Changes in Toxin Production, Morphology and Viability of Gymnodinium catenatum Associated with Allelopathy of Chattonella marina var. marina and Gymnodinium impudicum
Abstract
:1. Introduction
2. Results
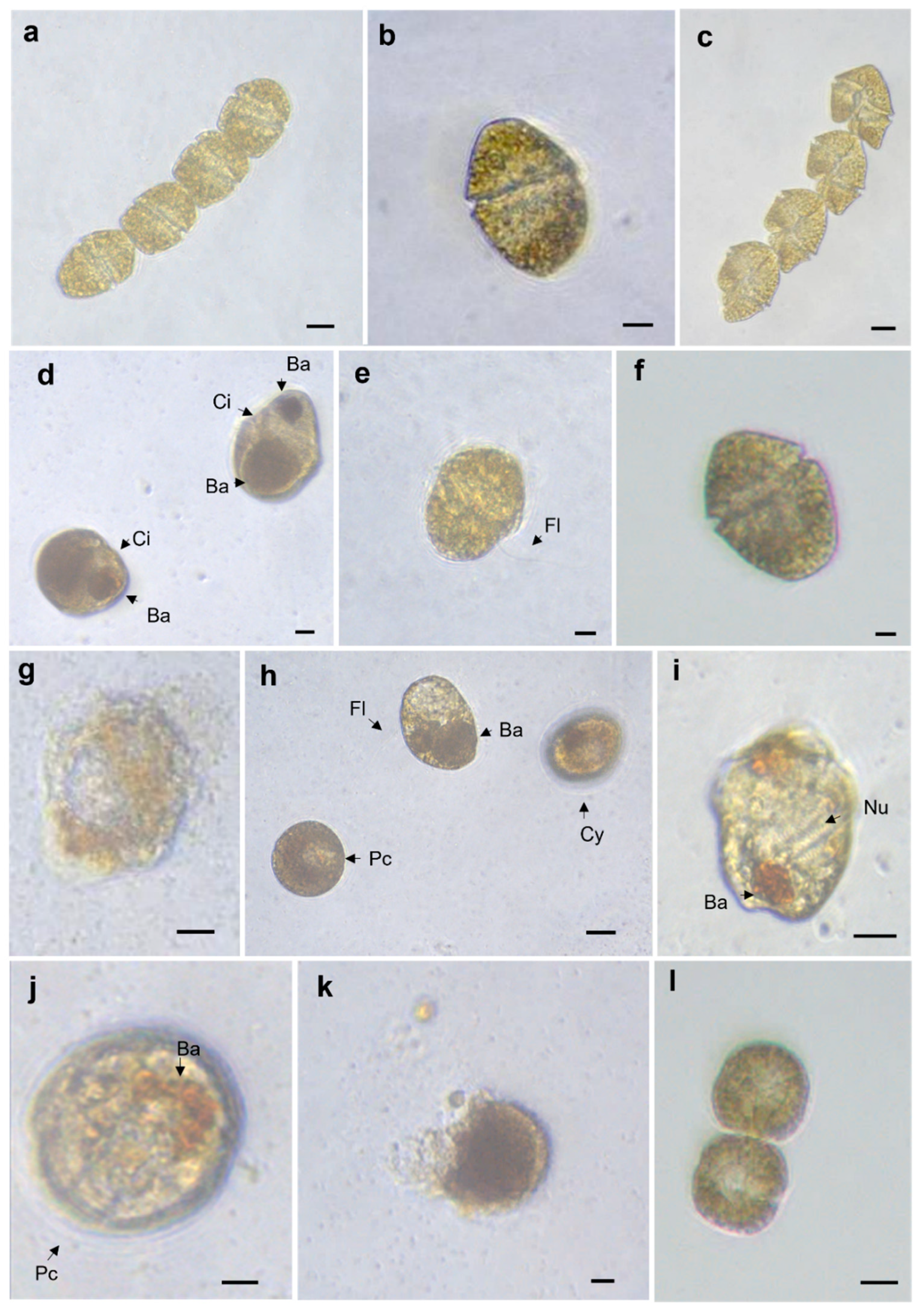
2.1. Allelopathy Experiment: Changes in Cell Shape and Volume
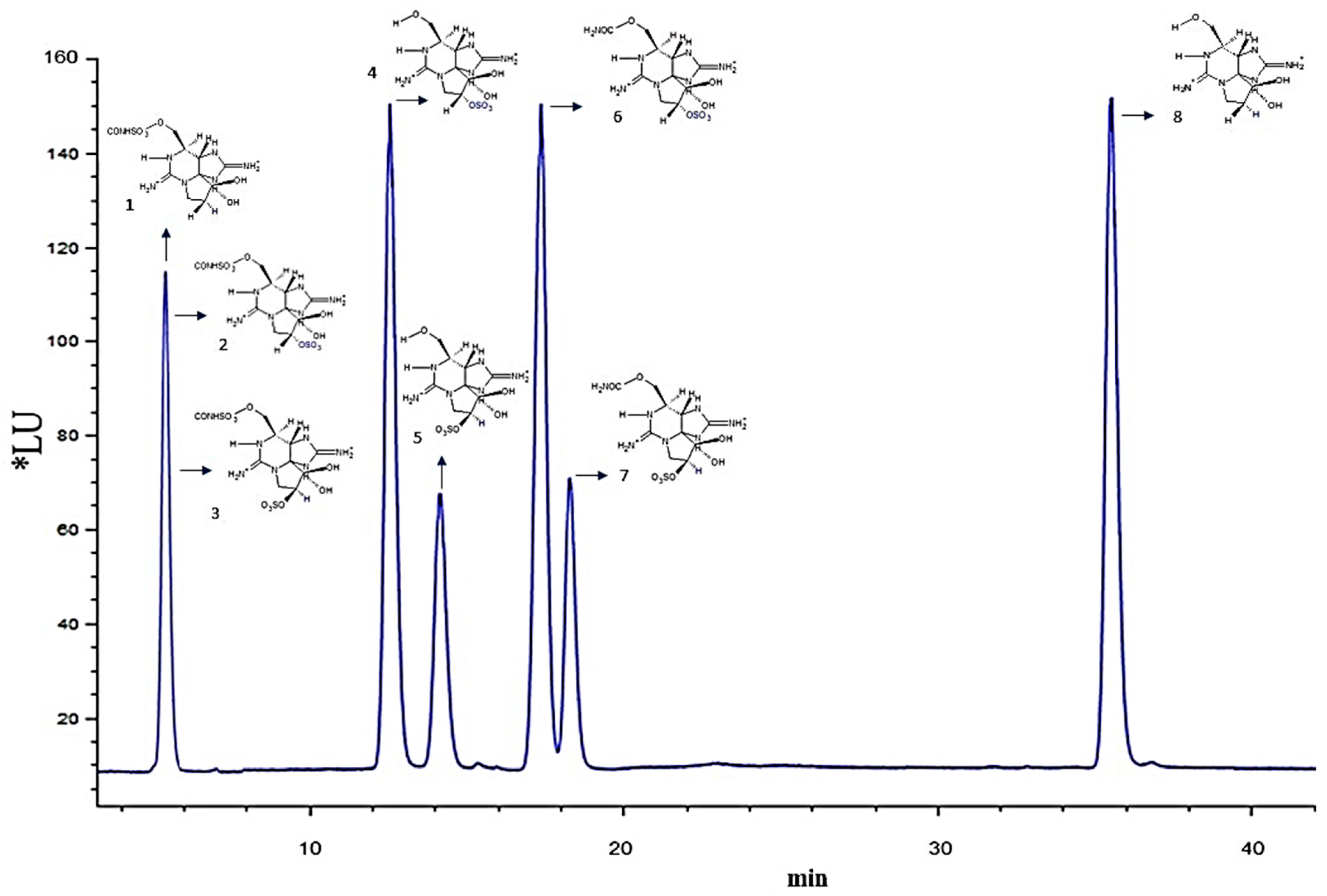
2.2. Paralytic Toxin Profile
2.3. Viability, Growth Rate, and Generation Time
2.3.1. Single Cell
2.3.2. Four-Cell Chains
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Allelopathy Experiment: Changes in Cell Shape and Volume
5.2. Paralytic Toxin Profile
5.3. Viability, Growth Rate, and Generation Time of Single and Four-Cell Chains of G. catenatum exposed to Allelochemicals
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, E.M. Allelopathy of Aquatic Autotrophs. CRC Crit Rev. Plant Sci. 2010, 33, 313–339. [Google Scholar] [CrossRef]
- Hattenrath-Lehmann, T.K.; Gobler, C.J. Allelopathic Inhibition of Competing Phytoplankton by North American Strains of the Toxic Dinoflagellate, Alexandrium fundyense: Evidence from Field Experiments, Laboratory Experiments, and Bloom Events. Harmful Algae 2011, 11, 106–116. [Google Scholar] [CrossRef]
- Molish, H. Der Einfluss Einer Pflanze Auf Die Andere: Allelopathie; Fisher: Jena, Germany, 1937; p. 106. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 423. [Google Scholar]
- Granéli, E.; Weberg, M.; Salomon, P.S. Harmful Algal Blooms of Allelopathic Microalgal Species: The Role of Eutrophication. Harmful Algae 2008, 8, 94–102. [Google Scholar] [CrossRef]
- Ianora, A.; Boersma, M.; Casotti, R.; Fontana, A.; Harder, J.; Hoffmann, F.; Pavia, H.; Potin, P.; Poulet, S.A.; Toth, G. New Trends in Marine Chemical Ecology. Estuaries Coasts 2006, 29, 531–551. [Google Scholar] [CrossRef]
- Gross, E.M. Aquatic Chemical Ecology Meets Ecotoxicology. Aquat. Ecol. 2022, 56, 493–511. [Google Scholar] [CrossRef]
- Pichierri, S.; Accoroni, S.; Pezzolesi, L.; Guerrini, F.; Romagnoli, T.; Pistocchi, R.; Totti, C. Allelopathic Effects of Diatom Filtrates on the Toxic Benthic Dinoflagellate Ostreopsis Cf. ovata. Mar. Environ. Res. 2017, 131, 116–133. [Google Scholar] [CrossRef]
- Guo, X.; Han, T.; Tan, L.; Zhao, T.; Zhu, X.; Huang, W.; Lin, K.; Zhang, N.; Wang, J. The Allelopathy and Underlying Mechanism of Skeletonema Costatum on Karenia Mikimotoi Integrating Transcriptomics Profiling. Aquat. Toxicol. 2022, 242, 106042. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Legrand, C.; Rengefors, K.; Granéli, E. Temporary Cyst Formation in Phytoplankton: A Response to Allelopathic Competitors? Environ. Microbiol. 2004, 6, 791–798. [Google Scholar] [CrossRef]
- Tillmann, U.; Hansen, P.J. Allelopathic Effects of Alexandrium tamarense on Other Algae: Evidence from Mixed Growth Experiments. Aquat. Microb. Ecol. 2009, 57, 101–112. [Google Scholar] [CrossRef]
- Tillmann, U.; Alpermann, T.L.; da Purificação, R.C.; Krock, B.; Cembella, A. Intra-Population Clonal Variability in Allelochemical Potency of the Toxigenic Dinoflagellate Alexandrium tamarense. Harmful Algae 2009, 8, 759–769. [Google Scholar] [CrossRef]
- Yang, J.; Deng, X.; Xian, Q.; Qian, X.; Li, A. Allelopathic Effect of Microcystis aeruginosa on Microcystis wesenbergii: Microcystin-LR as a Potential Allelochemical. Hydrobiologia 2014, 737, 65–73. [Google Scholar] [CrossRef]
