Impact of Endogenous Pneumococcal Hydrogen Peroxide on the Activity and Release of Pneumolysin
Abstract
:1. Introduction
2. Results
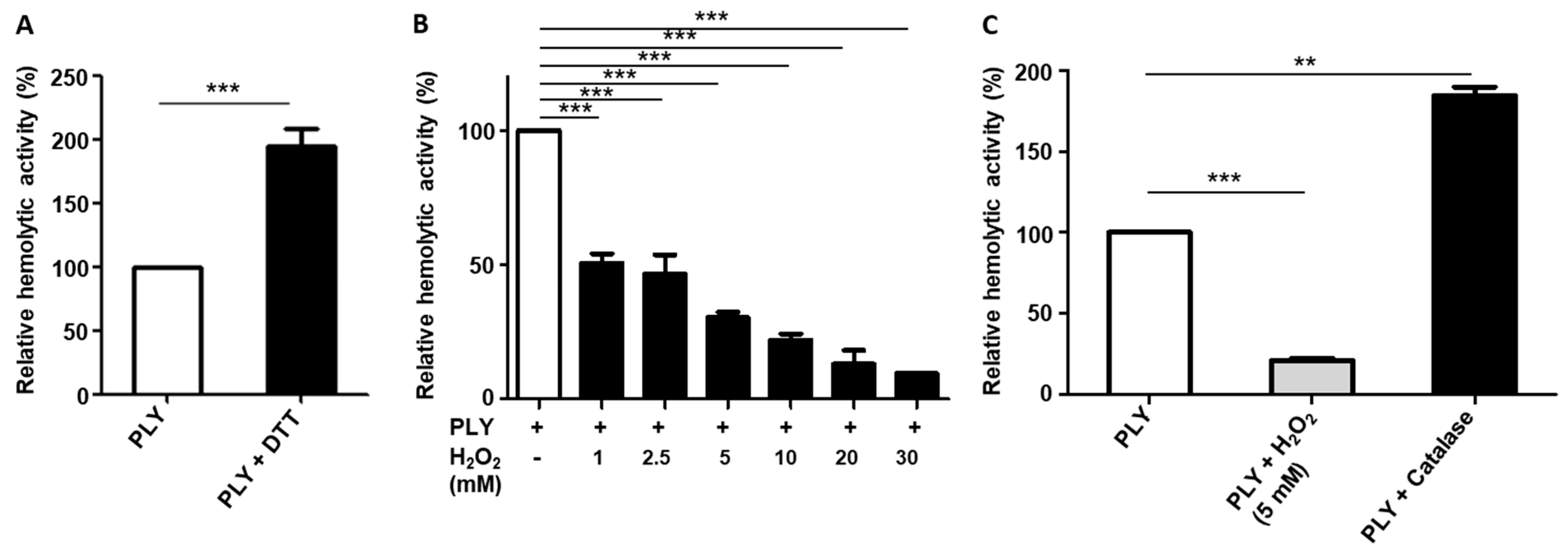
2.1. H2O2 Affects the Haemolytic Activity of PLY
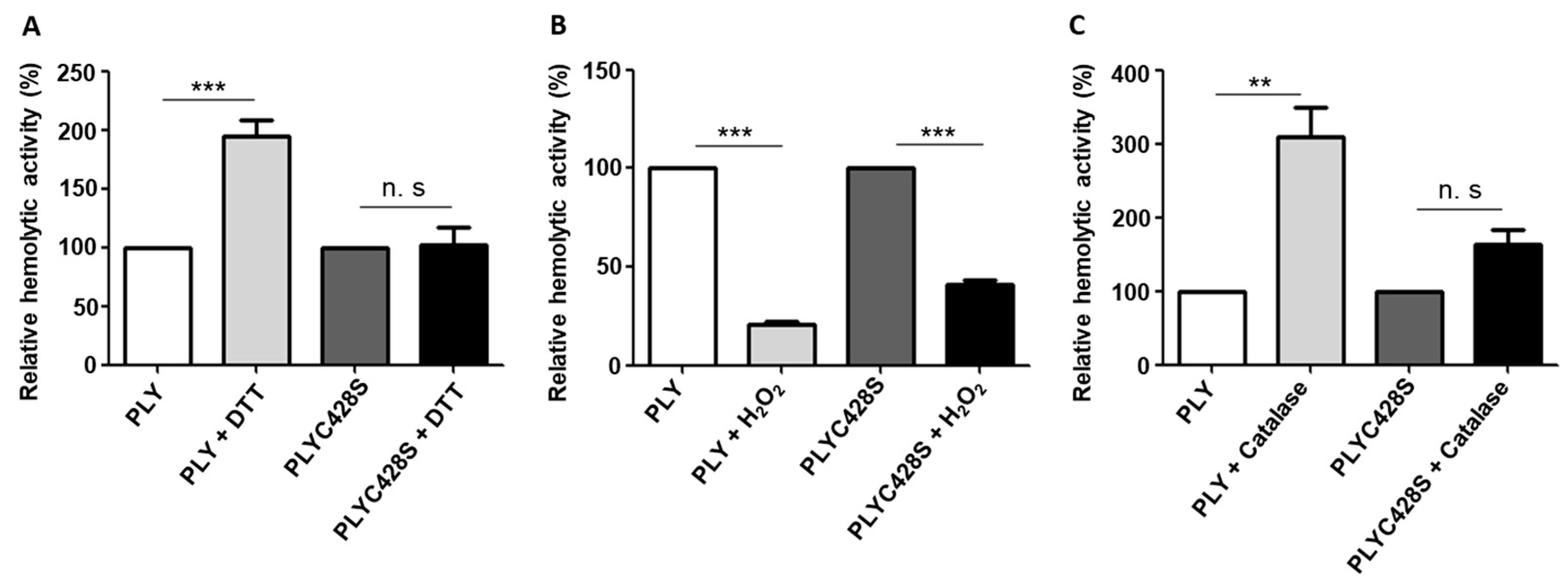
2.2. The Unique Cysteine Residue of PLY Contributes to the H2O2-Dependent Decrease in Its Haemolytic Activity
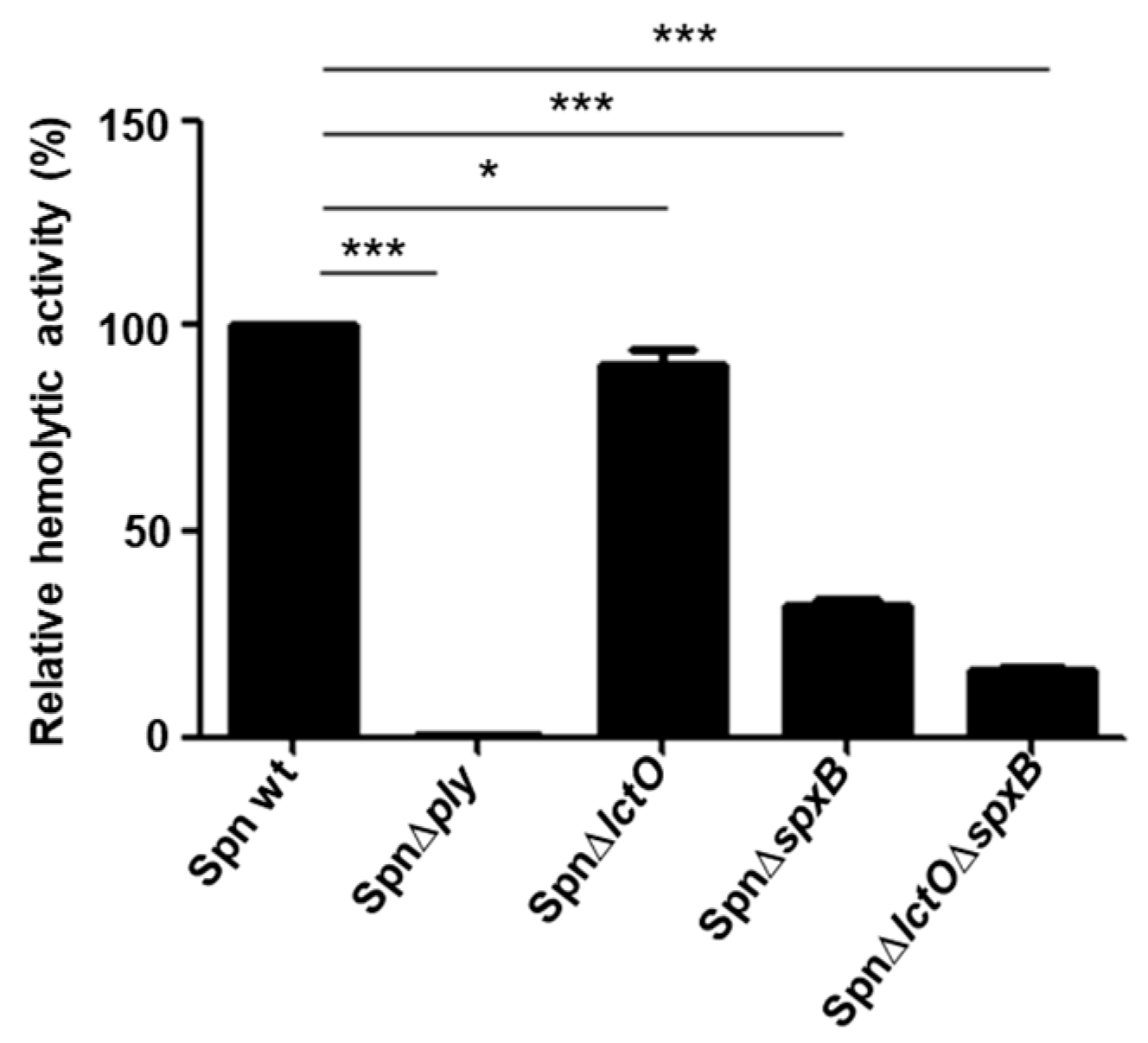
2.3. Supernatants of S. pneumoniae Mutant Strains with Impaired H2O2 Production Have Reduced Haemolytic Activity
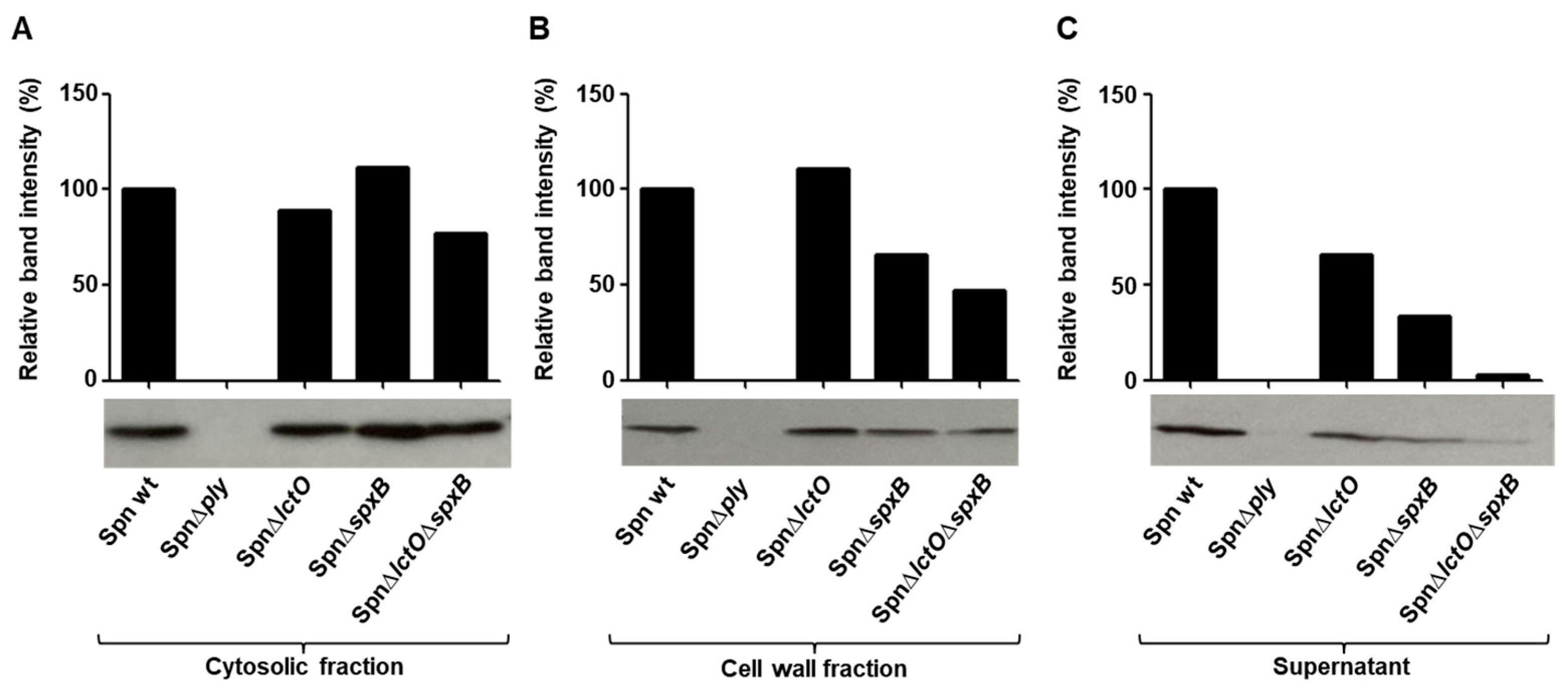
2.4. Endogenously Produced Pneumococcal H2O2 Affects PLY Release
2.5. Upregulation of Cell-Wall Synthesis and Choline-Binding Genes in S. pneumoniae Mutants Impaired in H2O2 Production
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains and Growth Conditions
5.2. Generation of PLYC428S and Isolation of PLY
5.3. Haemolysis Assay
5.4. Detection and Quantification of PLY
5.5. Minimum Inhibitory Concentration (MIC) Determination
5.6. Cell Culture Conditions
5.7. Adhesion Assays
5.8. RNA Isolation
5.9. Quantitative RT-PCR
5.10. Library Preparation for RNA-Sequencing
5.11. RNA-Seq Analyses
