Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro
Abstract
:1. Introduction
2. Results
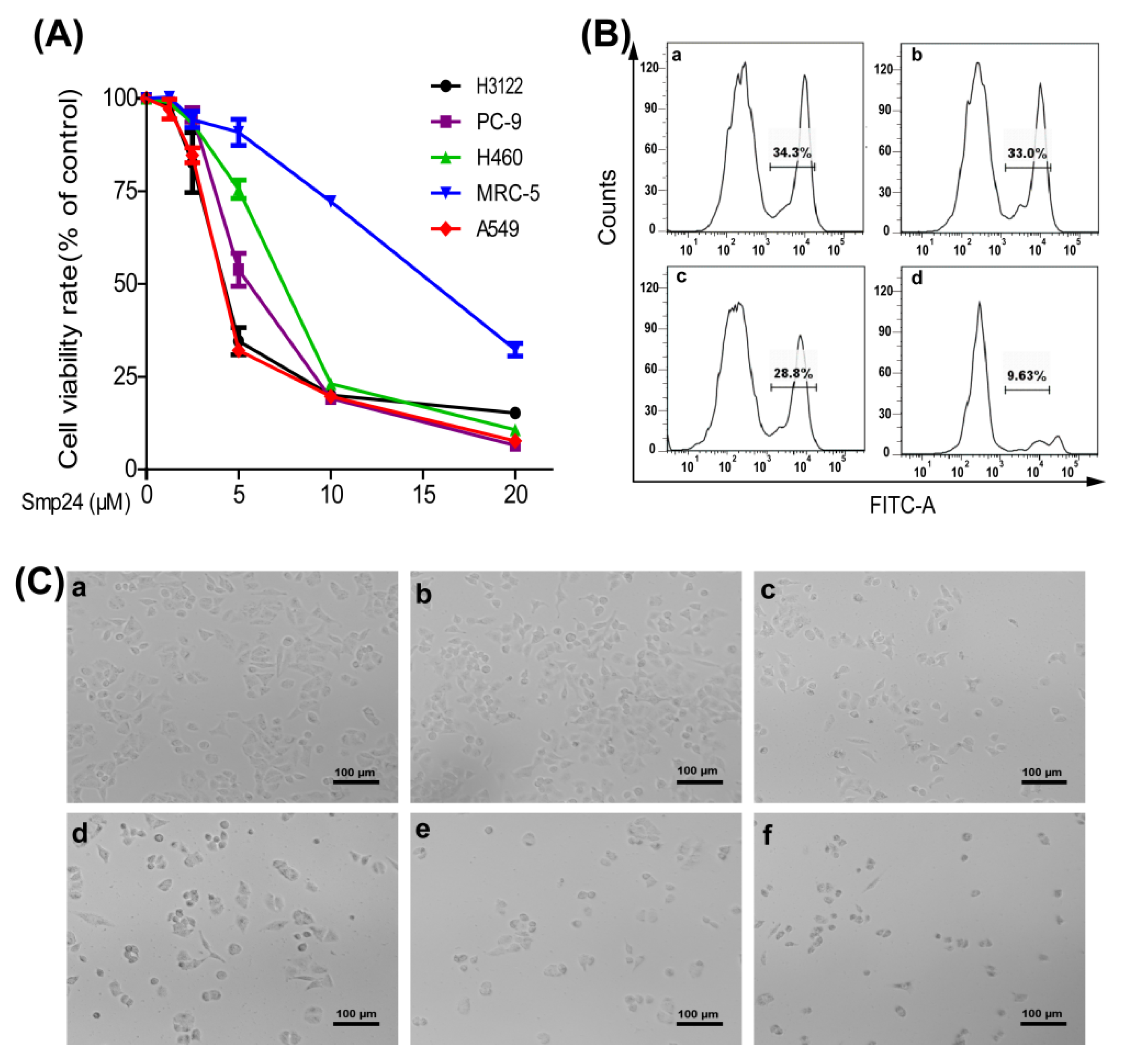
2.1. Smp24 Inhibits the Proliferation of Human Lung Cancer Cells
2.2. Smp24 Induces Necrosis of A549 Cells by Membrane Damage
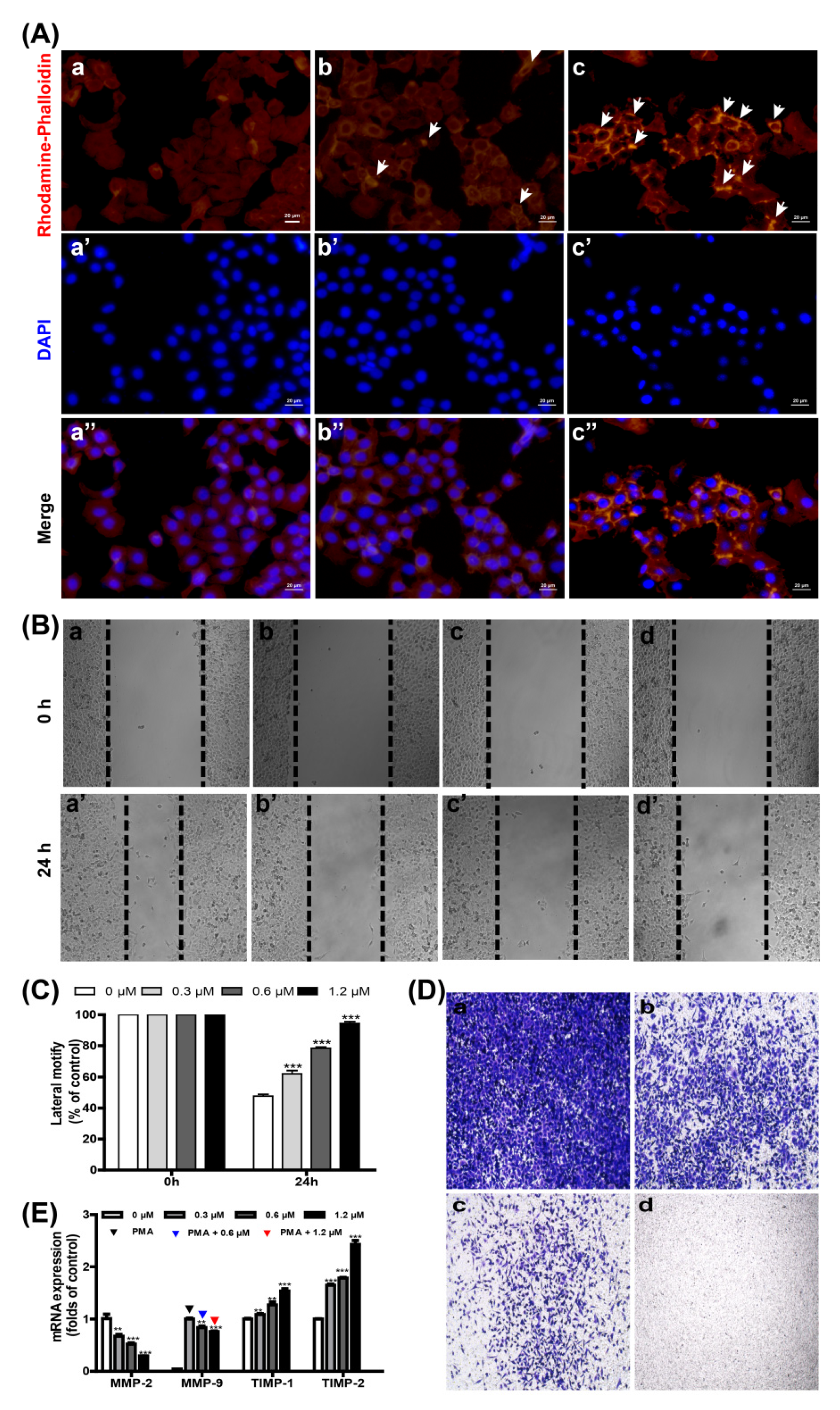
2.3. Smp24 Inhibits Motility by Damaging the Cytoskeleton and Altering MMP-2/-9 and TIMP-1/-2 Expression
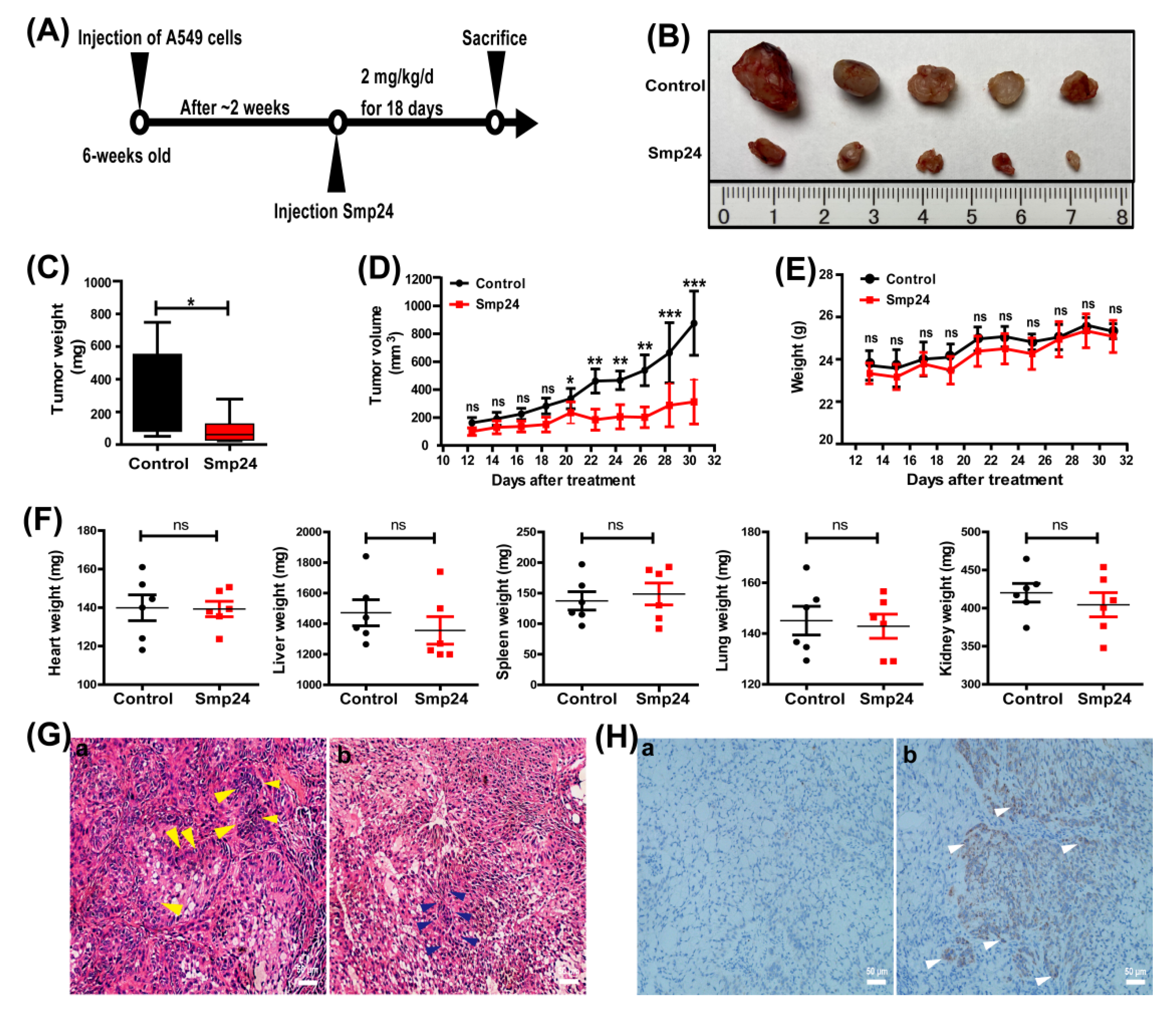
2.4. Smp24 Inhibits Tumor Growth in Mice
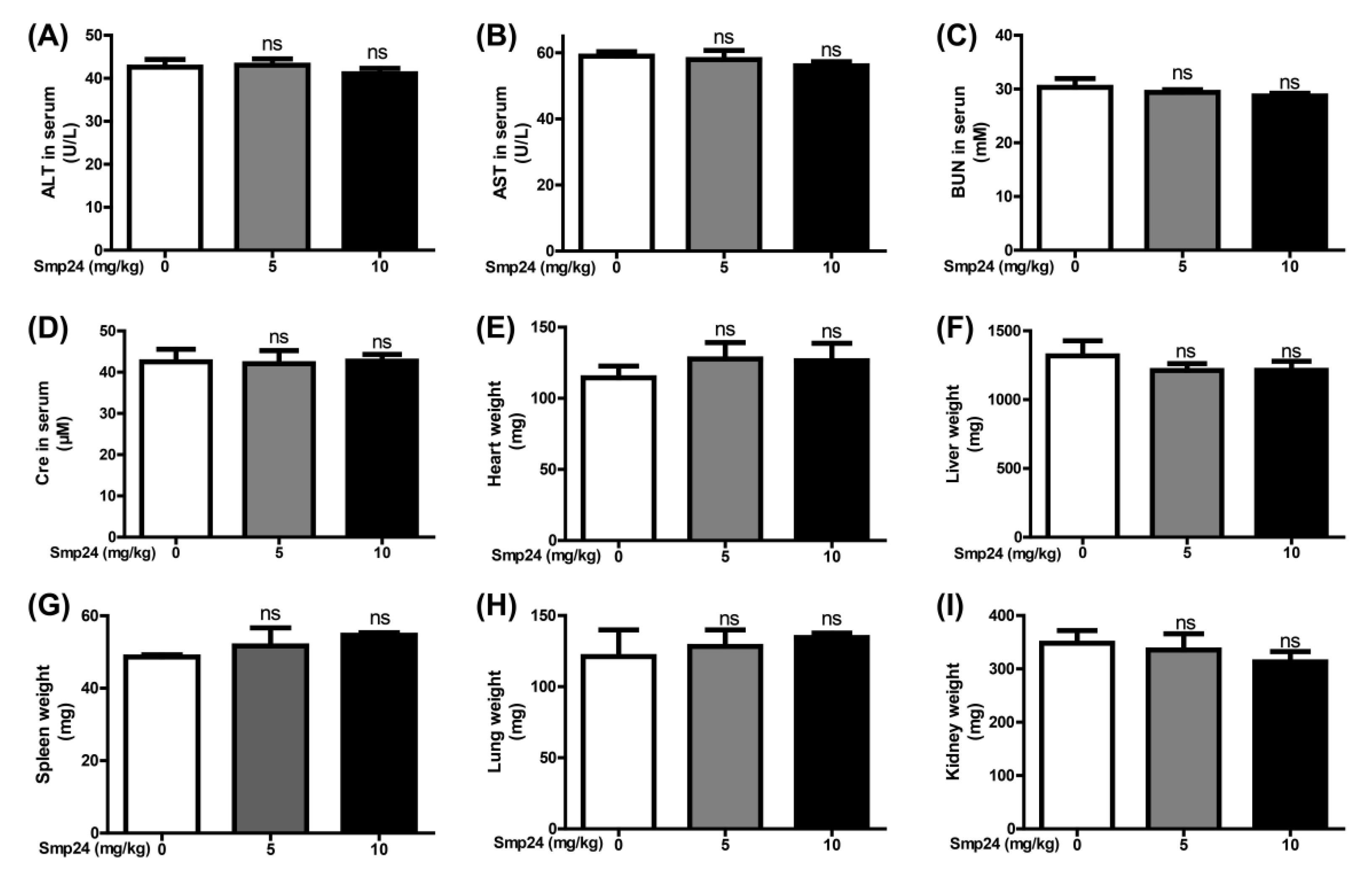
2.5. Smp24 Has No Hepatotoxicity and Nephrotoxicity in Acute Toxicity Doses
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Ethics Statement
5.2. Chemicals and Cell Culture
5.3. Cell Viability and Proliferation Assays
5.4. Membrane Integrity Assay
5.5. Cell Morphology Observation
5.6. LDH Release Assay
5.7. Scanning Electron Microscopy Analysis
5.8. Fluorescence Microscopy Analysis
5.9. Cell Motility Assay
5.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
5.11. In Vivo Antitumor Experiments
5.12. Acute Toxicity Analysis
5.13. Statistical Analysis
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Zervantonakis, I.K.; Kamm, R.D. Tumor cell migration in complex microenvironments. Cell Mol. Life Sci. 2013, 70, 1335–1356. [Google Scholar] [CrossRef] [Green Version]
- Khamessi, O.; Ben, M.H.; Elfessi-Magouri, R.; Kharrat, R. RK1, the first very short peptide from Buthus occitanus tunetanus inhibits tumor cell migration, proliferation and angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef]