- Kubanek, J.; Hicks, M.K.; Naar, J.; Villareal, T.A. Does the Red Tide Dinoflagellate Karenia brevis Use Allelopathy to Outcompete Other Phytoplankton? Limnol. Oceanogr. 2005, 50, 883–895. [Google Scholar] [CrossRef]
- Prince, E.K.; Myers, T.L.; Naar, J.; Kubanek, J. Competing Phytoplankton Undermines Allelopathy of a Bloom-Forming Dinoflagellate. Proc. R. Soc. B Biol. Sci. 2008, 275, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Gobler, C.J. Allelopathic Effects of Cochlodinium polykrikoides Isolates and Blooms from the Estuaries of Long Island, New York, on Co-Occurring Phytoplankton. Mar. Ecol. Prog. Ser. 2010, 406, 19–31. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Zumaya-Higuera, M.G.; López-Cortés, D.J.; Leyva-Valencia, I.; Quijano-Scheggia, S.I.; Hernández-Guerrero, C.J. Allelopathic Effects of Margalefidinium polykrikoides and Gymnodinium impudicum in the Growth of Gymnodinium catenatum. Harmful Algae 2020, 96, 101846. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Tang, Y.; Xu, N. Allelopathic Effects of Mixotrophic Dinoflagellate Akashiwo sanguinea on Co-Occurring Phytoplankton: The Significance of Nutritional Ecology. J. Oceanol. Limnol. 2020, 39, 903–917. [Google Scholar] [CrossRef]
- Fernández-Herrera, L.J.; Band-Schmidt, C.J.; López-Cortés, D.J.; Hernández-Guerrero, C.J.; Bustillos-Guzmán, J.J.; Núñez-Vázquez, E. Allelopathic Effect of Chattonella marina var. marina (Raphidophyceae) on Gymnodinium catenatum (Dinophycea). Harmful Algae 2016, 51, 1–9. [Google Scholar] [CrossRef]
- Fernández-Herrera, L.J.; Band-Schmidt, C.J.; Zenteno-Savín, T.; Leyva-Valencia, I.; Hernández-Guerrero, C.J.; Muñoz-Ochoa, M. Cell Death and Metabolic Stress in Gymnodinium catenatum Induced by Allelopathy. Toxins 2021, 13, 506. [Google Scholar] [CrossRef]
- Marshall, J.A.; Hovenden, M.; Oda, T.; Hallegraeff, G.M. Photosynthesis Does Influence Superoxide Production in the Ichthyotoxic Alga Chattonella marina (Raphidophyceae). J. Plankton Res. 2003, 24, 1231–1236. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; Nichols, P.D.; David Waite, T.; Hallegraeff, G.M. Strain Variability in Fatty Acid Composition of Chattonella marina (Raphidophyceae) and Its Relation to Differing Ichthyotoxicity toward Rainbow Trout Gill Cells. J. Phycol. 2013, 49, 427–438. [Google Scholar] [CrossRef]
- Aquino-Cruz, A.; Band-Schmidt, C.J.; Zenteno-Savín, T. Superoxide Production Rates and Hemolytic Activity Linked to Cellular Growth Phases in Chattonella Species (Raphidophyceae) and Margalefidinium polykrikoides (Dinophyceae). J. Appl. Phycol. 2020, 32, 4029–4046. [Google Scholar] [CrossRef]
- Shikata, T.; Yuasa, K.; Kitatsuji, S.; Sakamoto, S.; Akita, K.; Fujinami, Y.; Nishiyama, Y.; Kotake, T.; Tanaka, R.; Yamasaki, Y. Superoxide Production by the Red Tide-Producing Chattonella marina Complex (Raphidophyceae) Correlates with Toxicity to Aquacultured Fishes. Antioxidants 2021, 10, 1635. [Google Scholar] [CrossRef]
- Fraga, S.; Bravo, I.; Delgado, M.; Franco, J.M.; Zapata, M. Gyrodinium impudicum sp. Nov. (Dinophyceae), a Non Toxic, Chain-Forming, Red Tide Dinoflagellate. Phycologia 1995, 34, 514–521. [Google Scholar] [CrossRef]
- Reguera, B. Establecimiento de un Programa de Seguimiento de Microalgas Toxicas. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2003; p. 24. (In Spanish) [Google Scholar]