5.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullahi, O.; Karani, A.; Tigoi, C.C.; Mugo, D.; Kungu, S.; Wanjiru, E.; Jomo, J.; Musyimi, R.; Lipsitch, M.; Scott, J.A.G. The Prevalence and Risk Factors for Pneumococcal Colonization of the Nasopharynx among Children in Kilifi District, Kenya. PLoS ONE 2012, 7, e30787. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, R.Y.; den Heijer, C.; van Bijnen, E.M.; Paget, W.J.; Pringle, M.; Goossens, H.; Bruggeman, C.A.; Schellevis, F.G.; Stobberingh, E.E.; APRES Study Team. Prevalence and antibiotic resistance of commensal Streptococcus pneumoniae in nine European countries. Future Microbiol. 2016, 11, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.J.; Orlova, E.V.; Gilbert, R.J.; Andrew, P.W.; Saibil, H.R. Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 2005, 121, 247–256. [Google Scholar] [CrossRef] [PubMed]
- van Pee, K.; Neuhaus, A.; D’Imprima, E.; Mills, D.J.; Kuhlbrandt, W.; Yildiz, O. CryoEM structures of membrane pore and prepore complex reveal cytolytic mechanism of Pneumolysin. eLife 2017, 6, e23644. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejas, M.J. Pneumococcal virulence factors: Structure and function. Microbiol. Mol. Biol. Rev. 2001, 65, 187–207. [Google Scholar] [CrossRef]
- Johnson, M.K.; Aultman, K.S. Studies on the mechanism of action of oxygen-labile haemolysins. J. Gen. Microbiol. 1977, 101, 237–241. [Google Scholar] [CrossRef]
- Berry, A.M.; Lock, R.A.; Hansman, D.; and Paton, J.C. Contribution of autolysin to virulence of Streptococcus pneumoniae. Infect. Immun. 1989, 57, 2324–2330. [Google Scholar] [CrossRef]
