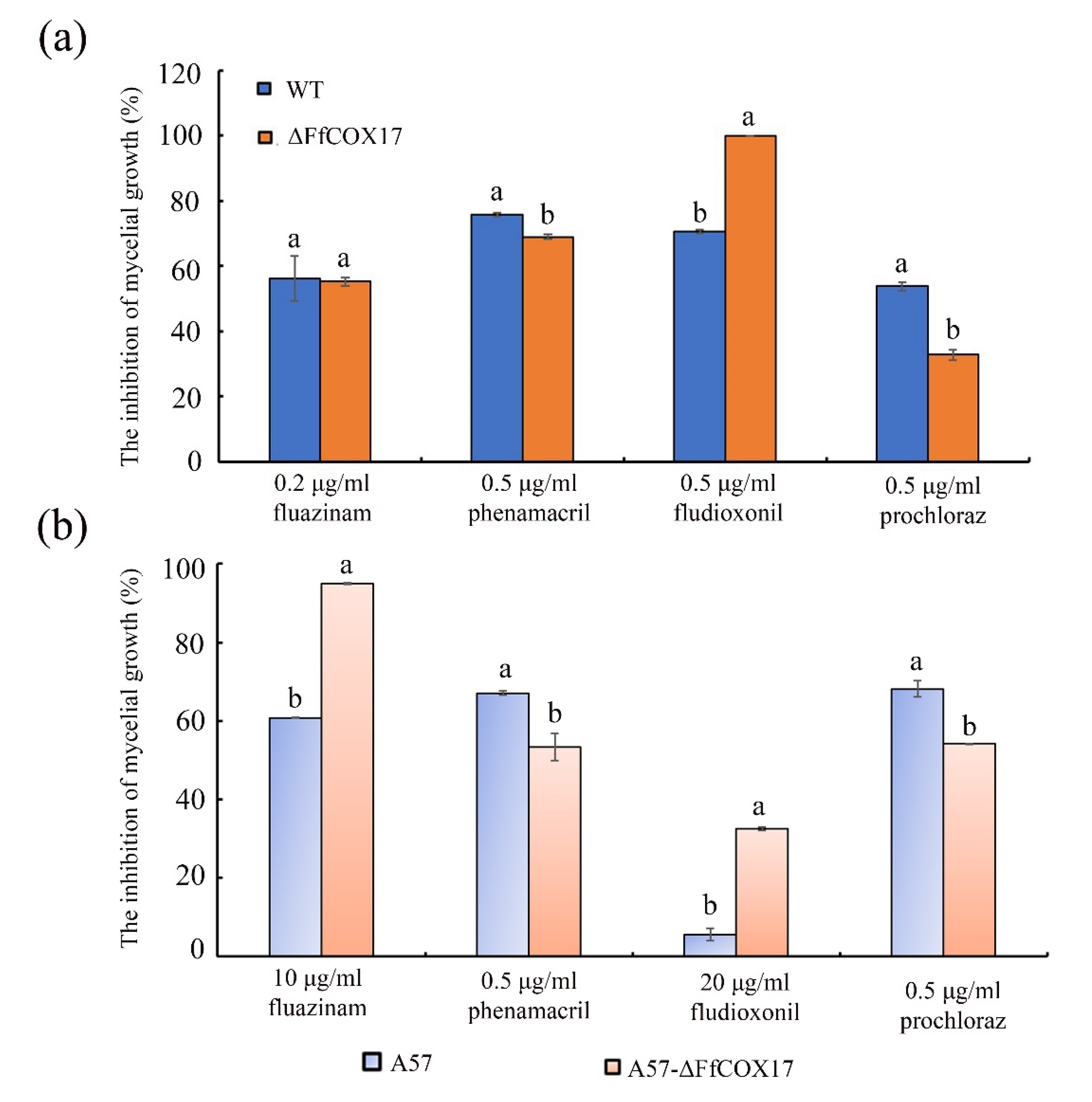
2.7. FfCOX17 Regulates the Sensitivity to Different Fungicides
2.8. Subcellular Localization of GFP-FfCOX17 Fusion Protein
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains, Media, and Culture Conditions
5.2. Construction of Vectors, Fungal Transformation and Generation of Gene Deletion
5.3. Test for Vegetative Growth and Asexual Reproduction
5.4. Pathogenicity Assays
5.5. FUM and GA Content Assay
5.6. Quantitative RT-PCR (qPCR)
5.7. Sensitivity of the ΔFfCOX17 Mutants to Different Stress
5.8. Determination of the Sensitivity of F. fujikuroi to Different Fungicides
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Janevska, S.; Güldener, U.; Sulyok, M.; Tudzynski, B.; Studt, L. Set1 and Kdm5 are antagonists for H3K4 methylation and regulators of the major conidiation-specific transcription factor gene ABA1 in Fusarium fujikuroi. Environ. Microbiol. 2018, 20, 3343–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Mao, C.X.; Zhai, X.Y.; Jamieson, P.A.; Zhang, C. Mutation in cyp51b and overexpression of cyp51a and cyp51b confer multiple resistant to DMIs fungicide prochloraz in Fusarium fujikuroi. Pest Manag. Sci. 2021, 77, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Matic, S.; Gullino, M.L.; Spadaro, D. The puzzle of bakanae disease through interactions between Fusarium fujikuroi and rice. Front. Biosci. 2017, 9, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.X.; Inagaki, S.; Tatebayashi, S.; Sultana, S.; Shimizu, M.; Kageyama, K.; Suga, H. Expression difference of P450-1 and P450-4 between G- and F-groups of Fusarium fujikuroi. Eur. J. Plant Pathol. 2021, 159, 27–36. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Brown, D.W.; Seo, J.A.; Lee, Y.W. Discontinuous Distribution of the Fumonisin Biosynthetic Gene Cluster in Fusarium. Mycol. Res. 2004, 108, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, P.; Brown, D.W.; Kleigrewe, K.; Jin, W.B.; Keller, N.P.; Humpf, H.U.; Tudzynski, B. FfVel1 and FfLae1, components of a velvet-like complex in Fusarium fujikuroi, affect differentiation, secondary metabolism and virulence. Mol. Microbiol. 2010, 77, 972–994. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Wu, H.X.; Gu, Q.; Tang, G.F.; Liu, W.D. Glycosyltransferase FvCpsA Regulates Fumonisin Biosynthesis and Virulence in Fusarium verticillioides. Toxins 2021, 13, 718. [Google Scholar] [CrossRef]
