A Systematic Review and Meta-Analysis of the Inhibitory Effects of Plant-Derived Sterilants on Rodent Population Abundance
Abstract
:1. Introduction
2. Results
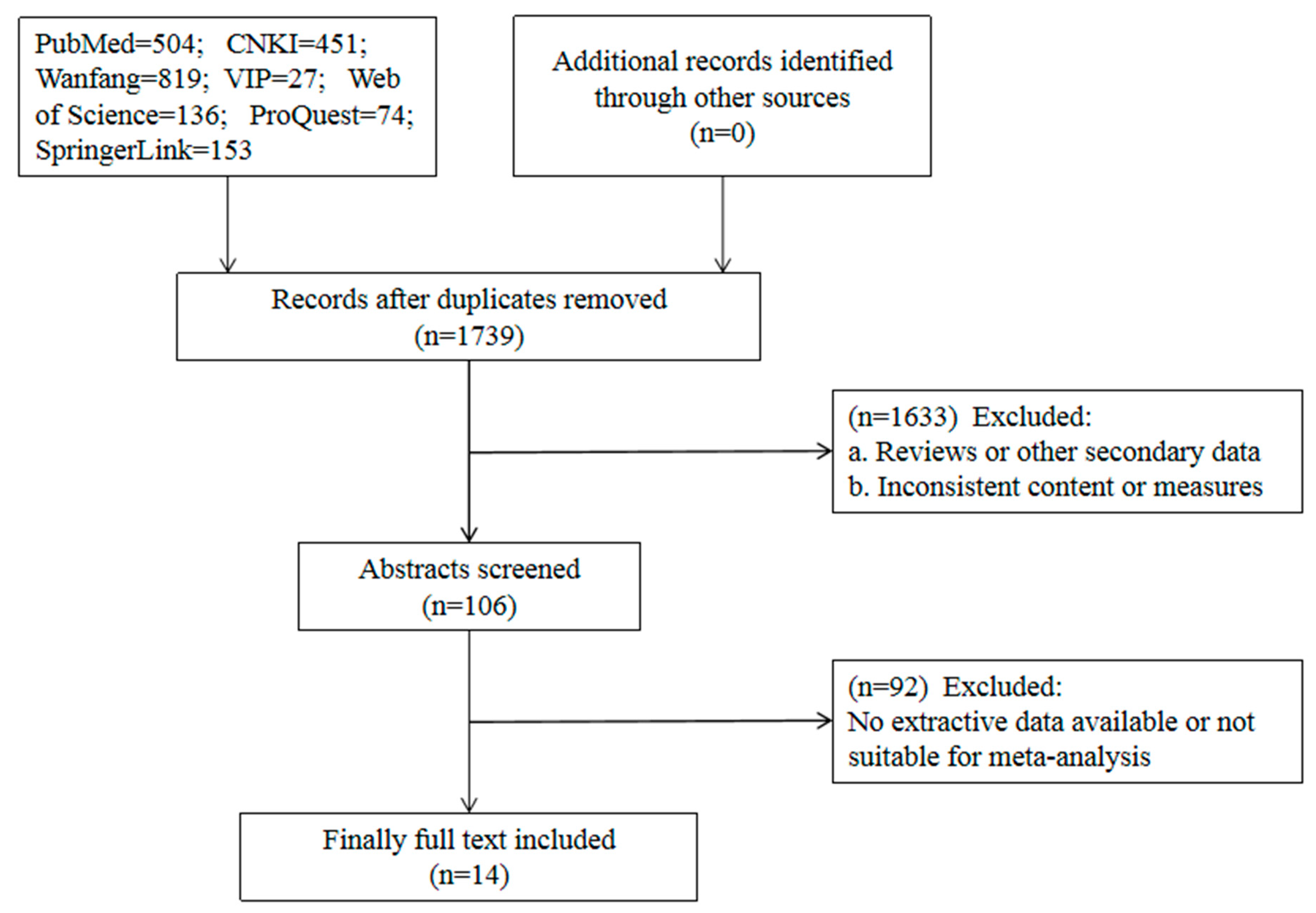
2.1. Literature Search
2.2. Basic Characteristics of the Included Studies
2.3. Meta-Analysis
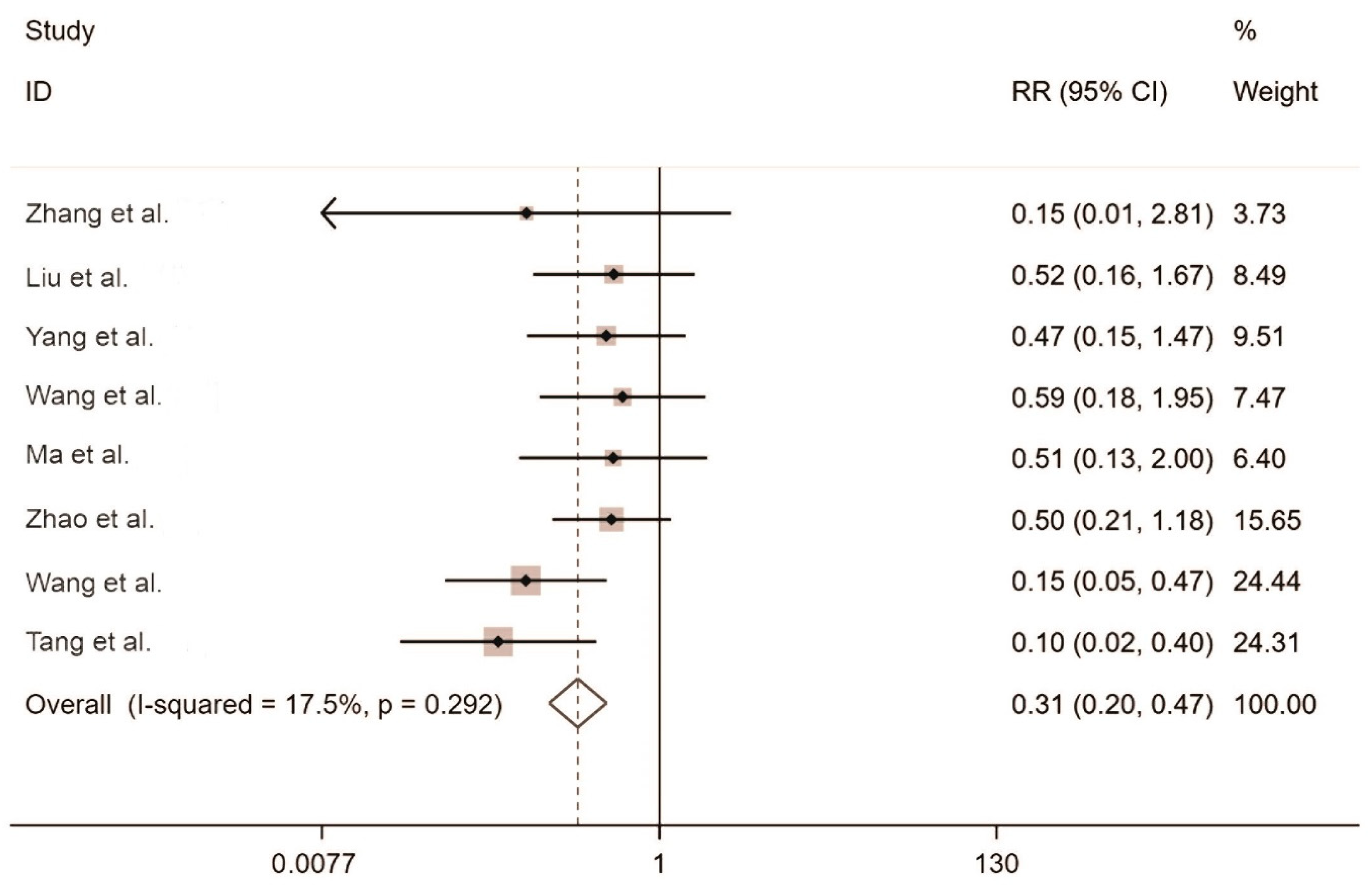
2.3.1. Meta-Analysis of the Rodent Capture Rate
2.3.2. Meta-Analysis of the Rodent Pregnancy Rate
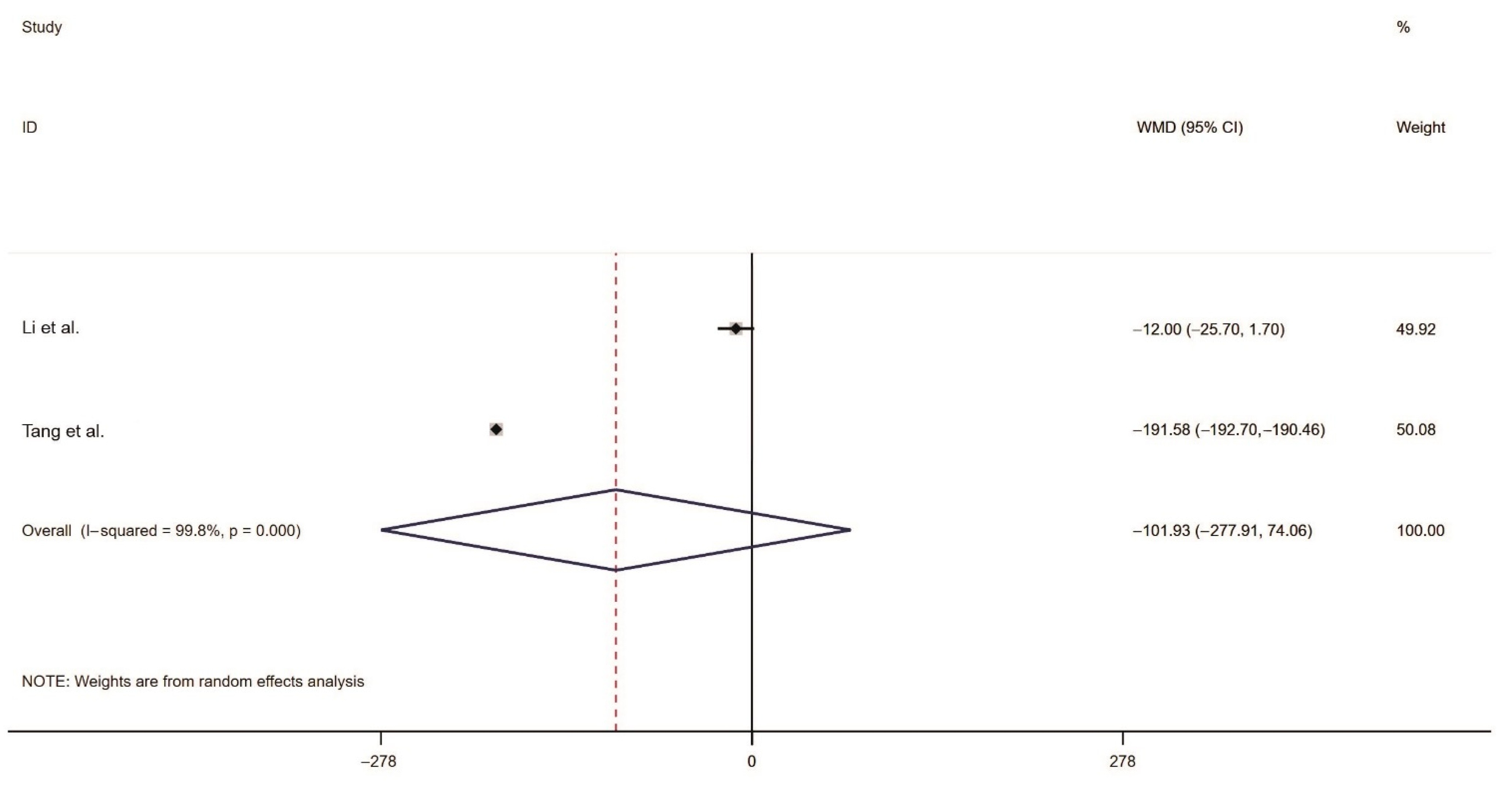
2.3.3. Meta-Analysis of the Rodent Sperm Survival Rate
2.3.4. Meta-Analysis of the Number of Effective Rodent Holes
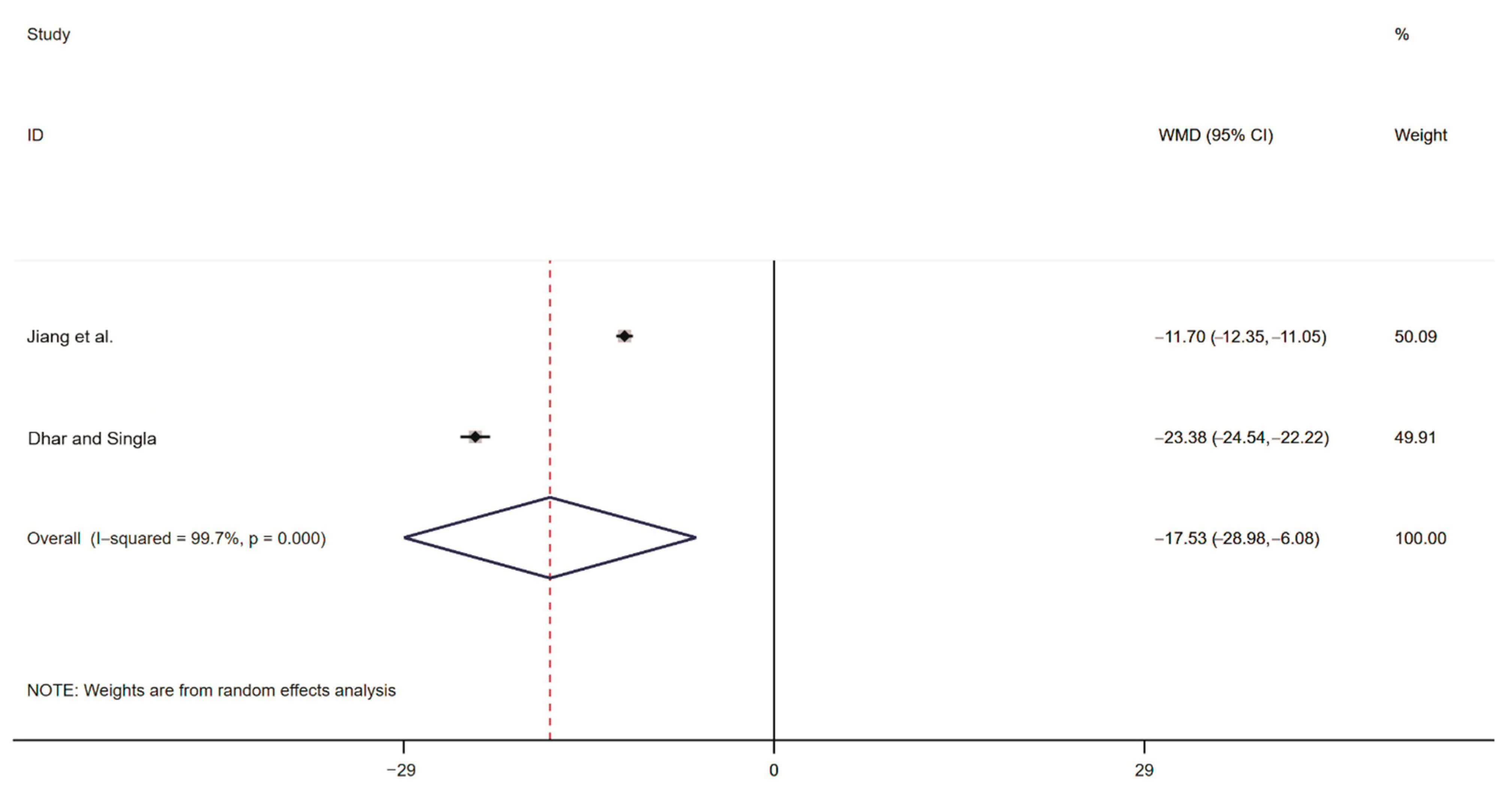
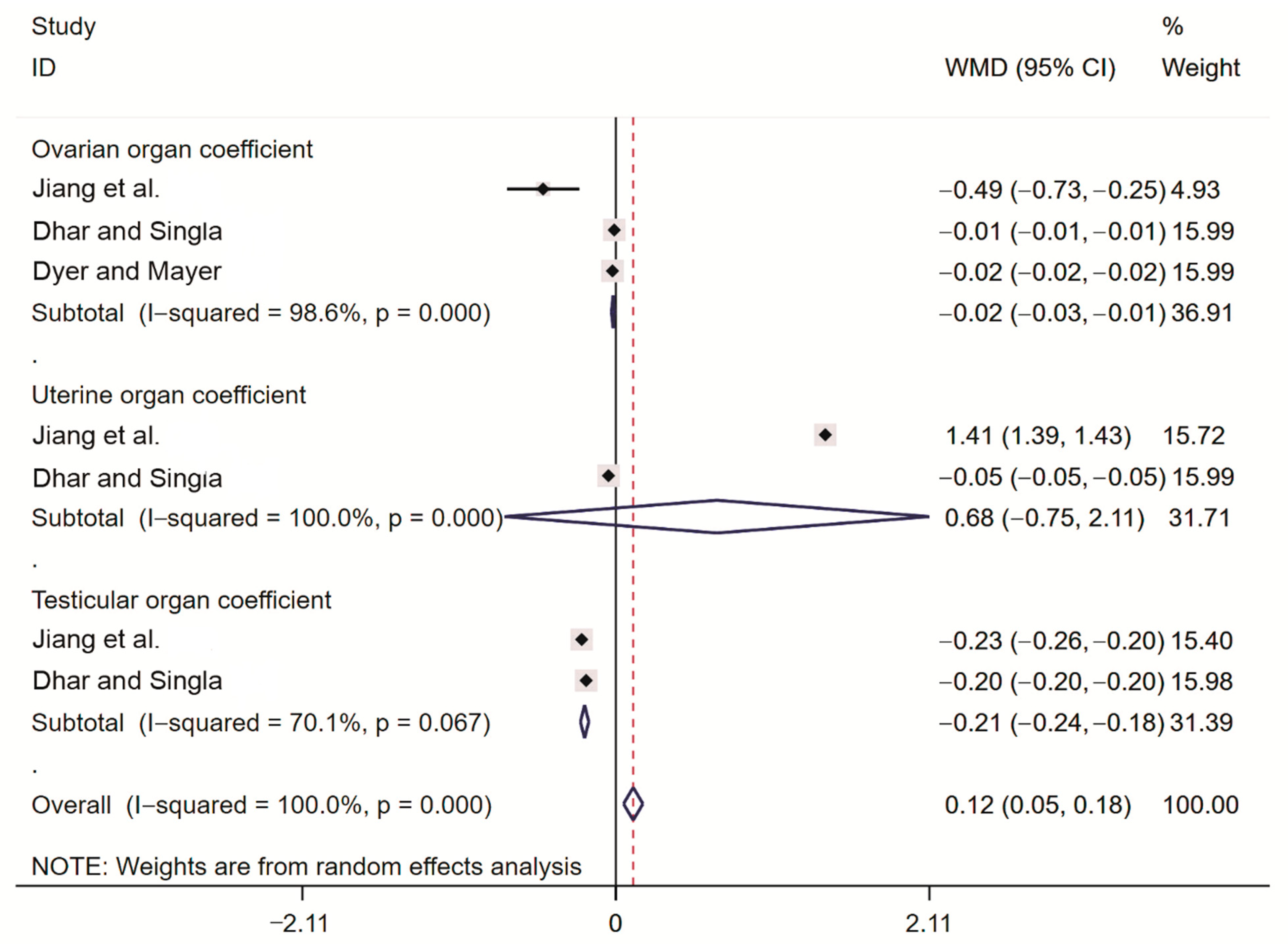
2.3.5. Meta-Analysis of Rodent Organ Coefficients
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Registration
5.2. Inclusion and Exclusion Criteria
5.3. Search Strategies
5.4. Literature Screening and Data Extraction
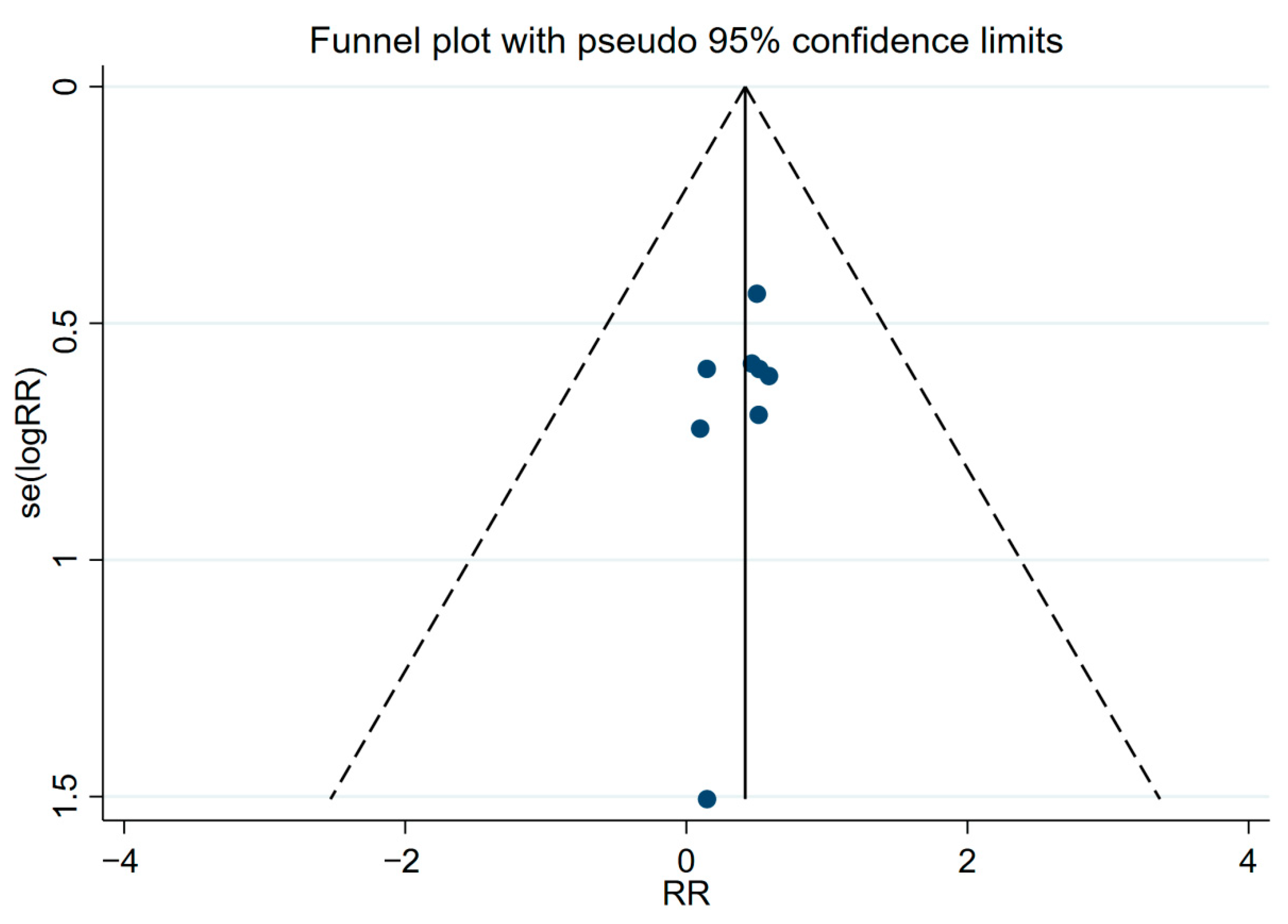
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, cities, people, and pathogens: A systematic review and narrative synthesis of literature regarding the ecology of rat-associated zoonoses in urban centers. Vector-Borne Zoonotic Dis. 2013, 13, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.H.; Zarnoch, S.J. A test of the predator satiation hypothesis, acorn predator size, and acorn preference. Can. J. For. Res. 2018, 48, 237–245. [Google Scholar] [CrossRef]
- Lee, J.-K.; Hwang, H.-S.; Eum, T.-K.; Bae, H.-K.; Rhim, S.-J. Cascade effects of slope gradient on ground vegetation and small-rodent populations in a forest ecosystem. Anim. Biol. 2019, 70, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Lohr, M.T.; Davis, R.A. Anticoagulant rodenticide use, non-target impacts and regulation: A case study from Australia. Sci. Total Environ. 2018, 634, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Senac, T. Human pharmaceutical products in the environment—The “problem” in perspective. Chemosphere 2014, 115, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Memmott, K.; Murray, M.; Rutberg, A. Use of anticoagulant rodenticides by pest management professionals in Massachusetts, USA. Ecotoxicology 2017, 26, 90–96. [Google Scholar] [CrossRef]