- Diaz, J.M.; Plummer, S. Production of Extracellular Reactive Oxygen Species by Phytoplankton: Past and Future Directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Xu, Y.; Leaw, C.P.; Lim, P.T.; Wang, J.; Chen, J.; Deng, Y.; Hu, Z.; Tang, Y.Z. Potent Allelopathy and Non-PSTs, Non-Spirolides Toxicity of the Dinoflagellate Alexandrium leei to Phytoplankton, Finfish and Zooplankton Observed from Laboratory Bioassays. Sci. Total Environ. 2021, 780, 146484. [Google Scholar] [CrossRef]
- Ji, X.; Han, X.; Zheng, L.; Yang, B.; Yu, Z.; Zou, J. Allelopathic Interactions between Prorocentrum micans and Skeletonema costatum or Karenia mikimotoi in Laboratory Cultures. Chin. J. Oceanol. Limnol. 2011, 29, 840–848. [Google Scholar] [CrossRef]
- Monti, M.; Cecchin, E. Comparative Growth of Three Strains of Ostreopsis ovata at Different Light Intensities with Focus on Inter-Specific Allelopathic Interactions. Cryptogam. Algol. 2013, 33, 113–119. [Google Scholar] [CrossRef]
- He, D.; Liu, J.; Hao, Q.; Ran, L.; Zhou, B.; Tang, X. Interspecific Competition and Allelopathic Interaction between Karenia mikimotoi and Dunaliella salina in Laboratory Culture. Chin. J. Oceanol. Limnol. 2016, 34, 301–313. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Nagasoe, S.; Matsubara, T.; Shikata, T.; Shimasaki, Y.; Oshima, Y.; Honjo, T. Allelopathic Interactions between the Bacillariophyte Skeletonema costatum and the Raphidophyte Heterosigma akashiwo. Mar. Ecol. Prog. Ser. 2007, 339, 83–92. [Google Scholar] [CrossRef]
- Qiu, X.; Yamasaki, Y.; Shimasaki, Y.; Gunjikake, H.; Honda, M.; Kawaguchi, M.; Matsubara, T.; Nagasoe, S.; Etoh, T.; Matsui, S.; et al. Allelopathy of the Raphidophyte Heterosigma akashiwo against the Dinoflagellate Akashiwo sanguinea Is Mediated via Allelochemicals and Cell Contact. Mar. Ecol. Prog. Ser. 2013, 446, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Tang, Y.Z.; Qin, J.; Duan, S.; Gobler, C.J. Ability of the Marine Diatoms Pseudo-nitzschia multiseries and P. pungens to Inhibit the Growth of Co-Occurring Phytoplankton via Allelopathy. Aquat. Microb. Ecol. 2015, 74, 29–41. [Google Scholar] [CrossRef]
- Ianora, A.; Bentley, M.G.; Caldwell, G.S.; Casotti, R.; Cembella, A.D.; Engström-Öst, J.; Halsband, C.; Sonnenschein, E.; Legrand, C.; Llewellyn, C.A.; et al. The Relevance of Marine Chemical Ecology to Plankton and Ecosystem Function: An Emerging Field. Mar. Drugs 2011, 9, 1625–1648. [Google Scholar] [CrossRef] [PubMed]
- Sieg, R.D.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical Ecology of the Marine Plankton. Nat. Prod. Rep. 2011, 28, 388–399. [Google Scholar] [CrossRef]
- Schwartz, E.R.; Poulin, R.X.; Mojib, N.; Kubanek, J. Chemical Ecology of Marine Plankton. Nat. Prod. Rep. 2016, 33, 843–860. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful Algal Blooms: Their Ecophysiology and General Relevance to Phytoplankton Blooms in the Sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Cembella, A.D. Chemical Ecology of Eukaryotic Microalgae in Marine Ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Legrand, C.; Granéli, E. Allelopathic Effect of Prymnesium parvum on a Natural Plankton Community. Mar. Ecol. Prog. Ser. 2003, 255, 115–125. [Google Scholar] [CrossRef]