- Myung, K.; Li, S.J.; Butchko, R.A.E.; Busman, M.; Proctor, R.H.; Abbas, H.K.; Calvo, A.M. FvVE1 Regulates Biosynthesis of Fumonisins and Fusarins in Fusarium verticillioides. J. Agric. Food Chem. 2009, 57, 5089–5094. [Google Scholar] [CrossRef] [Green Version]
- Gelderblom, W.C.A.; Jaskiewicz, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, M.J.; Vleggaar, R.; Kriek, N.P.J. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.; Sullards, M.C.; Allegood, J.; Wang, E.; Schmelz, E.M.; Hartl, M.; Humpf, H.U.; Liotta, D.C.; Peng, Q.; Merrill, A.H., Jr. Fumonisins and fumonisin analogs as inhibitors of ceramide synthase and inducers of apoptosis. Biochim. Biophys. Acta 2002, 1585, 188–192. [Google Scholar] [CrossRef]
- Sultana, S.; Kitajim, M.; Kobayash, H.; Nakagawa, H.; Shimizu, M.; Kageyama, K.; Suga, H. A Natural Variation of Fumonisin Gene Cluster Associated with Fumonisin Production Difference in Fusarium fujikuroi. Toxins 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woloshuk, C.P.; Shim, W.B. Aflatoxins, fumonisins, and trichothecenes: A convergence of knowledge. FEMS Microbiol. Rev. 2013, 37, 94–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fanelli, F.; Iversen, A.; Logrieco, A.F.; Mulè, G. Relationship between fumonisin production and FUM gene expression in Fusarium verticillioides under different environmental conditions. Food Addit. Contam. 2013, 30, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Choi, Y.E.; Zou, X.; Xu, J.R. The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis, and fumonisin production in Fusarium verticillioides. Fungal Genet. Biol. 2011, 48, 71–79. [Google Scholar] [CrossRef]
- Wu, Y.; Li, T.T.; Gong, L.; Wang, Y.; Jiang, Y.M. Effects of Different Carbon Sources on Fumonisin Production and FUM Gene Expression by Fusarium proliferatum. Toxins 2019, 11, 289. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Ji, T.T.; Sun, X.; Huang, H.; Zhang, H.; Lu, X.; Wu, L.; Huo, R.; Wu, H.; Gao, X.W. Histone H3 lysine 9 methyltransferase FvDim5 regulates fungal development, pathogenicity and osmotic stress responses in Fusarium verticillioides. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [Green Version]