- Nakagawa, L.; de Masi, E.; Narciso, E.; Neto, H.M.; Papini, S. Palatability and efficacy of bromadiolone rodenticide block bait previously exposed to environmental conditions. Pest Manag. Sci. 2015, 71, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Cheng, X.; Dong, Y.; Wang, Q.; Lin, X. Analysis of the activity rhythms of the great gerbil (Rhombomys opimus) and its predators and their correlations based on infrared camera technology. Glob. Ecol. Conserv. 2020, 24, e01337. [Google Scholar] [CrossRef]
- Meerburg, B.G.; Brom, F.W.; Kijlstra, A. The ethics of rodent control. Pest Manag. Sci. 2008, 64, 1205–1211. [Google Scholar] [CrossRef]
- Grilo, A.; Moreira, A.; Carrapico, B.; Belas, A.; Sao Braz, B. Epidemiological study of pesticide poisoning in domestic animals and wildlife in Portugal: 2014–2020. Front. Vet. Sci. 2020, 7, 616293. [Google Scholar] [CrossRef]
- McGee, C.F.; McGilloway, D.A.; Buckle, A.P. Anticoagulant rodenticides and resistance development in rodent pest species–A comprehensive review. J. Stored Prod. Res. 2020, 88, 101688. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A.; Prakash, O. Potential antifertility agents from plants: A comprehensive review. J. Ethnopharmacol. 2012, 140, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Su, P.; Zhang, Z.; Gao, L.; Wang, J.; Hu, T.; Zhou, J.; Zhang, Y.; Zhao, Y.; Liu, Y.; et al. Genome of Tripterygium wilfordii and identification of cytochrome P450 involved in triptolide biosynthesis. Nat. Commun. 2020, 11, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikim, A.P.; Lue, Y.H.; Wang, C.; Reutrakul, V.; Sangsuwan, R.; Swerdloff, R.S. Posttesticular antifertility action of triptolide in the male rat: Evidence for severe impairment of cauda epididymal sperm ultrastructure. J. Androl. 2000, 21, 431–437. [Google Scholar]
