Solanum nigrum Fruit Extract Modulates Immune System Activity of Mealworm Beetle, Tenebrio molitor L.
Abstract
:1. Introduction
2. Results
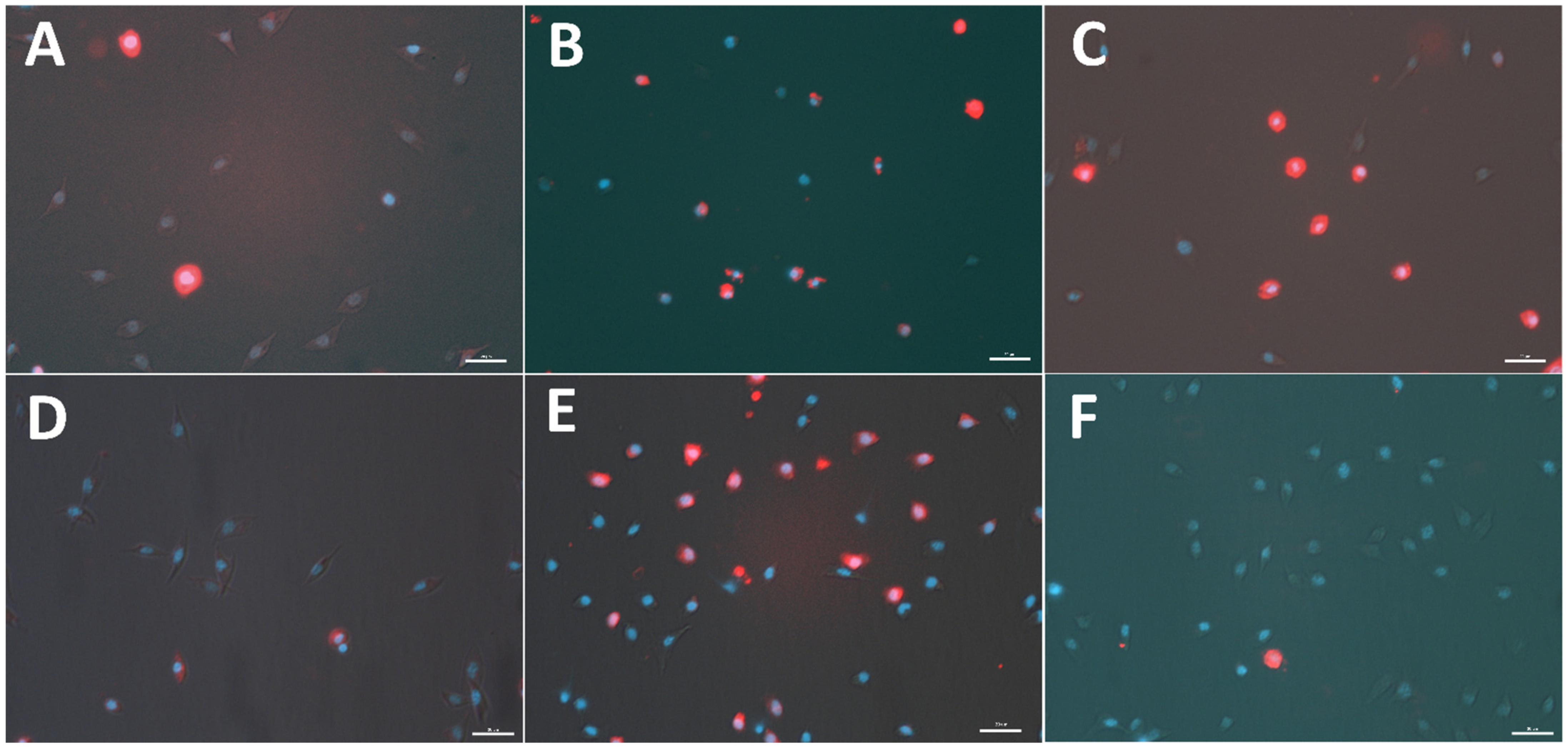
2.1. Apoptotic Ratio
2.2. Total Haemocyte Count
2.3. Phenoloxidase Activity
2.4. Lysozyme-like Antimicrobial Activity of T. molitor Haemolymph
2.5. Expression of Immune-Related Genes
2.5.1. Cecropin
2.5.2. Tenecin 3
2.5.3. Toll Receptor
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects
5.2. Solanum Nigrum Fruit Extract
5.3. Injection and Tissue Collection
5.4. Total Haemocyte Count
5.5. Apoptosis
5.6. Phenoloxidase Activity
5.7. The Lysozyme-like Activity of T. molitor Haemolymph
5.8. Quantitative Analysis of Expression Level of Immune-Related Genes
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Douglas, A.E. Strategies for enhanced crop resistance to insect pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [Green Version]
- Spochacz, M.; Chowański, S.; Walkowiak-Nowicka, K.; Szymczak, M.; Adamski, Z. Plant-derived substances used against beetles–pests of stored crops and food–and their mode of action: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1339–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lengai, G.M.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Chowański, S.; Adamski, Z.; Marciniak, P.; Rosiński, G.; Büyükgüzel, E.; Büyükgüzel, K.; Falabella, P.; Scrano, L.; Ventrella, E.; Lelario, F. A review of bioinsecticidal activity of Solanaceae alkaloids. Toxins 2016, 8, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spochacz, M.; Chowański, S.; Szymczak-Cendlak, M.; Marciniak, P.; Lelario, F.; Salvia, R.; Nardiello, M.; Scieuzo, C.; Scrano, L.; Bufo, S.A. Solanum nigrum extract and solasonine affected hemolymph metabolites and ultrastructure of the fat body and the midgut in Galleria mellonella. Toxins 2021, 13, 617. [Google Scholar] [CrossRef]
- Spochacz, M.; Szymczak, M.; Chowański, S.; Bufo, S.A.; Adamski, Z. Solanum nigrum Fruit extract increases toxicity of fenitrothion—A synthetic insecticide, in the mealworm beetle Tenebrio molitor larvae. Toxins 2020, 12, 612. [Google Scholar] [CrossRef]
- Chowański, S.; Chudzińska, E.; Lelario, F.; Ventrella, E.; Marciniak, P.; Miądowicz-Kobielska, M.; Spochacz, M.; Szymczak, M.; Scrano, L.; Bufo, S.A. Insecticidal properties of Solanum nigrum and Armoracia rusticana extracts on reproduction and development of Drosophila melanogaster. Ecotoxicol. Environ. Saf. 2018, 162, 454–463. [Google Scholar] [CrossRef]
- Yang, L.; Gao, S.; Su, Z.; Qin, X.; Li, Z. Identification of the constituents and the cancer-related targets of the fruit of Solanum nigrum based on molecular docking and network pharmacology. J. Pharm. Biomed. Anal. 2021, 200, 114067. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Syu, K.-Y.; Lin, J.-K. Chemical composition of Solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in AU565 breast cancer cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Vommaro, M.L.; Kurtz, J.; Giglio, A. Morphological Characterisation of Haemocytes in the Mealworm Beetle Tenebrio molitor (Coleoptera, Tenebrionidae). Insects 2021, 12, 423. [Google Scholar] [CrossRef]