- Canvin, J.R.; Marvin, A.P.; Sivakumaran, M.; Paton, J.C.; Boulnois, G.J.; Andrew, P.W.; Mitchell, T.J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 1995, 172, 119–123. [Google Scholar] [CrossRef]
- Benton, K.A.; Paton, J.P.; Briles, D.E. Differences in Virulence for Mice among Streptococcus of Capsular Types 2, 3, 4, 5, and 6 Are Not Attributable to Differences in Pneumolysin Production. Infect. Immun. 1997, 65, 1237–1244. [Google Scholar] [CrossRef]
- Balachandran, P.; Hollingshead, S.K.; Paton, J.C.; Briles, D.E. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 2001, 183, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Yang, G.; Gorshkov, B.A.; Zemskov, E.A.; Sridhar, S.; Umapathy, N.S.; Jezierska-Drutel, A.; Alieva, I.B.; Leustik, M.; Hossain, H.; et al. Protein kinase C-α and arginase I mediate pneumolysin-induced pulmonary endothelial hyperpermeability. Am. J. Respir. Cell Mol. Biol. 2012, 47, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.M.; Xu, S.; Leong, J.M.; Sousa, S. The Yin and Yang of Pneumolysin during Pneumococcal Infection. Front. Immunol. 2022, 13, 878244. [Google Scholar] [CrossRef] [PubMed]