- Raposo, C. Scorpion and spider venoms in cancer treatment: State of the art, challenges, and perspectives. J. Clini. Transl. Res. 2017, 3, 233–249. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Strong, P.N.; Tawfik, M.M.; Miller, K. Characterisation of three alpha-helical antimicrobial peptides from the venom of Scorpio maurus palmatus. Toxicon 2016, 117, 30–36. [Google Scholar] [CrossRef]
- Harrison, P.L.; Heath, G.R.; Johnson, B.; Abdel-Rahman, M.A.; Strong, P.N.; Evans, S.D.; Miller, K. Phospholipid dependent mechanism of smp24, an alpha-helical antimicrobial peptide from scorpion venom. Biochim. Biophys. Acta 2016, 1858, 2737–2744. [Google Scholar] [CrossRef] [Green Version]
- Elrayess, R.A.; Mohallal, M.E.; El-Shahat, Y.M.; Ebaid, H.M.; Miller, K.; Strong, P.N.; Abdel-Rahman, M.A. Cytotoxic effects of smp24 and smp43 scorpion venom antimicrobial peptides on tumour and non-tumour cell lines. Int. J. Pept. Res. Ther. 2020, 26, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Gefen, A.; Weihs, D. Mechanical cytoprotection: A review of cytoskeleton-protection approaches for cells. J. Biomech. 2016, 49, 1321–1329. [Google Scholar] [CrossRef]
- Gefen, A.; Weihs, D. Cytoskeleton and plasma-membrane damage resulting from exposure to sustained deformations: A review of the mechanobiology of chronic wounds. Med. Eng. Phys. 2016, 38, 828–833. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef]
- Svitkina, T. The actin cytoskeleton and Actin-Based motility. Cold Spring Harb. Perspect. Biol. 2018, 10, a18267. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, J.Z.; Lin, Z.X.; Yuan, Q.J.; Li, Y.C.; Liang, J.L.; Zhan, J.Y.; Xie, Y.L.; Su, Z.R.; Liu, Y.H. Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnen against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2019, 234, 44–56. [Google Scholar] [CrossRef]