- Suikkanen, S.; Fistarol, G.O.; Granéli, E. Effects of Cyanobacterial Allelochemicals on a Natural Plankton Community. Mar. Ecol. Prog. Ser. 2005, 287, 1–9. [Google Scholar] [CrossRef]
- Fistarol, G.O.; Legrand, C.; Selander, E.; Hummert, C.; Stolte, W.; Granéli, E. Allelopathy in Alexandrium Spp.: Effect on a Natural Plankton Community and on Algal Monocultures. Aquat. Microb. Ecol. 2004, 35, 45–56. [Google Scholar] [CrossRef]
- Legrand, C.; Rengefors, K.; Fistarol, G.; Graneli, E. Allelopathy in Phytoplankton-Biochemical, Ecological and Evolutionary Aspects. Phycologya 2003, 42, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Granéli, E.; Hansen, P.J. Allelopathy in Harmful Algae: A Mechanism to Compete for Resources? In Ecology Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 189–201. [Google Scholar] [CrossRef]
- Tillmann, U.; John, U.; Cembella, A. On the Allelochemical Potency of the Marine Dinoflagellate Alexandrium ostenfeldii against Heterotrophic and Autotrophic Protists. J. Plankton Res. 2007, 29, 527–543. [Google Scholar] [CrossRef]
- Jonsson, P.R.; Pavia, H.; Toth, G. Formation of Harmful Algal Blooms Cannot Be Explained by Allelopathic Interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 11177–11182. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Kim, G.Y.; Choi, B.D.; Rhodes, L.L.; Kim, T.J.; Kim, G.H.; Lee, J.D. A Comparative Study of the Harmful Dinoflagellates Cochlodinium polykrikoides and Gyrodinium impudicum Using Transmission Electron Microscopy, Fatty Acid Composition, Carotenoid Content, DNA Quantification and Gene Sequences. Bot. Mar. 2001, 44, 57–66. [Google Scholar] [CrossRef]
- Lee, C.K.; Kim, H.C.; Lee, S.-G.; Jung, C.S.; Kim, H.G.; Lim, W.A. Abundance of Harmful Algae, Cochlodinium polykrikoides, Â Gyrodinium impudicum and Gymnodinium catenatum in the Coastal Area of South Sea of Korea and Their Effects of Temperature, Salinity, Irradiance and Nutrient on the Growth in Culture. J. Korean Fish. Soc. Pusan 2001, 34, 536–544. [Google Scholar]
- Oh, S.J.; Kwon, H.K.; Noh, I.H.; Yang, H.S. Dissolved Organic Phosphorus Utilization and Alkaline Phosphatase Activity of the Dinoflagellate Gymnodinium impudicum Isolated from the South Sea of Korea. Ocean Sci. J. 2010, 45, 171–178. [Google Scholar] [CrossRef]
- López-Cortés, D.J.; Band-Schmidt, C.J.; Gárate-Lizárraga, I.; Bustillos-Guzmán, J.J.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J. Co-Ocurrencia de Chattonella marina y Gymnodinium catenatum En La Bahía de La Paz, Golfo de California (Primavera 3009). Hidrobiologica 2011, 21, 185–196. [Google Scholar]
- Benico, G.A.; Azanza, R.V. Molecular Phylogeny of Three Unarmored Dinoflagellates from Masinloc Bay, Zambales, Central Luzon, with a Description of the Morphology of Gymnodinium catenatum H.W. Graham. Philipp. J. Sci. 2021, 151, 61–72. [Google Scholar]
- Prince, E.K.; Poulson, K.L.; Myers, T.L.; Sieg, R.D.; Kubanek, J. Characterization of Allelopathic Compounds from the Red Tide Dinoflagellate Karenia brevis. Harmful Algae 2010, 10, 39–48. [Google Scholar] [CrossRef]
- Poulson-Ellestad, K.L.; Jones, C.M.; Roy, J.; Viant, M.R.; Fernández, F.M.; Kubanek, J.; Nunn, B.L. Metabolomics and Proteomics Reveal Impacts of Chemically Mediated Competition on Marine Plankton. Proc. Natl. Acad. Sci. USA 2014, 111, 9009–9014. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Bustillos-Guzmán, J.J.; Morquecho, L.; Band-Schmidt, C.J.; Alonso-Rodríguez, R.; Erler, K.; Luckas, B.; Reyes-Salinas, A.; Góngora-González, D.T. Comparative Paralytic Shellfish Toxin Profiles in the Strains of Gymnodinium catenatum Graham from the Gulf of California, Mexico. Mar. Pollut. Bull. 2005, 50, 208–236. [Google Scholar] [CrossRef]
