Exploring the Interplay between the Clinical and Presumed Effect of Botulinum Injections for Cervical Dystonia: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Demographical and Treatment Related Data
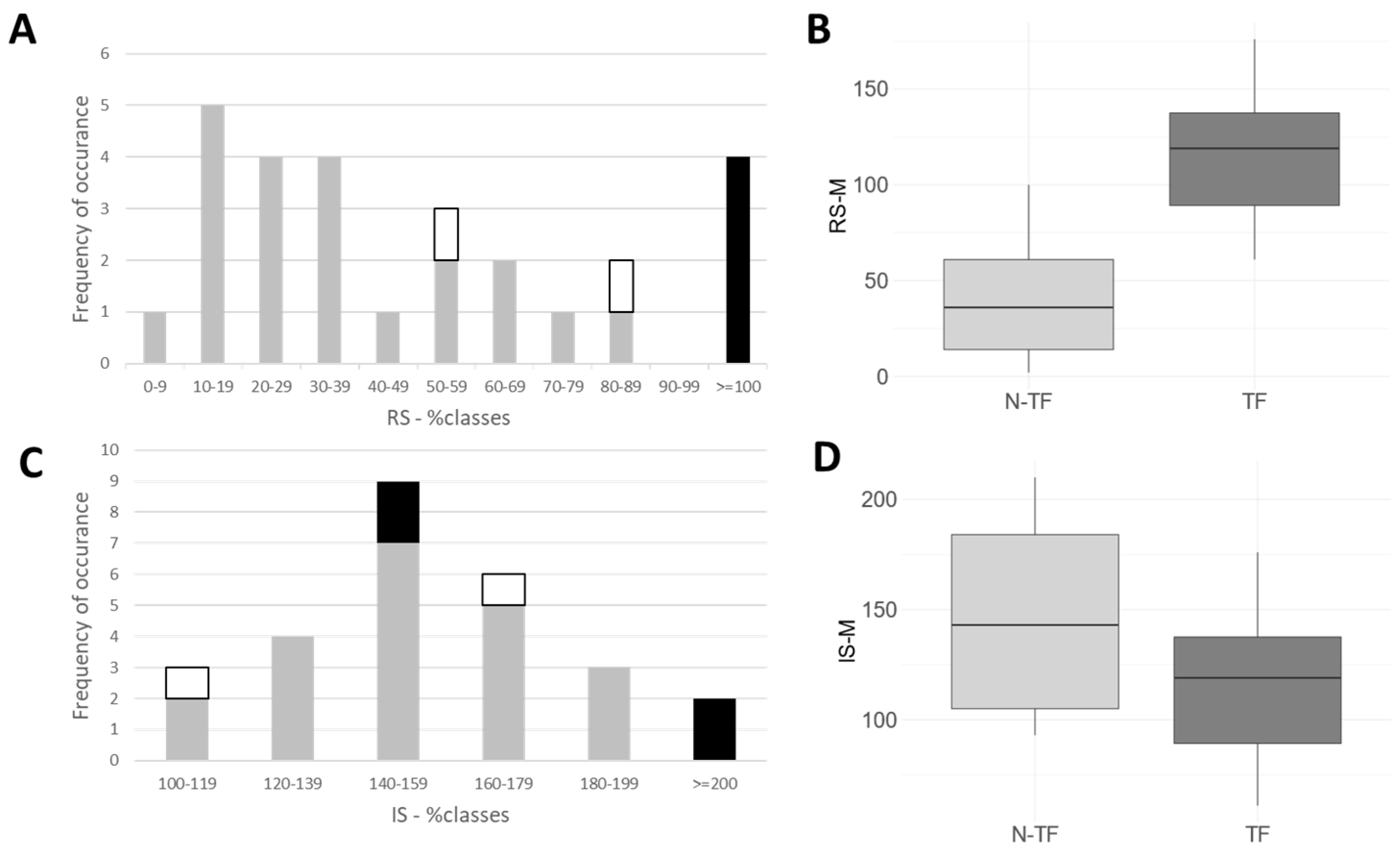
2.2. Long-Term Outcome, as Assessed by the Treating Physician and Patient
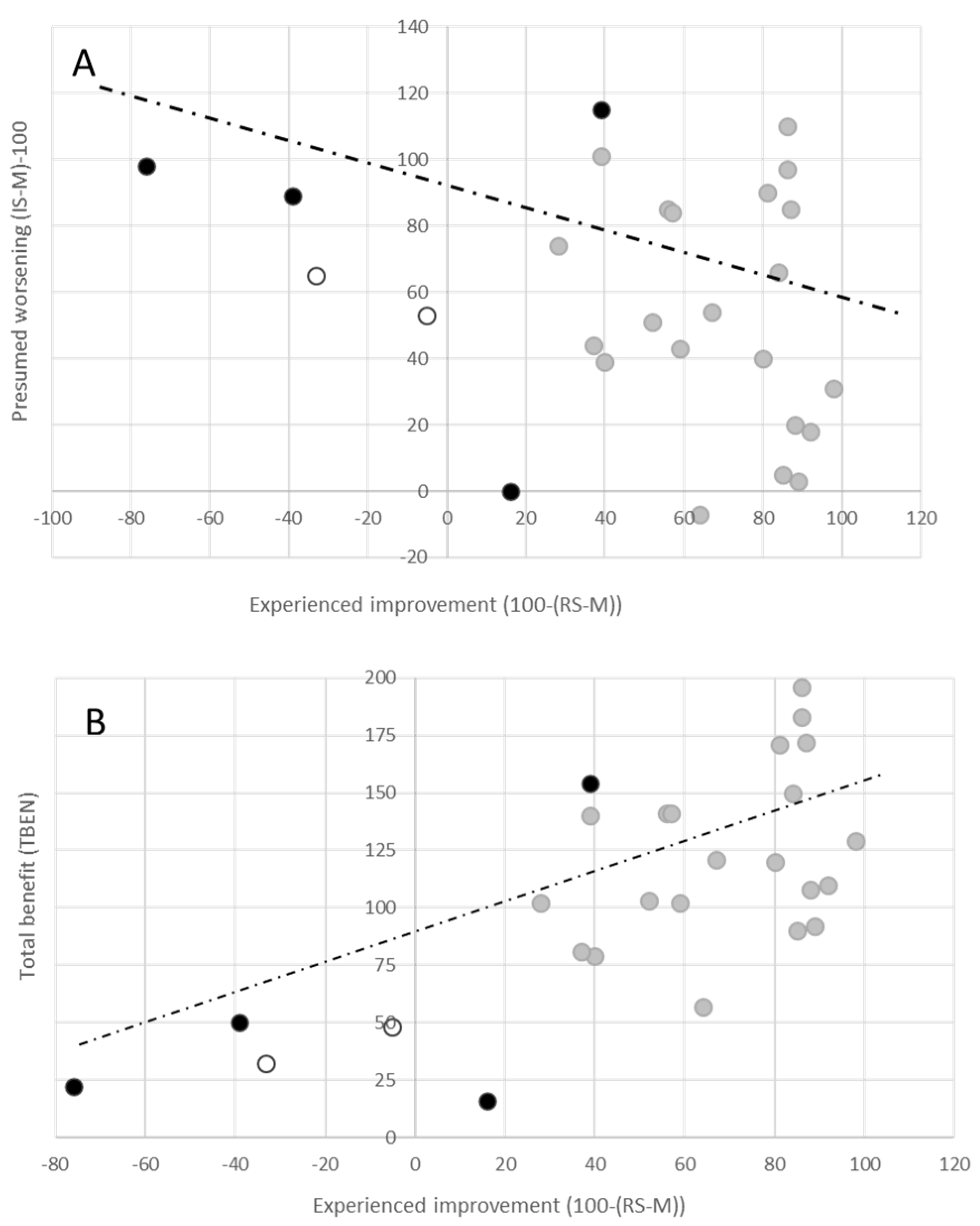
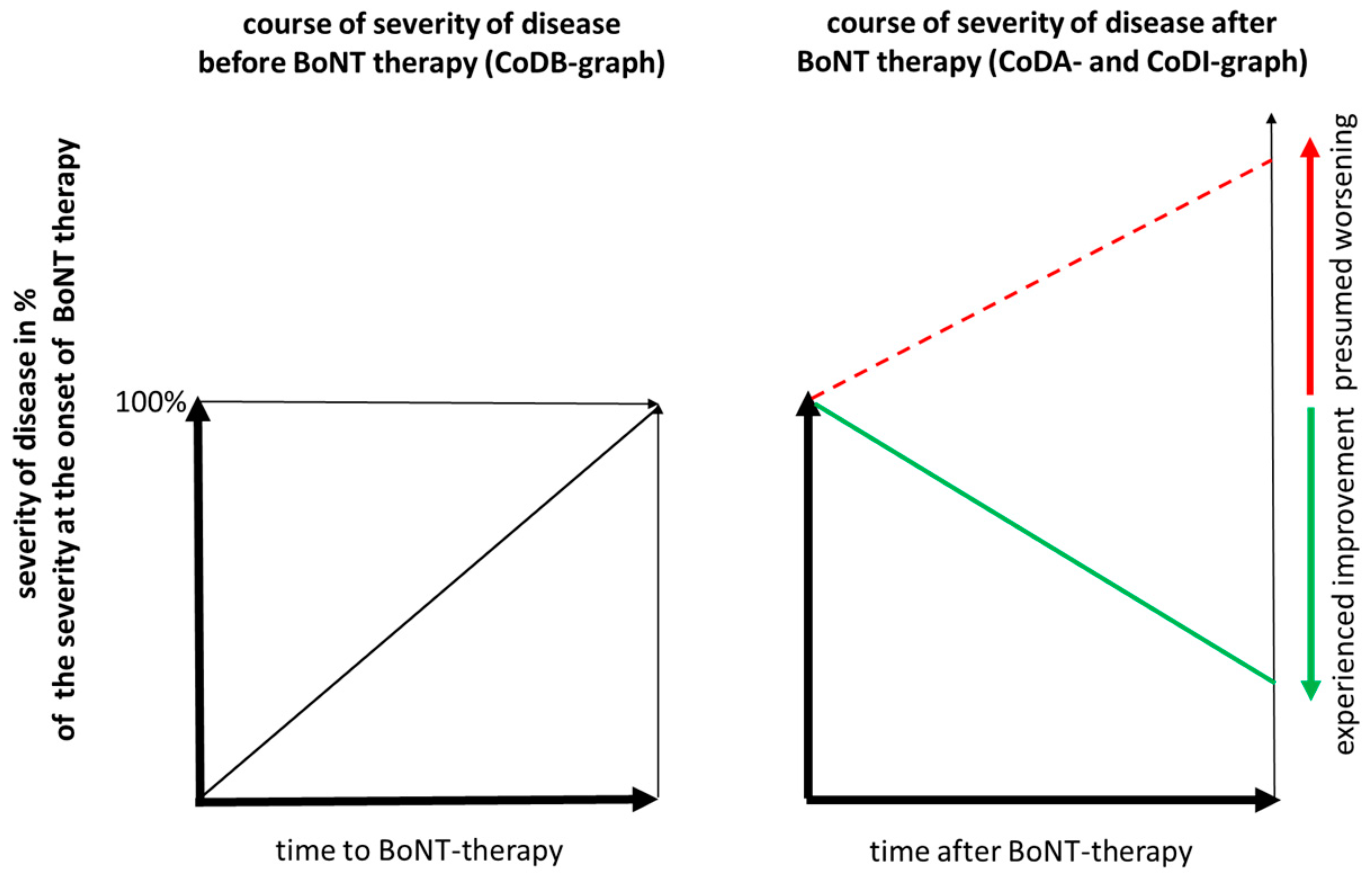
2.3. Assessment of Presumed Outcome without BoNT Injection Therapy or DBS Operation
2.4. Correlation Analysis
3. Discussion
3.1. Reasons for the Excellent Outcome in the Present Cohort
3.2. Realistic Estimation of the Progress of CD without Specific Therapy
3.3. Consequences of an “Presumed” Progression of CD without Specific Therapy
4. Conclusions
Strengths and Limitations of the Study
5. Materials and Methods
Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians BSoRM. Spasticity in Adults: Management Using Botulinum Toxin. In National Guidelines; Royal College of Physicians: London, UK, 2018; pp. 1–72. [Google Scholar]
- Skogseid, I.M.; Kerty, E. The course of cervical dystonia and patient satisfaction with long-term botulinum toxin A treatment. Eur. J. Neurol. 2005, 12, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Jinnah, H.A.; Goodmann, E.; Rosen, A.R.; Evatt, M.; Freeman, A.; Factor, S. Botulinum toxin treatment failures in cervical dystonia: Causes, management, and outcomes. J. Neurol. 2016, 263, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jansen, A.; Samadzadeh, S.; Kahlen, U.; Moll, M.; Ringelstein, M.; Soncin, G.; Bigalke, H.; Aktas, O.; Moldovan, A.; et al. Long-term adherence and response to botulinum toxin in different indications. Ann. Clin. Transl. Neurol. 2021, 8, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Sethi, K.D.; Rodriguez, R.; Olayinka, B. Satisfaction with botulinum toxin treatment: A cross-sectional survey of patients with cervical dystonia. J. Med. Econ. 2012, 15, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Schomaecker, I.; Schomaecker, M.; Ürer, B.; Brauns, R.; Rosenthal, D.; Albrecht, P.; Samadzadeh, S. Lessons about Botulinum Toxin A Therapy from Cervical Dystonia Patients Drawing the Course of Disease: A Pilot Study. Toxins 2023, 15, 431. [Google Scholar] [CrossRef]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology 2020, 94, e2109–e2120. [Google Scholar] [CrossRef]