- Deng, C.; Ji, J.; Li, N.; Yu, Y.; Duan, G.; Zhang, X. Fast determination of curcumol, curdione and germacrone in three species of Curcuma rhizomes by microwave-assisted extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2006, 1117, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.C.; Stolter, C.; Imholt, C.; Jacob, J. Like or dislike: Response of rodents to the odor of plant secondary metabolites. Integr. Zool. 2017, 12, 428–436. [Google Scholar] [CrossRef]
- Risi, E. Control of reproduction in ferrets, rabbits and rodents. Reprod. Domest. Anim. 2014, 49 (Suppl. 2), 81–86. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, R.J.; Visser, P.; Louw, H.; Ryan, P.G. Palatability and efficacy of rodent baits for eradicating house mice (Mus musculus) from Gough Island, Tristan da Cunha. Wildl. Res. 2011, 38, 196–203. [Google Scholar] [CrossRef]
- Henderson, R.J.; Frampton, C.M.; Morgan, D.R.; Hickling, G.J. The efficacy of baits containing 1080 for control of brushtail possums. J. Wildl. Manag. 1999, 63, 1138–1151. [Google Scholar] [CrossRef]
- Dhar, P.; Singla, N. Effect of triptolide on reproduction of female lesser bandicoot rat, Bandicota bengalensis. Drug Chem. Toxicol. 2014, 37, 448–458. [Google Scholar] [CrossRef]
- Dyer, C.; Mayer, L. Sprague dawley female rat consumption of a liquid bait containing vinylcyclohexene diepoxide and triptolide leads to subfertility. Proc. Vertebr. Pest Conf. 2014, 26, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.E.; Guo, Y.W.; Shi, D.Z.; Du, G.L. The anti-fertility effect of curcumol baits on Lasiopodomys brandti. Chin. J. Vector Biol. Control 2011, 22, 22–25. [Google Scholar]
- Li, Y.; Huang, Y.Z.; Zhao, R.L. Expermental research on controlling forest harmful rats with 0.2% curcumol bait. J. Chang. Univ. 2010, 20, 20–22. [Google Scholar]