- Maraming, P.; Klaynongsruang, S.; Boonsiri, P.; Peng, S.F.; Daduang, S.; Leelayuwat, C.; Pientong, C.; Chung, J.G.; Daduang, J. The cationic cell-penetrating KT2 peptide promotes cell membrane defects and apoptosis with autophagy inhibition in human HCT 116 colon cancer cells. J. Cell Physiol. 2019, 234, 22116–22129. [Google Scholar] [CrossRef]
- Leite, N.B.; Aufderhorst-Roberts, A.; Palma, M.S.; Connell, S.D.; Ruggiero, N.J.; Beales, P.A. PE and PS lipids synergistically enhance membrane poration by a peptide with anticancer properties. Biophys. J. 2015, 109, 936–947. [Google Scholar] [CrossRef] [Green Version]
- Paredes-Gamero, E.J.; Martins, M.N.; Cappabianco, F.A.; Ide, J.S.; Miranda, A. Characterization of dual effects induced by antimicrobial peptides: Regulated cell death or membrane disruption. Biochim. Biophys. Acta 2012, 1820, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Ju, X.; Fan, D.; Kong, L.; Yang, Q.; Zhu, Y.; Zhang, S.; Su, G.F.; Li, Y. Antimicrobial peptide Brevinin-1RL1 from frog skin secretion induces apoptosis and necrosis of tumor cells. Molecules 2021, 26, 2059. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tsai, T.L.; Ye, X.H.; Lin, T.H. Anti-proliferative effect on a colon adenocarcinoma cell line exerted by a membrane disrupting antimicrobial peptide KL15. Cancer Biol. Ther. 2015, 16, 1172–1183. [Google Scholar] [CrossRef] [Green Version]