- Punter, F.A.; Glerum, D. Mutagenesis reveals a specific role for Cox17p in copper transport to cytochrome oxidase. J. Biol. Chem. 2003, 278, 30875–30880. [Google Scholar] [CrossRef] [Green Version]
- Babcock, G.T.; Wikström, M.J.N. Oxygen activation and the conservation of energy in cell respiration. Nature 1992, 356, 301–309. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kako, K.; Kashiwabara, S.; Takehara, A.; Inada, Y.; Arai, H.; Nakada, K.; Kodama, H.; Hayashi, J.; Baba, T. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol. Cell. Biol. 2002, 22, 7614–7621. [Google Scholar] [CrossRef] [Green Version]
- Shoubridge, E. Cytochrome c oxidase deficiency. Am. J. Med. Genet. 2001, 106, 46–52. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Hadjiloi, T.; Martinelli, M.; Palumaa, P. Mitochondrial copper (I) transfer from Cox17 to Sco1 is coupled to electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6803–6808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, L.; Welchen, E.; Gey, U.; Arce, A.L.; Steinebrunner, I.; Gonzalez, D.H. The cytochrome c oxidase biogenesis factor AtCOX17 modulates stress responses in Arabidopsis. Plant Cell Environ. 2016, 39, 628–644. [Google Scholar] [CrossRef] [PubMed]
- Balandin, T.; Castresana, C. AtCOX17, an Arabidopsis homolog of the yeast copper chaperone COX17. Plant Physiol. 2002, 129, 1852–1857. [Google Scholar] [CrossRef] [Green Version]
- Anabosia, D.; Meira, Z.; Shadkchana, Y.; Handelmana, M.; Abou-Kandila, A.; Yapb, A.; Urlingsa, D.; Golda, M.S.; Krappmannc, S.; Haasb, H.; et al. Transcriptional response of Aspergillus fumigatus to copper and the role of the Cu chaperones. Virulence 2021, 12, 2186–2200. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.C.; Winge, D.R.; Cobine, P. “Pulling the plug” on cellular copper: The role of mitochondria in copper export. Biochim. Biophys. Acta 2009, 1793, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronova, A.; Meyer-Klaucke, W.; Meyer, T.; Rompel, A.; Krebs, B.; Kazantseva, J.; Sillard, R.; Palumaa, P. Oxidative switches in functioning of mammalian copper chaperone Cox17. Biochem. J. 2007, 408, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Maxfield, A.B.; Heaton, D.N.; Winge, D. Cox17 is functional when tethered to the mitochondrial inner membrane. J. Biol. Chem. 2004, 279, 5072–5080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiemann, P.; Willmann, A.; Straeten, M.; Kleigrewe, K.; Beyer, M.; Humpf, H.U.; Tudzynski, B. Biosynthesis of the red pigment bikaverin in Fusarium fujikuroi: Genes, their function and regulation. Mol. Microbiol. 2009, 72, 931–946. [Google Scholar] [CrossRef]