- Lange, O.; Bigalke, H.; Dengler, R.; Wegner, F.; de Groot, M.; Wohlfarth, K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: Much ado about nothing. Clin. Neuropharmacol. 2009, 32, 213–218. [Google Scholar] [CrossRef]
- Fabbri, M.; Leodori, G.; Fernandes, R.M.; Bhidayasiri, R.; Marti, M.J.; Colosimo, C.; Ferreira, J.J. Neutralizing Antibody and Botulinum Toxin Therapy: A Systematic Review and Meta-Analysis. Neurotox. Res. 2016, 29, 105–117. [Google Scholar] [CrossRef]
- Bellows, S.; Jankovic, J. Immunogenicity associated with botulinum toxin treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef]
- Benecke, R.; Moore, P.; Dressler, D.; Naumann, M. Cervical and axial dystonia. In Handbook of Botulinum Toxin Treatment, 2nd ed.; Moore, P., Naumann, M., Eds.; Blackwell Science: Malden, MA, USA, 2003; pp. 158–194. [Google Scholar]
- Moll, M.; Rosenthal, D.; Hefter, H. Quality of life in long-term botulinum toxin treatment of cervical dystonia: Results of a cross sectional study. Park. Relat. Disord. 2018, 57, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Meares, R. Natural history of spasmodic torticollis, and effect of surgery. Lancet 1971, 298, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.; Kang, U.J.; Fahn, S. Spread of symptoms in idiopathic torsion dystonia. Mov. Disord. 1995, 10, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.A.; Jinnah, H.A.; Espay, A.J.; Klein, C.; Brüggemann, N.; Barbano, R.L.; Malaty, I.A.C.; Rodriguez, R.L.; Vidailhet, M.; Roze, E.; et al. Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov. Disord. 2016, 31, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Hefter, H.; Schoemaeker, I.; Schomaecker, M.; Hefter, H. Disease progression of idiopathic cervical dystonia in spite of improvement after botulinum toxin therapy. Front. Neurol. 2020, 11, 588395. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.K.; Eisen, A.; Stoessl, A.J.; Calne, S.; Calne, D.B. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet 1986, 2, 245–247. [Google Scholar] [CrossRef]