- Urbański, A.; Adamski, Z.; Rosiński, G. Developmental changes in haemocyte morphology in response to Staphylococcus aureus and latex beads in the beetle Tenebrio molitor L. Micron 2018, 104, 8–20. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune defenses of a beneficial pest: The mealworm beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerenius, L.; Söderhäll, K. Immune properties of invertebrate phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M. Beetles as model organisms in physiological, biomedical and environmental studies—A review. Front. Physiol. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harikrishnan, R.; Balasundaram, C.; Jawahar, S.; Heo, M.-S. Solanum nigrum enhancement of the immune response and disease resistance of tiger shrimp, Penaeus monodon against Vibrio harveyi. Aquaculture 2011, 318, 67–73. [Google Scholar] [CrossRef]
- Adamo, S. Why should an immune response activate the stress response? Insights from the insects (the cricket Gryllus texensis). Brain Behav. Immun. 2010, 24, 194–200. [Google Scholar] [CrossRef]
- Urbański, A.; Konopińska, N.; Lubawy, J.; Walkowiak-Nowicka, K.; Marciniak, P.; Rolff, J. A possible role of tachykinin-related peptide on an immune system activity of mealworm beetle, Tenebrio molitor L. Dev. Comp. Immunol. 2021, 120, 104065. [Google Scholar] [CrossRef]
- Sun, H.; Lv, C.; Yang, L.; Wang, Y.; Zhang, Q.; Yu, S.; Kong, H.; Wang, M.; Xie, J.; Zhang, C. Solanine induces mitochondria-mediated apoptosis in human pancreatic cancer cells. BioMed Res. Int. 2014, 2014, 805926. [Google Scholar] [CrossRef]
- Chen, Z.; Li, C.; Yuan, A.; Gu, T.; Zhang, F.; Fan, X.; Wu, X.; Xiong, X.; Yang, Q. α-Solanine causes cellular dysfunction of human trophoblast cells via apoptosis and autophagy. Toxins 2021, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.-W.; Hsu, S.-H.; Li, Y.-P.; Lin, W.-L.; Liu, L.-F.; Chang, L.-C.; Lin, C.-C.; Lin, C.-N.; Sheu, H.-M. Anticancer activity evaluation of the Solanum glycoalkaloid solamargine: Triggering apoptosis in human hepatoma cells. Biochem. Pharmacol. 2000, 60, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Chung, P.J.; Wu, C.H.; Lan, K.P.; Yang, M.Y.; Wang, C.J. Solanum nigrum L. polyphenolic extract inhibits hepatocarcinoma cell growth by inducing G2/M phase arrest and apoptosis. J. Sci. Food Agric. 2011, 91, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In vitro polyphenol effects on apoptosis: An update of literature data. Semin. Cancer Biol. 2017, 46, 119–131. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Q.; Zeng, F.; Cai, M.; Ding, D. The protective effect of gentisic acid on rheumatoid arthritis via the RAF/ERK signaling pathway. J. Orthop. Surg. Res. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Spochacz, M.; Chowański, S.; Szymczak, M.; Lelario, F.; Bufo, S.A.; Adamski, Z. Sublethal effects of Solanum nigrum fruit extract and its pure glycoalkaloids on the physiology of Tenebrio molitor (Mealworm). Toxins 2018, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Garvey, M.; Bredlau, J.; Kester, K.; Creighton, C.; Kaplan, I. Toxin or medication? Immunotherapeutic effects of nicotine on a specialist caterpillar. Funct. Ecol. 2021, 35, 614–626. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Feng, Q. Fat body biology in the last decade. Annu. Rev. Entomol. 2019, 64, 315–333. [Google Scholar] [CrossRef]
- Shaukat, Z.; Liu, D.; Gregory, S. Sterile inflammation in Drosophila. Mediat. Inflamm. 2015, 2015, 369286. [Google Scholar] [CrossRef] [Green Version]
- Krautz, R.; Arefin, B.; Theopold, U. Damage signals in the insect immune response. Front. Plant Sci. 2014, 5, 342. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Berry, R., III; López-Martínez, G. A dose of experimental hormesis: When mild stress protects and improves animal performance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110658. [Google Scholar] [CrossRef]
- Cutler, G.C.; Rix, R.R. Can poisons stimulate bees? Appreciating the potential of hormesis in bee–pesticide research. Pest Manag. Sci. 2015, 71, 1368–1370. [Google Scholar] [CrossRef]
- Haddi, K.; Oliveira, E.E.; Faroni, L.R.; Guedes, D.C.; Miranda, N.N. Sublethal exposure to clove and cinnamon essential oils induces hormetic-like responses and disturbs behavioral and respiratory responses in Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, Y.; Ni, J.; Song, J.; Li, L.; Yu, Z.; Pang, L.; Qi, H. Biphasic dose–response of components from Coptis chinensis on feeding and detoxification enzymes of Spodoptera litura larvae. Dose-Response 2020, 18, 1559325820916345. [Google Scholar] [CrossRef] [PubMed]
- Dubovskiy, I.; Grizanova, E.; Ershova, N.; Rantala, M.; Glupov, V. The effects of dietary nickel on the detoxification enzymes, innate immunity and resistance to the fungus Beauveria bassiana in the larvae of the greater wax moth Galleria mellonella. Chemosphere 2011, 85, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Lee, K.G.; Lee, H.H.; Lee, H.J.; An, H.J.; Nam, J.H.; Jang, D.S.; Lee, K.T. α-Solanine isolated from Solanum tuberosum L. Cv jayoung abrogates LPS-induced inflammatory responses via NF-κb Inactivation in RAW 264.7 Macrophages and Endotoxin-Induced Shock Model in Mice. J. Cell. Biochem. 2016, 117, 2327–2339. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Di, S.; Xu, Q.; Ren, Q.; Chen, S.; Huang, N.; Jia, D.; Shen, X. Steroidal alkaloid solanine A from Solanum nigrum Linn. exhibits anti-inflammatory activity in lipopolysaccharide/interferon γ-activated murine macrophages and animal models of inflammation. Biomed. Pharmacother. 2018, 105, 606–615. [Google Scholar] [CrossRef]
- El-Shazely, B.; Urbański, A.; Johnston, P.; Rolff, J. In vivo exposure of insect AMP resistant Staphylococcus aureus to an insect immune system. Insect Biochem. Mol. Biol. 2019, 110, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, T.R.; Lelario, F.; Bufo, S.A. Analysis of tomato glycoalkaloids by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2005, 19, 3103–3110. [Google Scholar] [CrossRef]