- Michielse, C.B.; Studt, L.; Janevska, S.; Sieber, C.M.; Arndt, B.; Espino, J.J.; Humpf, H.U.; Güldener, U.; Tudzynski, B. The global regulator FfSge 1 is required for expression of secondary metabolite gene clusters but not for pathogenicity in Fusarium fujikuroi. Environ. Microbiol. 2015, 17, 2690–2708. [Google Scholar] [CrossRef]
- Brakhage, A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Niehaus, E.M.; Rindermann, L.; Janevska, S.; Münsterkötter, M.; Güldener, U.; Tudzynski, B. biotechnology: Analysis of the global regulator Lae1 uncovers a connection between Lae1 and the histone acetyltransferase HAT1 in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2018, 102, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Huang, J.; Zhang, H.; Shim, W.B. A Rab GTPase protein FvSec4 is necessary for fumonisin B1 biosynthesis and virulence in Fusarium verticillioides. Curr. Genet. 2019, 66, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Palumaa, P. Copper chaperones. The concept of conformational control in the metabolism of copper. FEBS Lett. 2013, 587, 1902–1910. [Google Scholar] [CrossRef] [Green Version]
- Palumaa, P.; Kangur, L.; Voronova, A.; Sillard, R. Mechanism of metal binding by Cox17, a copper chaperone for cytochrome c oxidase. Biochem. J. 2004, 382, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnesano, F.; Balatri, E.; Banci, L.; Bertini, I.; Winge, D.R. Folding studies of Cox17 reveal an important interplay of cysteine oxidation and copper binding. Structure 2005, 13, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.Y.; Ren, W.C.; Zhang, Y.; Hou, Y.P.; Duan, Y.B.; Wang, J.X.; Zhou, M.G.; Chen, C.J. Baseline sensitivity of natural pop-ulations and characterization of resistant strains of Botrytis cinerea to fluazinam. Australas. Plant Pathol. 2015, 44, 375–383. [Google Scholar] [CrossRef]
- Mao, X.W.; Li, J.S.; Chen, Y.L.; Song, X.S.; Duan, Y.B.; Wang, J.X.; Chen, C.J.; Zhou, M.G.; Hou, Y.P. Resistance risk assessment for fluazinam in Sclerotinia sclerotiorum. Pestic. Biochem. Physiol. 2018, 144, 27–35. [Google Scholar] [CrossRef]