- Kessler, K.; Skutta, M.; Benecke, R. Long-term treatment of cervical dystonia with botulinum toxin A: Efficacy, safety, and antibody frequency. J. Neurol. 1999, 246, 265–274. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.-I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.-P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef]
- Hefter, H.; Rosenthal, D.; Moll, M. High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Mov. Disord. Clin. Pract. 2016, 3, 500–506. [Google Scholar] [CrossRef]
- Hefter, H.; Ürer, B.; Brauns, R.; Rosenthal, D.; Meuth, S.G.; Lee, J.-I.; Albrecht, P.; Samadzadeh, S. Significant long-lasting improvement after switch to incobotulinum toxin in cervical dystonia patients with secondary treatment failure. Toxins 2022, 14, 44. [Google Scholar] [CrossRef]
- Isaias, I.U.; Volkmann, J.; Kupsch, A.; Burgunder, J.-M.; Ostrem, J.L.; Alterman, R.L.; Mehdorn, H.M.; Schönecker, T.; Krauss, J.K.; Starr, P.; et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: The role of age and disease duration. J. Neurol. 2011, 258, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, J.; Mueller, J.; Deuschl, G.; A Kühn, A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal neurostimulation in patients with mediation-refractory cervical dystonia: A randomized, sham-controlled trial. Lancet Neurol. 2014, 13, 875–884. [Google Scholar] [CrossRef]
- Boyce, M.J.; Canning, C.G.; Mahant, N.; Morris, J.; Latimer, J.; Fung, V.S. The Toronto Western Spasmodic Torticollis Rating Scale: Reliability in neurologists and physiotherapists. Park. Relat. Disord. 2012, 18, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Krystkowiak, P.; du Montcel, S.T.; Vercueil, L.; Houeto, J.-L.; Lagrange, C.; Cornu, P.; Blond, S.; Benabid, A.