- Band-Schmidt, C.; Bustillos-Guzmán, J.; Morquecho, L.; Gárate-Lizárraga, I.; Alonso-Rodríguez, R.; Reyes-Salinas, A.; Erler, K.; Luckas, B. Variations of PSP Toxin Profiles During Different Growth Phases in Gymnodinium catenatum (Dinophyceae) Strains Isolated from Three Locations in the Gulf of California, Mexico. J. Phycol. 2006, 42, 757–768. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; López-Cortés, D.J.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E. Ecological and Physiological Studies of Gymnodinium catenatum in the Mexican Pacific: A Review. Mar. Drugs 2010, 8, 1935–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, R.L.; Santos, M.E.V.D.; Pacheco, A.B.F.; Azevedo, S.M.F.O. Effects of Light Intensity and Light Quality on Growth and Circadian Rhythm of Saxitoxins Production in Cylindrospermopsis raciborskii (Cyanobacteria). J. Plankton Res. 2009, 31, 481–488. [Google Scholar] [CrossRef]
- Bustillos-Guzmán, J.J.; Band-Schmidt, C.J.; López-Cortés, D.J.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E. Variations in Growth and Toxicity in Gymnodinium catenatum Graham from the Gulf of California under Different Ratios of Nitrogen and Phosphorus. Ciencias Mar. 2013, 38, 101–117. [Google Scholar] [CrossRef]
- Mesquita, M.C.B.; Lürling, M.; Dorr, F.; Pinto, E.; Marinho, M.M. Combined Effect of Light and Temperature on the Production of Saxitoxins in Cylindrospermopsis raciborskii Strains. Toxins 2019, 11, 38. [Google Scholar] [CrossRef]
- Bui, Q.T.N.; Kim, H.; Park, H.; Ki, J.S. Salinity Affects Saxitoxins (STXs) Toxicity in the Dinoflagellate Alexandrium pacificum, with Low Transcription of SXT-Biosynthesis Genes SxtA4 and SxtG. Toxins 2021, 13, 733. [Google Scholar] [CrossRef]
- Tillmann, U.; John, U. Toxic Effects of Alexandrium Spp. on Heterotrophic Dinoflagellates: An Allelochemical Defence Mechanism Independent of PSP-Toxin Content. Mar. Ecol. Prog. Ser. 2002, 230, 47–58. [Google Scholar] [CrossRef]
- Yang, W.D.; Xie, J.; van Rijssel, M.; Li, H.Y.; Liu, J.S. Allelopathic Effects of Alexandrium Spp. on Prorocentrum donghaiense. Harmful Algae 2010, 10, 116–120. [Google Scholar] [CrossRef]
- Selander, E.; Kubanek, J.; Hamberg, M.; Andersson, M.X.; Cervin, G.; Pavia, H. Predator Lipids Induce Paralytic Shellfish Toxins in Bloom-Forming Algae. Proc. Natl. Acad. Sci. USA 2015, 112, 6395–6400. [Google Scholar] [CrossRef]
- Gharbia, H.B.; Yahia, O.K.D.; Cecchi, P.; Masseret, E.; Amzil, Z.; Herve, F.; Rovillon, G.; Nouri, H.; M’Rabet, C.; Couet, D.; et al. New Insights on the Species-Specific Allelopathic Interactions between Macrophytes and Marine HAB Dinoflagellates. PLoS ONE 2017, 12, e0187963. [Google Scholar] [CrossRef]
- Long, M.; Peltekis, A.; González-Fernández, C.; Hégaret, H.; Bailleul, B. Allelochemicals of Alexandrium minutum: Kinetics of Membrane Disruption and Photosynthesis Inhibition in a Co-Occurring Diatom. Harmful Algae 2021, 103, 101997. [Google Scholar] [CrossRef] [PubMed]