- Li, B.; Sang, Z.; Xu, J.; Xu, Z.; Lai, X.; Peng, Z.; Shen, G.; Zhou, X.; Zha, X.; Zhang, M. Control effects of 2 sterilants and 1 anticoagulant to Ochotona curzoniae in the Northern Tibet. Plant Prot. 2015, 41, 230–234. [Google Scholar]
- Liu, D.; Zheng, Q.; Li, Z.; Zhao, R.; Liu, S.; Zhang, L.; Wang, Z.; Zuo, C. Efficacy of 0.2% curcumol bait against forest rodents. J. Pestic. Sci. Adm. 2006, 2, 12–15. [Google Scholar]
- Ma, J.; Zhang, D.; Zhuang, W.; Li, B.; Liu, W.; Zhao, H.; Shang, Z.; Liu, S. Demonstration of curcumol bait against rodents in farmland. China Plant Prot. 2007, 1, 34–36. [Google Scholar]
- Tang, X.; Qin, Y.; Lu, J.; Zuo, J. Investigation and control experiment of rodent (rabbit) pests in the newly planted forests in semi-arid valley in Lhasa. For. Pest Dis. 2011, 30, 31–34. [Google Scholar]
- Tian, B.P.; Li, H.Y.; Wang, Y.B.; Wang, Z.M.; Tan, C.Y. Studies on control effects of triptolide baits and curcumol baits on Spermophilus dauricus. Chin. J. Vector Biol. Control. 2014, 25, 461–463. [Google Scholar]
- Wang, T.L.; Zhang, H.D.; Zhao, R.L.; Ning, Z.D.; Zou, B.; Chang, W.Y.; Yang, X.G.; Hou, Y.; Liu, S.Q. Study on the effect of curcumol, one of sterilants, control territorial Rodents. J. Shanxi Agric. Sci. 2007, 10, 34–36. [Google Scholar]
- Wang, S.; Yuan, W.; Chen, C.; Li, Z.; Deng, D.; Luo, H. Control effect of triptolide on farmland rats in Sanya City. Plant Dis. Pests 2017, 8, 30–33. [Google Scholar]
- Yang, X.G.; Hou, Y.; Zhu, W.Y.; Wang, T.L.; Ning, Z.D.; Zou, B.; Chang, W.Y. Control effects of triptolide male infertility agents to vermin field mice. J. Shanxi Agric. Sci. 2012, 40, 1095–1098. [Google Scholar]
- Zhang, C.; Yang, J.; Zhang, T.; Zhao, J.; Zhao, S.; Zhang, Z. Experiment against forest pest rats with 0.2% curcumol sterilant. For. Pest Dis. 2009, 28, 9–11. [Google Scholar]