-L.; Pollak, P.; Vidailhet, M.; et al. Reliability of the Burke-Fahn-Marsden scale in a multicenter trial for dystonia. Mov. Disord. 2007, 22, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Wissel, J.; Kemmler, G.; Voller, B.; Bodner, T.; Schneider, A.; Wenning, G.K.; Poewe, W. Craniocervical dystonia questionnaire (CDQ-24): Development and validation of a diseasespecific quality of life instrument. J. Neurol. Neurosurg. Psychiatry 2004, 75, 749–753. [Google Scholar] [CrossRef]
- Comella, C.L.; Leurgans, S.; Wuu, J.; Stebbins, G.T.; Chmura, T.; The Dystonia Study Group. Rating scales for dystonia: A multicenter assessment. Mov. Disord. 2003, 18, 303–312. [Google Scholar] [CrossRef]
- Benecke, R.; Jost, W.H.; Kanovsky, P.; Ruzicka, E.; Comes, G.; Grafe, S. A new botulinum toxin type A free of complexing proteins for treatment of cervical Dystonia. Neurology 2005, 64, 1949–1951. [Google Scholar] [CrossRef]
- Dressler, D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov. Disord. 2004, 19, S92–S100. [Google Scholar] [CrossRef]
- Martino, D.; Berardelli, A.; Abbruzzese, G.; Bentivoglio, A.R.; Esposito, M.; Fabbrini, G.; Guidubaldi, A.; Girlanda, P.; Liguori, R.; Marinelli, L.; et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov. Disord. 2012, 27, 1447–1450. [Google Scholar] [CrossRef]
- Münchau, A.; Schrag, A.; Chuang, C.; MacKinnon, C.D.; Bhatia, K.P.; Quinn, N.P.; Rothwell, J.C. Arm tremor in cervical dystonia differs from essential tremor and can be classified by onset age and spread of symptoms. Brain 2001, 124, 1765–1776. [Google Scholar] [CrossRef]
- Yahalom, G.; Fay-Karmon, T.; Livneh, V.; Israeli-Korn, S.; Ephraty, L.; Hassin-Baer, S. Botulinum Injections for Idiopathic Cervical Dystonia: A Longitudinal Study. Neurotox. Res. 2021, 39, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Mejia, N.I.; Vuong, K.D.; Jankovic, J. Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov. Disord. 2005, 20, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Contarino, M.F.; Van Den Dool, J.; Balash, Y.; Bhatia, K.; Giladi, N.; Koelman, J.H.; Lokkegaard, A.; Marti, M.J.; Postma, M.; Relja, M.; et al. Clinical Practice: Evidence-Based Recommendations for the Treatment of Cervical Dystonia with Botulinum Toxin. Front. Neurol. 2017, 8, 35. [Google Scholar] [CrossRef] [PubMed]