- Lisher, J.P.; Tsui, H.C.T.; Ramos-Montañez, S.; Hentchel, K.L.; Martin, J.E.; Trinidad, J.C.; Winkler, M.E.; Giedroc, D.P. Biological and Chemical Adaptation to Endogenous Hydrogen Peroxide Production in Streptococcus pneumoniae D39. mSphere 2017, 2, e00291-16. [Google Scholar] [CrossRef] [PubMed]
- Mraheil, M.A.; Toque, H.A.; La Pietra, L.; Hamacher, J.; Phanthok, T.; Verin, A.; Gonzales, J.; Su, Y.; Fulton, D.; Eaton, D.C.; et al. Dual Role of Hydrogen Peroxide as an Oxidant in Pneumococcal Pneumonia. Antioxid. Redox Signal. 2021, 34, 962–978. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Trzcinski, K.; Thompson, C.M.; Malley, R.; Lipsitch, M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 4996–5001. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Trzcinski, K.; Thompson, C.M.; Lipsitch, M.; Malley, R. SpxB is a suicide gene of Streptococcus pneumoniae and confers a selective advantage in an in vivo competitive colonization model. J. Bacteriol. 2007, 189, 6532–6539. [Google Scholar] [CrossRef]
- Pericone, C.D.; Overweg, K.; Hermans, P.W.; Weiser, J.N. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 2000, 68, 3990–3997. [Google Scholar] [CrossRef]
- Yesilkaya, H.; Andisi, V.F.; Andrew, P.W.; Bijlsma, J.J.E. Streptococcus pneumoniae and reactive oxygen species: An unusual approach to living with radicals. Trends Microbiol. 2013, 21, 187–195. [Google Scholar] [CrossRef]