- Ochiai, N.; Fujimura, M.; Oshima, M.; Motoyama, T.; Ichiishi, A.; Yamada-Okabe, H.; Yamaguchi, I. Effects of iprodione and fludioxonil on glycerol synthesis and hyphal development in Candida albicans. Biosci. Biotechnol. Biochem. 2002, 66, 2209–2215. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.T.; Gao, T.; Zhang, Y.; Hou, Y.P.; Wang, J.X.; Zhou, M.G. FgFim, a key protein regulating resistance to the fungicide JS 399-19, asexual and sexual development, stress responses and virulence in Fusarium graminearum. Mol. Plant Pathol. 2014, 15, 488–499. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhang, Y.S.; Liu, N.; Ren, W.C.; Hou, Y.P.; Duan, Y.B.; Song, X.S.; Zhou, M.G. The Dis1/Stu2/XMAP215 Family Gene FgStu2 Is Involved in Vegetative Growth, Morphology, Sexual and Asexual Reproduction, Pathogenicity and DON Production of Fusarium graminearum. Front. Microbiol. 2020, 11, 545015. [Google Scholar] [CrossRef]
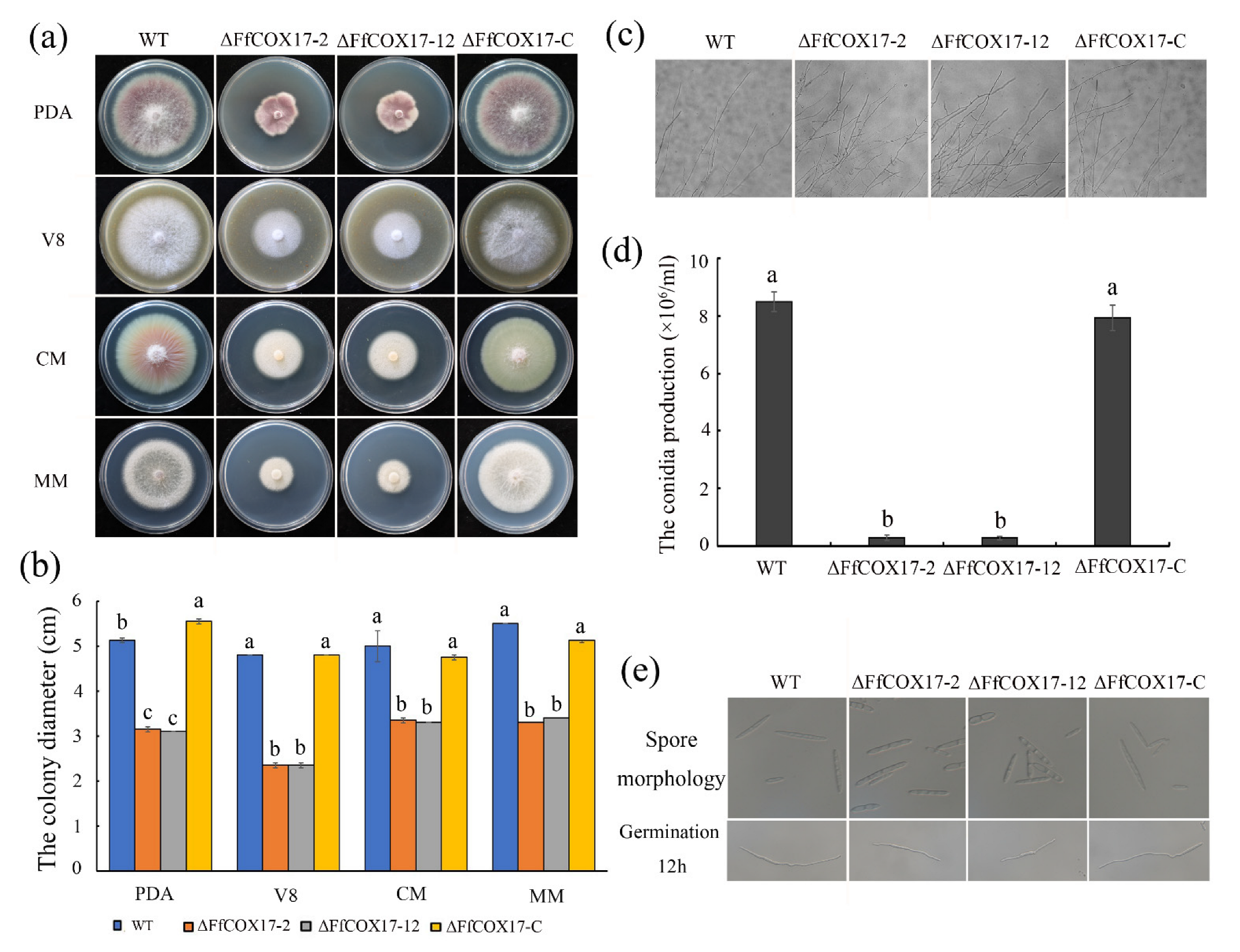



| Strain | Vegetative Growth (cm) | Conidiation (×106/mL) | Pathogenicity | |||
|---|---|---|---|---|---|---|
| PDA | V8 | CM | MM | Lesion Length (cm) | ||
| WT | 5.13 ± 0.05 b | 4.80 a | 5.0 ± 0.35 a | 5.50 a | 8.50 ± 0.35 a | 4.67 ± 0.73 a |
| ΔFfCOX17-2 | 3.15 ± 0.06 c | 2.35 ± 0.06 b | 3.35 ± 0.06 b | 3.30 b | 0.28 ± 0.10 b | 4.48 ± 0.48 a |
| ΔFfCOX17-12 | 3.10 c | 2.35 ± 0.06 b | 3.30 b | 3.40 b | 0.27 ± 0.06 b | 4.48 ± 0.28 a |
| ΔFfCOX17-C | 5.55 ± 0.06 a | 4.80 a | 4.75 ± 0.06 a | 5.13 ± 0.05 a | 7.94 ± 0.44 a | 4.61 ± 0.36 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Wu, Z.; Chen, F.; Zhou, M.; Hou, Y. FfCOX17 is Involved in Fumonisins Production, Growth, Asexual Reproduction, and Fungicide Sensitivity in Fusarium fujikuroi. Toxins 2022, 14, 427. https://doi.org/10.3390/toxins14070427
Mao X, Wu Z, Chen F, Zhou M, Hou Y. FfCOX17 is Involved in Fumonisins Production, Growth, Asexual Reproduction, and Fungicide Sensitivity in Fusarium fujikuroi. Toxins. 2022; 14(7):427. https://doi.org/10.3390/toxins14070427
Chicago/Turabian StyleMao, Xuewei, Zhiwen Wu, Furong Chen, Mingguo Zhou, and Yiping Hou. 2022. "FfCOX17 is Involved in Fumonisins Production, Growth, Asexual Reproduction, and Fungicide Sensitivity in Fusarium fujikuroi" Toxins 14, no. 7: 427. https://doi.org/10.3390/toxins14070427