- Zhao, X.J.; Zhao, R.L.; Ji, H.Z.; Li, Y.L. Study on the anti-fertility effect of different curcumol forms on plateau pika. Chin. J. Hyg. Insectic. Equip. 2017, 23, 414–418. [Google Scholar]
- Shuster, S.M.; Pyzyna, B.; Mayer, L.P.; Dyer, C.A. The opportunity for sexual selection and the evolution of non-responsiveness to pesticides, sterility inducers and contraceptives. Heliyon 2018, 29, e00943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoblinnert, K.; Jacob, J.; Zhang, Z.; Hinds, L.A. The status of fertility control for rodents—recent achievements and future directions. Integr. Zool. 2021. [Google Scholar] [CrossRef]
- Nwaehujor, C.O.; Ode, J.O.; Ekwere, M.R.; Udegbunam, R.I. Anti-fertility effects of fractions from carica papaya (pawpaw) linn. methanol root extract in male wistar rats. Arab. J. Chem. 2019, 12, 1563–1568. [Google Scholar] [CrossRef] [Green Version]
- Jana, K.; Samanta, P.K. Evaluation of single intratesticular injection of calcium chloride for nonsurgical sterilization in adult albino rats. Contraception 2006, 73, 289–300. [Google Scholar] [CrossRef]
- Zhang, C.M.; Wang, R.H. Advance in the research of sterilants against rodents. J. For. Res. 2002, 13, 77–81. [Google Scholar] [CrossRef]
- Akbarsha, M.A.; Murugaian, P. Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: Effect on the testis and the cauda epididymidal spermatozoa. Phytother. Res. 2000, 14, 432–435. [Google Scholar] [CrossRef]
- Mishra, R.K.; Singh, S.K. Reversible antifertility effect of aqueous rhizome extract of Curcuma longa L. in male laboratory mice. Contraception 2009, 79, 479–487. [Google Scholar] [CrossRef]
- Rao, M.V.; Shah, K.D.; Rajani, M. Contraceptive effects of Phyllanthus amarus extract in the male mouse (Mus musculus). Phytother. Res. 1997, 11, 594–596. [Google Scholar] [CrossRef]