- Šoln, K.; Klemenčič, M.; Koce, J.D. Plant Cell Responses to Allelopathy: From Oxidative Stress to Programmed Cell Death. Protoplasma 2022, 259, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Morrill, L.C. Ecdysis and the Location of the Plasma Membrane in the Dinoflagellate Heterocapsa niei. Protoplasma 1984 1191 1984, 119, 8–20. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Skarlato, S. Dinoflagellate Amphiesma at Different Stages of the Life Cycle. Protistology 2013, 7, 108–115. [Google Scholar]
- Kalinina, V.; Matantseva, O.; Berdieva, M.; Skarlato, S. Trophic Strategies in Dinoflagellates: How Nutrients Pass through the Amphiesma. Protistology 2018, 23, 3–11. [Google Scholar] [CrossRef]
- Matantseva, O. Cellular Mechanisms of Dinoflagellate Cyst Development and Ecdysis–Many Questions to Answer. Protistology 2019, 12, 47–56. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Matantseva, O.; Skarlato, S.; Pozdnyakov, I.; Matantseva, O.; Skarlato, S. Consensus Channelome of Dinoflagellates Revealed by Transcriptomic Analysis Sheds Light on Their Physiology. Algae 2021, 36, 315–326. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A Review on Control of Harmful Algal Blooms by Plant-Derived Allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Wu, J.T.; Chiang, Y.R.; Huang, W.Y.; Jane, W.N. Cytotoxic Effects of Free Fatty Acids on Phytoplankton Algae and Cyanobacteria. Aquat. Toxicol. 2006, 80, 338–345. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, M.; Wang, Y.; Tang, X.; Zhao, Y. Allelopathic Effects of Ulva linza on Marine Phytoplankton and Identification of the Allelochemicals. Environ. Sci. Pollut. Res. 2021, 28, 45714–45723. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Rojas-Posadas, D.I.; Morquecho, L.; Hernández-Saavedra, N.Y. Heterogeneity of LSU RDNA Sequences and Morphology of Gymnodinium catenatum Dinoflagellate Strains in Bahía Concepción, Gulf of California, Mexico. J. Plankton Res. 2008, 30, 755–763. [Google Scholar] [CrossRef]
- Graham, H.W. Gymnodinium catenatum, a New Dinoflagellate from the Gulf of California. Trans. Am. Microsc. Soc. 1943, 63, 259–261. [Google Scholar] [CrossRef]
- Blackburn, S.I.; Hallegraeff, G.M.; Bolch, C.J. Vegetative Reproduction and Sexual Life Cycle of the Toxic Dinoflagellate Gymnodinium catenatum from Tasmania, Australia. J. Phycol. 1989, 25, 577–590. [Google Scholar] [CrossRef]
- Munir, S.; Burhan, Z.N.; Nias, T.; Morton, S.L.; Siddiqui, P.J.A. Morphometric Forms, Biovolume and Cellular Carbon Content of Dinoflagellates from Polluted Waters on The. Indian J. Geo-Marine Sci. 2015, 44, 19–25. [Google Scholar]
- Figueroa, R.I.; Bravo, I.; Garcés, E.; Ramilo, I. Nuclear Features and Effect of Nutrients on Gymnodinium catenatum (Dinophyceae) Sexual Stages. J. Phycol. 2006, 42, 67–77. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Bravo, I.; Ramilo, I.; Pazos, Y.; Moroño, A. New Life-Cycle Stages of Gymnodinium catenatum (Dinophyceae): Laboratory and Field Observations. Aquat. Microb. Ecol. 2008, 52, 13–33. [Google Scholar] [CrossRef]
- Bravo, I.; Figueroa, R.I. Towards an Ecological Understanding of Dinoflagellate Cyst Functions. Microorganisms 2014, 2, 11–32. [Google Scholar] [CrossRef]
- Hakanen, P.; Suikkanen, S.; Kremp, A. Allelopathic Activity of the Toxic Dinoflagellate Alexandrium ostenfeldii: Intra-Population Variability and Response of Co-Occurring Dinoflagellates. Harmful Algae 2014, 39, 287–294. [Google Scholar] [CrossRef]