| Parameter | TF Group | N-TF Group | ALL | Wilcoxon p-Value |
|---|---|---|---|---|
| N= | 6 | 21 | 27 | |
| SEX (f/m) | 2/4 | 7/14 | 9/18 | |
| AOS (years) | 40.8/12.9 | 51.7/13.5 | 49.2/14.3 | 0.175 |
| AOT (years) | 48.7/12.4 | 54.1/13.4 | 53.1/12.7 | 0.670 |
| AGE (years) | 62.5/4.5 | 69.8/12.5 | 68.1/11.9 | 0.097 |
| DURS (years) | 9.00/10.00 | 2.43/2.16 | 3.89/5.66 | 0.252 |
| DURT (years) | 12.6/5.2 | 15.5/9.0 | 14.9/8.5 | 0.798 |
| ATSUI | 5.33/1.96 | 3.00/1.90 | 3.41/2.21 | 0.024 |
| ADOSE (uDU) | 411/128 | 330/86 | 341/108 | 0.202 |
| RS-% | 105/37.1 | 29.0/19.4 | 46.0/37.7 | 0.001 |
| RS-M | 116/40.6 | 31.0/20.6 | 50.0/41.7 | 0.0009 |
| IS-% | 164/37.2 | 147/21.9 | 151/26.3 | 0.239 |
| IS-M | 170/40.9 | 154/33.9 | 158/35.1 | 0.307 |
| AOS | AOT | AGE | DURS | DURT | RS-% | RS-M | IS-% | IS-M | ADOSE | ATSUI | 100-(RS-%) | (IS-%)-100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AOS | r = 0.91 * | r = 0.67 * | r = −0.44 * | r = −0.38 * | r = −0.19 | r = −0.19 | r = −0.08 | r = 0.14 | r = −0.35 | r = −0.27 | r = 0.19 | r = −0.08 | |
| AOT | p = 0.000 | r = 0.76 * | r = −0.04 | r = −0.38 | r = 0.11 | r = 0.13 | r = 0.04 | r = 0.27 | r = −0.36 | r = −0.07 | r = −0.11 | r = 0.04 | |
| AGE | p = 0.000 | p = 0.000 | r = 0.04 | r = 0.31 | r = 0.02 | r = 0.02 | r = 0.01 | r = 0.14 | r = −0.34 | r = 0.07 | r = −0.02 | r = 0.01 | |
| DURS | p = 0.022 | p = 0.858 | p = 0.860 | r = 0.10 | r = 0.72 * | r = 0.73 * | r = 0.30 | r = 0.25 | r = 0.04 | r = 0.50 * | r = −0.72 * | r = 0.30 | |
| DURT | p = 0.050 | p = 0.053 | p = 0.116 | p = 0.604 | r = −0.13 | r = −0.16 | r = −0.05 | r = −0.18 | r = 0.05 | r = 0.21 | r = 0.13 | r = −0.05 | |
| RS-% | p = 0.331 | p = 0.584 | p = 0.905 | p = 0.000 | p = 0.524 | r = 0.99 * | r = 0.27 | r = 0.23 | r = 0.21 | r = 0.56 * | r = −1.00 * | r = 0.27 | |