- Singla, N.; Kaur, G.; Babbar, B.K.; Sandhu, B.S. Potential of triptolide in reproductive management of the house rat, Rattus rattus. Integr. Zool. 2013, 8, 260–276. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Zhang, J.; Shi, D.; Wu, X. Effects of levonorgestrel-quinestrol (EP-1) treatment on Mongolian gerbil wild populations: A case study. Integr. Zool. 2013, 8, 277–284. [Google Scholar] [CrossRef]
- Roy, V.K.; Peki, V.; Devi, M.S.; Sanjeev, S.; Khusboo, M.; Zothansanga, R.; Ibrahim, K.S.; Kumar, N.S.; Gurusubramanian, G. Biosterilant effects of Bacillus thuringiensis kurstaki HD-73 extract on male wistar albino rats. Theriogenology 2017, 88, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ylönen, H. Rodent plagues, immunocontraception and the mousepox virus. Trends Ecol. Evol. 2001, 16, 418–420. [Google Scholar] [CrossRef]
- Coeurdassier, M.; Riols, R.; Decors, A.; Mionnet, A.; David, F.; Quintaine, T.; Truchetet, D.; Scheifler, R.; Giraudoux, P. Unintentional wildlife poisoning and proposals for sustainable management of rodents. Conserv. Biol. 2014, 28, 315–321. [Google Scholar] [CrossRef]
- Shilova, S.A.; Tchabovsky, A.V. Population response of rodents to control with rodenticides. Curr. Zool. 2009, 55, 81–91. [Google Scholar] [CrossRef]
- Singleton, G.R.; Farroway, L.N.; Chambers, L.K.; Lawson, M.A.; Smith, A.L.; Hinds, L.A. Ecological basis for fertility control in the house mouse (Mus domesticus) using immunocontraceptive vaccines. Reprod. Suppl. 2002, 60, 31–39. [Google Scholar]
- Dominguez, J.C.; Calero-Riestra, M.; Olea, P.P.; Malo, J.E.; Burridege, C.P.; Proft, K.; Illanas, S.; Viñuela, J.; García, J.T. Lack of detectable genetic isolation in the cyclic rodent Microtus arvalis despite large landscape fragmentation owing to transportation infrastructures. Sci. Rep. 2021, 11, 12534. [Google Scholar] [CrossRef]