- Doblin, M.A.; Thompson, P.A.; Revill, A.T.; Butler, E.C.V.; Blackburn, S.I.; Hallegraeff, G.M. Vertical Migration of the Toxic Dinoflagellate Gymnodinium catenatum under Different Concentrations of Nutrients and Humic Substances in Culture. Harmful Algae 2006, 5, 665–677. [Google Scholar] [CrossRef]
- Band-Schmidt, C.J.; Martínez-López, A.; Bustillos-Guzmán, J.J.; Carréon-Palau, L.; Morquecho, L.; Olguín-Monroy, N.O.; Zenteno-Savín, T.; Mendoza-Flores, A.; González-Acosta, B.; Hernández-Sandoval, F.E.; et al. Morphology, biochemistry and growth of Raphidophyte strains from the Gulf of California. Hydrobiologia 2012, 693, 81–97. [Google Scholar] [CrossRef]
- Bustillos-Guzmán, J.J.; Band-Schmidt, C.J.; Durán-Riveroll, L.M.; Hernández-Sandoval, F.E.; López-Cortés, D.J.; Núñez-Vázquez, E.J.; Cembella, A.; Krock, B. Paralytic Toxin Profile of the Marine Dinoflagellate Gymnodinium catenatum Graham from the Mexican Pacific as Revealed by LC-MS/MS. Food Addit. Contam. 2015, 32, 381–394. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric Models for Calculating Cell Biovolume and Surface Area for Phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Hummert, C.; Ritscher, M.; Reinharde, K.; Luckas, B. Analysis of the Characteristic PSP Profiles of Pyrodinium bahamense and Several Strains of Alexandrium by HPLC Based on Ion-Pair Chromatographic Separation, Post-Column Oxidation, and Fluorescence Detection. Chromatographia 1997, 45, 312–316. [Google Scholar] [CrossRef]
- Yu, R.C.; Hummert, C.; Luckas, B.; Qian, P.Y.; Li, J.; Zhou, M.J. A Modified HPLC Method for Analysis of PSP Toxins in Algae and Shellfish from China. Chromatographia 1998, 48, 671–676. [Google Scholar] [CrossRef]
- Sieracki, M.; Poulton, N.; Crosbie, N. Automated Isolation Techniques for Microalgae. In Algal culturing techniques; Anderson, R.A., Ed.; Elsevier Academic Press: New York, NY, USA, 2005; pp. 101–116. [Google Scholar]
- Sinigalliano, C.D.; Winshell, J.; Guerrero, M.A.; Scorzetti, G.; Fell, J.W.; Eaton, R.W.; Brand, L.; Rein, K.S. Viable Cell Sorting of Dinoflagellates by Multiparametric Flow Cytometry. Phycologia 2009, 48, 249–257. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. Cult. Mar. Invertebr. Anim. 1975, 29–60. [Google Scholar] [CrossRef]
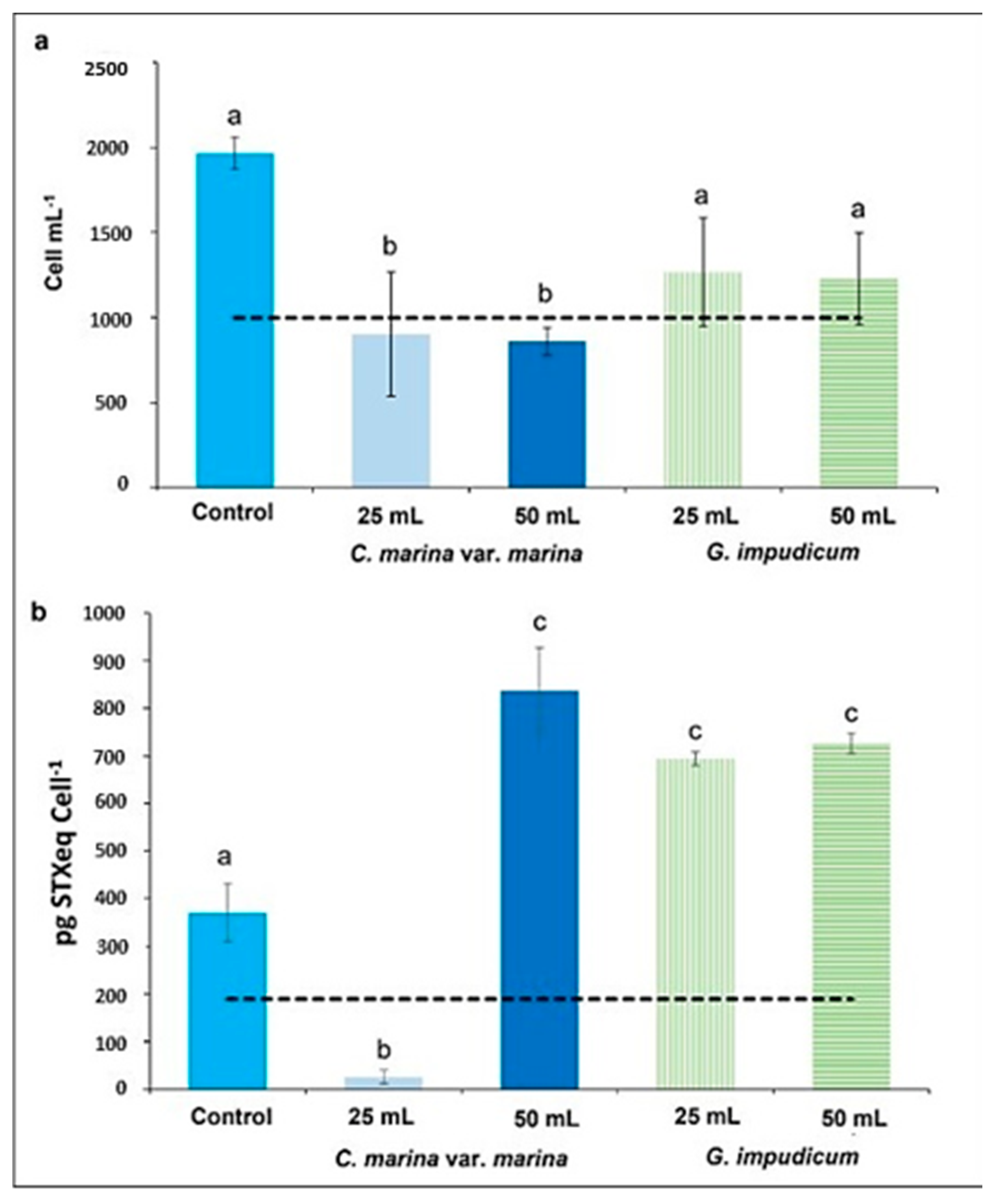
| Treatment | (n) | Abundance (Cells mL−1) | Width (µm) | Length (µm) | Biovolumen (µm3) |
|---|---|---|---|---|---|
| Control | 30 | 1966 ± 95 | 43.95 ± 5.55 | 49.08 ± 8.37 | 19,550 ± 8316 a |
| Cell-free media of Chattonella marina var. marina | |||||
| 25 mL | 30 | 902 ± 363 | 46.58 ± 10.91 | 52.04 ± 15.38 | 27,630 ± 19,520 b |
| 50 mL | 30 | 860 ± 79 | 45.34 ± 0.99 | 51.91 ± 15.78 | 27,502 ± 15,636 b |
| Cell-free media of Gymnodinium impudicum | |||||
| 25 mL | 30 | 1268 ± 316 | 46.43 ± 11.01 | 53.30 ± 17.39 | 24,425 ± 7628 a |
| 50 mL | 30 | 1229 ± 370 | 46.37 ± 10.78 | 52.17 ± 21.54 | 27,505 ± 2231 a |
| Treatment | GTX2/3 | dcSTX | dcGTX2/3 | B 1 | C 1/2 |
|---|---|---|---|---|---|
| Control | 1.43 ± 0.26 a | 0.67 ± 0.03 a | 3.33 ± 0.21 a | 0.09 ± 0.01 a | 94.44 ± 0.48 a |
| Cell-free media of Chattonella marina var. marina 25 mL | 5.6 ± 0.45 a | 1.58 ± 0.01 b | 7.0 ± 0.43 b,* | 0.53 ±0.09 b | 85.84 ± 3.46 b |
| 50 mL | 1.48 ± 0.06 a | 0.61 ± 0.08 a | 2.91 ± 0.16 c | 0.04 ±0.02 a | 94.96 ± 0.11 a |