| RS-M | p = 0.354 | p = 0.531 | p = 0.936 | p = 0.000 | p = 0.418 | p = 0.000 | r = 0.31 | r = 0.27 | r = 0.18 | r = 0.54 * | r = −0.99 * | r = 0.31 | |
| IS-% | p = 0.678 | p = 0.827 | p = 0.952 | p = 0.124 | p = 0.813 | p = 0.167 | p = 0.119 | r = 0.92 * | r = 0.49 * | r = 0.50 * | r = −0.27 | r = 1.00 * | |
| IS-M | p = 0.496 | p = 0.181 | p = 0.471 | p = 0.209 | p = 0.361 | 0.250 | p = 0.169 | p = 0.000 | r = 0.30 | r = 0.43 * | r = −0.23 | r = 0.92 * | |
| ADOSE | p = 0.078 | p = 0.062 | p = 0.081 | p = 0.832 | p = 0.820 | p = 0.293 | p = 0.380 | p = 0.009 | p = 0.123 | r = 0.55 * | r = −0.21 | r = 0.49 * | |
| ATSUI | p = 0.178 | p = 0.721 | p = 0.719 | p = 0.008 | p = 0.292 | p = 0.003 | p = 0.003 | p = 0.008 | p = 0.025 | p = 0.003 | r = −0.56 * | r = 0.50 * | |
| 100-(RS-%) | p = 0.331 | p = 0.584 | p = 0.905 | p = 0.000 | p = 0.524 | p = 0.000 | p = 0.000 | p = 0.167 | p = 0.250 | p = 0.293 | p = 0.003 | r = −0.27 | |
| (IS-%)-100 | p = 0.678 | p = 0.827 | p = 0.952 | p = 0.124 | p = 0.813 | p = 0.167 | p = 0.119 | p = 0.000 | p = 0.000 | p = 0.009 | p = 0.008 | p = 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefter, H.; Samadzadeh, S. Exploring the Interplay between the Clinical and Presumed Effect of Botulinum Injections for Cervical Dystonia: A Pilot Study. Toxins 2023, 15, 592. https://doi.org/10.3390/toxins15100592
Hefter H, Samadzadeh S. Exploring the Interplay between the Clinical and Presumed Effect of Botulinum Injections for Cervical Dystonia: A Pilot Study. Toxins. 2023; 15(10):592. https://doi.org/10.3390/toxins15100592
Chicago/Turabian StyleHefter, Harald, and Sara Samadzadeh. 2023. "Exploring the Interplay between the Clinical and Presumed Effect of Botulinum Injections for Cervical Dystonia: A Pilot Study" Toxins 15, no. 10: 592. https://doi.org/10.3390/toxins15100592
APA StyleHefter, H., & Samadzadeh, S. (2023). Exploring the Interplay between the Clinical and Presumed Effect of Botulinum Injections for Cervical Dystonia: A Pilot Study. Toxins, 15(10), 592. https://doi.org/10.3390/toxins15100592