- Urbański, A.; Walkowiak-Nowicka, K.; Nowicki, G.; Chowański, S.; Rosinski, G. Effect of short-term desiccation, recovery time and CAPA-PVK neuropeptides on the immune system of the burying beetle Nicrophorus vespilloides. Front. Physiol. 2021, 12, 845. [Google Scholar] [CrossRef]
- Czarniewska, E.; Mrowczynska, L.; Kuczer, M.; Rosinski, G. The pro-apoptotic action of the peptide hormone Neb-colloostatin on insect haemocytes. J. Exp. Biol. 2012, 215, 4308–4313. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, R.P.; Small, C.N.; Govind, S. Quantitative analysis of phenol oxidase activity in insect hemolymph. Biotechniques 2002, 32, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Urbański, A.; Czarniewska, E.; Baraniak, E.; Rosinski, G. Developmental changes in cellular and humoral responses of the burying beetle Nicrophorus vespilloides (Coleoptera, Silphidae). J. Insect Physiol. 2014, 60C, 98–103. [Google Scholar] [CrossRef]
- Arce, A.N.; Smiseth, P.T.; Rozen, D.E. Antimicrobial secretions and social immunity in larval burying beetles, Nicrophorus vespilloides. Anim. Behav. 2013, 86, 741–745. [Google Scholar] [CrossRef]
- Urbański, A.; Johnston, P.; Bittermann, E.; Keshavarz, M.; Paris, V.; Walkowiak-Nowicka, K.; Konopińska, N.; Marciniak, P.; Rolff, J. Tachykinin-related peptides modulate immune-gene expression in the mealworm beetle Tenebrio molitor L. Sci. Rep. 2022, 12, 1–19. [Google Scholar]
- Jacobs, C.G.; Gallagher, J.D.; Evison, S.E.; Heckel, D.G.; Vilcinskas, A.; Vogel, H. Endogenous egg immune defenses in the yellow mealworm beetle (Tenebrio molitor). Dev. Comp. Immunol. 2017, 70, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.A.; Patnaik, B.B.; Ali Mohammadie Kojour, M.; Kim, B.B.; Bae, Y.M.; Park, K.B.; Lee, Y.S.; Jo, Y.H.; Han, Y.S. Tm Spz-like plays a fundamental role in response to E. coli but not S. aureus or C. albican infection in Tenebrio molitor via regulation of antimicrobial peptide production. Int. J. Mol. Sci. 2021, 22, 10888. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbański, A.; Konopińska, N.; Bylewska, N.; Gmyrek, R.; Spochacz-Santoro, M.; Bufo, S.A.; Adamski, Z. Solanum nigrum Fruit Extract Modulates Immune System Activity of Mealworm Beetle, Tenebrio molitor L. Toxins 2023, 15, 68. https://doi.org/10.3390/toxins15010068
Urbański A, Konopińska N, Bylewska N, Gmyrek R, Spochacz-Santoro M, Bufo SA, Adamski Z. Solanum nigrum Fruit Extract Modulates Immune System Activity of Mealworm Beetle, Tenebrio molitor L. Toxins. 2023; 15(1):68. https://doi.org/10.3390/toxins15010068
Chicago/Turabian StyleUrbański, Arkadiusz, Natalia Konopińska, Natalia Bylewska, Radosław Gmyrek, Marta Spochacz-Santoro, Sabino Aurelio Bufo, and Zbigniew Adamski. 2023. "Solanum nigrum Fruit Extract Modulates Immune System Activity of Mealworm Beetle, Tenebrio molitor L." Toxins 15, no. 1: 68. https://doi.org/10.3390/toxins15010068