| Cell-free media of Gymnodinium impudicum 25 mL | 1.40 ± 0.02 a | 0.54 ± 0.11 a | 2.55 ± 0.28 a | 0.08 ±0.06 a | 95.42 ± 0.37 a |
| 50 mL | 1.62 ± 0.03 a | 0.48 ± 0.04 a | 2.25 ± 0.07 a | 0.12 ±0.02 a | 95.52 ± 0.06 a |
| Treatment | Abundance after 96 h (Cells) | Abundance after 192 h (Cells) | Growth Rate (Div Day−1) | Generations Day−1 | Pv Viability (%) | Pv Relative to Control (%) |
|---|---|---|---|---|---|---|
| Control | 3 ± 1 | 5 ± 2 | 1.57 ± 0.38 a | 0.14 ± 0.03 a | 46 ± 3 a | - |
| Cell-free media of Chattonella marina var. marina | ||||||
| 25 mL | 2 ± 2 | 3 ± 1 | 0.55 ± 0.33 b | 0.16 ± 0.02 a | 14 ± 4 b | −69.6 ± 2 |
| 50 mL | 1 ± 1 | 4 ± 3 | 1.03 ± 0.49 a | 0.32 ± 0.15 b | 8 ± 3 b | −82.6 ± 2 |
| Cell-free media of Gymnodinium impudicum | ||||||
| 25 mL | 2 ± 1 | 3 ± 2 | 0.45 ± 0.33 b | 0.33 ± 0.15 b | 24 ± 6 c | −47.9 ± 6 |
| 50 mL | 2 ± 1 | 3 ± 1 | 0.90 ± 0.17 a | 0.31 ± 0.02 b | 20 ± 2 c | −56.6 ± 3 |
| Treatment | Abundance after 96 h (Cells) | Abundance after 192 h (Cells) | Growth Rate (Div Day−1) | Generations Day−1 | Pv Viability (%) | Pv Relative to Control (%) |
|---|---|---|---|---|---|---|
| Control | 6 ± 2 | 35 ± 10 | 2.61 ± 0.59 a | 0.12 ± 0.02 a | 62 ± 3 a | - |
| Cell-free media of Chattonella marina var. marina | ||||||
| 25 mL | 2 ± 1 | 3 ± 1 | 1.53 ± 0.53 b | 0.22 ± 0.11 b | 54 ± 12 a | −13 ± 1 |
| 50 mL | 2 ± 1 | 8 ± 2 | 1.23 ± 0.21 b | 0.21 ± 0.13 b | 38 ± 2 b | −54.9 ± 4 |
| Cell-free media of Gymnodinium impudicum | ||||||
| 25 mL | 6 ± 1 | 15 ± 4 | 1.97 ± 0.43 a | 0.33 ± 0.15 c | 50 ± 7 a | −50.1 ± 2 |
| 50 mL | 5 ± 2 | 4 ± 1 | 1.75 ± 0.48 a | 0.11 ± 0.04 a | 41 ± 9 c | −33.9 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Herrera, L.J.; Band-Schmidt, C.J.; Zenteno-Savín, T.; Leyva-Valencia, I.; Hernández-Guerrero, C.J.; Hernández-Sandoval, F.E.; Bustillos-Guzmán, J.J. Changes in Toxin Production, Morphology and Viability of Gymnodinium catenatum Associated with Allelopathy of Chattonella marina var. marina and Gymnodinium impudicum. Toxins 2022, 14, 616. https://doi.org/10.3390/toxins14090616
Fernández-Herrera LJ, Band-Schmidt CJ, Zenteno-Savín T, Leyva-Valencia I, Hernández-Guerrero CJ, Hernández-Sandoval FE, Bustillos-Guzmán JJ. Changes in Toxin Production, Morphology and Viability of Gymnodinium catenatum Associated with Allelopathy of Chattonella marina var. marina and Gymnodinium impudicum. Toxins. 2022; 14(9):616. https://doi.org/10.3390/toxins14090616
Chicago/Turabian StyleFernández-Herrera, Leyberth José, Christine Johanna Band-Schmidt, Tania Zenteno-Savín, Ignacio Leyva-Valencia, Claudia Judith Hernández-Guerrero, Francisco Eduardo Hernández-Sandoval, and José Jesús Bustillos-Guzmán. 2022. "Changes in Toxin Production, Morphology and Viability of Gymnodinium catenatum Associated with Allelopathy of Chattonella marina var. marina and Gymnodinium impudicum" Toxins 14, no. 9: 616. https://doi.org/10.3390/toxins14090616
APA StyleFernández-Herrera, L. J., Band-Schmidt, C. J., Zenteno-Savín, T., Leyva-Valencia, I., Hernández-Guerrero, C. J., Hernández-Sandoval, F. E., & Bustillos-Guzmán, J. J. (2022). Changes in Toxin Production, Morphology and Viability of Gymnodinium catenatum Associated with Allelopathy of Chattonella marina var. marina and Gymnodinium impudicum. Toxins, 14(9), 616. https://doi.org/10.3390/toxins14090616