- Cichon, T.; Smolarczyk, R.; Matuszczak, S.; Barczyk, M.; Jarosz, M.; Szala, S. D-K6L 9 peptide combination with IL-12 inhibits the recurrence of tumors in mice. Arch. Immunol. Ther. Exp. 2014, 62, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Lu, L.; Shao, X.; Tang, C.; Zhao, X. Anti-melanoma activity of hybrid peptide P18 and its mechanism of action. Biotechnol. Lett. 2010, 32, 463–469. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, L.; Lin, X.; Zhu, J.; Qian, Y.; Liu, W.; Chen, J.; Zhou, C.; He, J. Therapeutic effects of synthetic triblock amphiphilic short antimicrobial peptides on human lung adenocarcinoma. Pharmaceutics 2022, 14, 929. [Google Scholar] [CrossRef]
- Radis-Baptista, G. Cell-penetrating peptides derived from animal venoms and toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef]
- Ma, R.; Wong, S.W.; Ge, L.; Shaw, C.; Siu, S.; Kwok, H.F. In vitro and MD simulation study to explore physicochemical parameters for antibacterial peptide to become potent anticancer peptide. Mol. Ther. Oncolytics 2020, 16, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Zoeteweij, J.P.; van de Water, B.; de Bont, H.J.; Mulder, G.J.; Nagelkerke, J.F. Calcium-induced cytotoxicity in hepatocytes after exposure to extracellular ATP is dependent on inorganic phosphate effects on mitochondrial calcium. J. Biol. Chem. 1993, 268, 3384–3388. [Google Scholar] [CrossRef]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef]
- Gu, S.; Kounenidakis, M.; Schmidt, E.M.; Deshpande, D.; Alkahtani, S.; Alarifi, S.; Föller, M.; Alevizopoulos, K.; Lang, F.; Stournaras, C. Rapid activation of FAK/mTOR/p70S6K/PAK1-signaling controls the early testosterone-induced actin reorganization in colon cancer cells. Cell Signal. 2013, 25, 66–73. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Choi, Y.; Shin, J.M.; Cho, H.J.; Kang, J.H.; Park, K.K.; Choe, J.Y.; Bae, Y.S.; Han, S.M.; Kim, C.H.; et al. Melittin suppresses EGF-induced cell motility and invasion by inhibiting PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem. Toxicol. 2014, 68, 218–225. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.M.; Li, Y.N.; Cao, J.L.; Xue, H.; Wang, C.; Jin, C.H. Atractylodin induces apoptosis and inhibits the migration of a549 lung cancer cells by regulating ROS-Mediated signaling pathways. Molecules 2022, 27, 2946. [Google Scholar] [CrossRef]
- Hua, R.; Pei, Y.; Gu, H.; Sun, Y.; He, Y. Antitumor effects of flavokawain-B flavonoid in gemcitabine-resistant lung cancer cells are mediated via mitochondrial-mediated apoptosis, ROS production, cell migration and cell invasion inhibition and blocking of PI3K/AKT Signaling pathway. J. BUON 2020, 25, 262–267. [Google Scholar]
- Sun, M.; Hong, S.; Li, W.; Wang, P.; You, J.; Zhang, X.; Tang, F.; Wang, P.; Zhang, C. MiR-99a regulates ROS-mediated invasion and migration of lung adenocarcinoma cells by targeting NOX4. Oncol. Rep. 2016, 35, 2755–2766. [Google Scholar] [CrossRef]
- Yu, L.; Chen, W.; Tang, Q.; Ji, K.Y. Micheliolide inhibits liver cancer cell growth via inducing apoptosis and perturbing actin cytoskeleton. Cancer Manag. Res. 2019, 11, 9203–9212. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.Y.; Shih, P.C.; Huang, P.W.; Lee, Y.H.; Chen, Y.F.; Tai, M.H.; Liu, C.H.; Wen, Z.H.; Kuo, H.M. Sinularin, an anti-cancer agent causing mitochondria-modulated apoptosis and cytoskeleton disruption in human hepatocellular carcinoma. Int. J. Mol. Sci. 2021, 22, 3946. [Google Scholar] [CrossRef]