- Dong, J.; Li, C.; Zhang, Z. Density-dependent genetic variation in dynamic populations of the greater long-tailed hamster (Tscherskia triton). J. Mammal. 2010, 91, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Kalykova, A.; Kustova, T.; Sakipova, Z.; Ibragimova, N.; Islamov, R.; Vetchý, D.; Ilin, A. Acute and subchronic toxicity studies of the original drug FS-1. Acta Vet. Brno 2016, 85, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Redwood, A.J.; Smith, L.M.; Lloyd, M.L.; Hinds, L.A.; Hardy, C.M.; Shellam, G.R. Prospects for virally vectored immunocontraception in the control of wild house mice (Mus domesticus). Wildl. Res. 2007, 34, 530–539. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Country | Study Type | Intervention Measure | Species | Outcome Measures |
|---|---|---|---|---|---|
| Zhang et al. [32] | China | randomized controlled trial | curcumol bait | Apodemus draco Eothenomys miletus Rhombomys opimus | capture rate pregnancy rate |
| Li et al. [23] | China | randomized controlled trial | curcumol bait | Apodemus draco Eothenomys miletus | pregnancy rate number of effective holes |
| Liu et al. [25] | China | randomized controlled trial | curcumol bait | Apodemus peninsulae | capture rate pregnancy rate |
| Li et al. [24] | China | randomized controlled trial | curcumol bait | Ochotona curzoniae Cricetulus longicaudatus | number of effective holes |
| Yang et al. [31] | China | randomized controlled trial | triptolide bait | Cricetulus longicaudatusApodemus peninsulae Niviventer niviventer Apodemus agrarius | capture rate pregnancy rate |
| Wang et al. [29] | China | randomized controlled trial | curcumol bait | Cricetulus longicaudatus | capture rate pregnancy rate |
| Ma et al. [26] | China | randomized controlled trial | curcumol bait | Tscherskia tritonde Apodemus agrarius | capture rate pregnancy rate |
| Zhao et al. [33] | China | randomized controlled trial | curcumol bait | Ochotona curzoniae | capture rate pregnancy rate |
| Tian et al. [28] | China | randomized controlled trial | curcumol bait, triptolide bait | Spermophilus dauricus | capture rate pregnancy rate |
| Jiang et al. [22] | China | randomized controlled trial | curcumol bait | Lasiopodomys brandtii | ovarian organ coefficient uterine organ coefficient testicular organ coefficient sperm survival rate |
| Wang et al. [30] | China | randomized controlled trial | triptolide bait | Rattus losea Rattus norvegicus Rattus flavipectus | capture rate pregnancy rate |
| Dhar and Singla [20] | India | randomized controlled trial | triptolide bait | Bandicota bengalensis | ovarian organ coefficient uterine organ coefficient testicular organ coefficient sperm survival rate |
| Dyer and Mayer [21] | USA | randomized controlled trial | triptolide bait | Rattus norvegicus | ovarian organ coefficient |
| Tang et al. [27] | China | randomized controlled trial | curcumol bait | Pitymys leucurus | capture rate number of effective holes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Yuan, S.; Li, L.; Dai, Q.; Yang, L.; Jiang, F.; Lin, X. A Systematic Review and Meta-Analysis of the Inhibitory Effects of Plant-Derived Sterilants on Rodent Population Abundance. Toxins 2022, 14, 487. https://doi.org/10.3390/toxins14070487
Wen X, Yuan S, Li L, Dai Q, Yang L, Jiang F, Lin X. A Systematic Review and Meta-Analysis of the Inhibitory Effects of Plant-Derived Sterilants on Rodent Population Abundance. Toxins. 2022; 14(7):487. https://doi.org/10.3390/toxins14070487
Chicago/Turabian StyleWen, Xuanye, Shuai Yuan, Limei Li, Quanhua Dai, Li Yang, Fan Jiang, and Xiao Lin. 2022. "A Systematic Review and Meta-Analysis of the Inhibitory Effects of Plant-Derived Sterilants on Rodent Population Abundance" Toxins 14, no. 7: 487. https://doi.org/10.3390/toxins14070487
APA StyleWen, X., Yuan, S., Li, L., Dai, Q., Yang, L., Jiang, F., & Lin, X. (2022). A Systematic Review and Meta-Analysis of the Inhibitory Effects of Plant-Derived Sterilants on Rodent Population Abundance. Toxins, 14(7), 487. https://doi.org/10.3390/toxins14070487