- Fife, C.M.; Mccarroll, J.A.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, J.; Kucik, D.F. The microtubule cytoskeleton participates in control of beta2 integrin avidity. J. Biol. Chem. 2001, 276, 44762–44769. [Google Scholar] [CrossRef] [Green Version]
- Ng, D.H.; Humphries, J.D.; Byron, A.; Millon-Fremillon, A.; Humphries, M.J. Microtubule-dependent modulation of adhesion complex composition. PLoS ONE 2014, 9, e115213. [Google Scholar] [CrossRef] [Green Version]
- Ezratty, E.J.; Partridge, M.A.; Gundersen, G.G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 2005, 7, 581–590. [Google Scholar] [CrossRef]
- Mui, K.L.; Bae, Y.H.; Gao, L.; Liu, S.L.; Xu, T.; Radice, G.L.; Chen, C.S.; Assoian, R.K. N-Cadherin induction by ECM stiffness and FAK overrides the spreading requirement for proliferation of vascular smooth muscle cells. Cell Rep. 2015, 10, 1477–1486. [Google Scholar] [CrossRef] [Green Version]
- Maes, H.; Van Eygen, S.; Krysko, D.V.; Vandenabeele, P.; Nys, K.; Rillaerts, K.; Garg, A.D.; Verfaillie, T.; Agostinis, P. BNIP3 supports melanoma cell migration and vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell Death Dis. 2014, 5, e1127. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Wu, Y.; Kang, S.; Ma, J.; Yang, J.; Zhang, F. CecropinXJ, a silkworm antimicrobial peptide, induces cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim. Biophys. Sin. 2014, 46, 867–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liscano, Y.; Onate-Garzon, J.; Delgado, J.P. Peptides with dual antimicrobial-anticancer activity: Strategies to overcome peptide limitations and rational design of anticancer peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Hao, D.J.; Zhang, Q.; An, J.; Zhao, J.J.; Chen, B.; Zhang, L.L.; Yang, H. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharm. 2016, 78, 1113–1130. [Google Scholar] [CrossRef]
- Zhang, S.F.; Chen, Z. Melittin exerts an antitumor effect on nonsmall cell lung cancer cells. Mol. Med. Rep. 2017, 16, 3581–3586. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.; Chai, J.; Zeng, Q.; Zhang, B.; Ye, T.; Chen, X.; Xu, X. The anticancer properties and mechanism of action of tablysin-15, the RGD-containing disintegrin, in breast cancer cells. Int. J. Biol. Macromol. 2019, 129, 1155–1167. [Google Scholar] [CrossRef]

| Genes | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ | 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
| MMP-2 | 5′-AGCGAGTGGATGCCGCCTTTAA-3′ | 5′-CATTCCAGGCATCTGCGATGAG-3′ |
| MMP-9 | 5′-GCCACTACTGTGCCTTTGAGTC-3′ | 5′-CCCTCAGAGAATCGCCAGTACT-3′ |
| TIMP-1 | 5′-CTGTTGTTGCTGTGGCTGATAG-3′ | 5′-CGCTGGTATAAGGTGGTCTGG-3′ |
| TIMP-2 | 5′-ACCCTCTGTGACTTCATCGTGC-3′ | 5′-GGAGATGTAGCACGGGATCATG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins 2022, 14, 438. https://doi.org/10.3390/toxins14070438
Guo R, Liu J, Chai J, Gao Y, Abdel-Rahman MA, Xu X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins. 2022; 14(7):438. https://doi.org/10.3390/toxins14070438
Chicago/Turabian StyleGuo, Ruiyin, Junfang Liu, Jinwei Chai, Yahua Gao, Mohamed A. Abdel-Rahman, and Xueqing Xu. 2022. "Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro" Toxins 14, no. 7: 438. https://doi.org/10.3390/toxins14070438
APA StyleGuo, R., Liu, J., Chai, J., Gao, Y., Abdel-Rahman, M. A., & Xu, X. (2022). Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins, 14(7), 438. https://doi.org/10.3390/toxins14070438