- Pericone, C.D.; Park, S.; Imlay, J.A.; Weiser, J.N. Factors Contributing to Hydrogen Peroxide Resistance in Streptococcus pneumoniae Include Pyruvate Oxidase (SpxB) and Avoidance of the Toxic Effects of the Fenton reaction. J. Bacteriol. 2003, 185, 6815–6825. [Google Scholar] [CrossRef]
- Rai, P.; Parrish, M.; Tay, I.J.J.; Li, N.; Ackerman, S.; He, F.; Kwang, J.; Engelward, B.P. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3421–E3430. [Google Scholar] [CrossRef] [PubMed]
- Erttmann, S.F.; Gekara, N. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef] [PubMed]
- Loose, M.; Hudel, M.; Zimmer, K.-P.; Garcia, E.; Hammerschmidt, S.; Lucas, R.; Chakraborty, T.; Pillich, H. Pneumococcal hydrogen peroxide-induced stress signaling regulates inflammatory genes. J. Infect. Dis. 2015, 211, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.; Apte, S.; Joseph, J.; Parthasarathy, A.; Madhavan, S.; Banerjee, A. Pyruvate Oxidase as a Key Determinant of Pneumococcal Viability during Transcytosis across Brain Endothelium. J. Bacteriol. 2021, 203, e0043921. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; He, F.; Kwang, J.; Engelward, B.P.; Chow, V.T.K. Pneumococcal Pneumolysin Induces DNA Damage and Cell Cycle Arrest. Sci. Rep. 2016, 6, 22972. [Google Scholar] [CrossRef]
- Lucas, R.; Hadizamani, Y.; Enkhbaatar, P.; Csanyi, G.; Caldwell, R.W.; Hundsberger, H.; Sridhar, S.; Lever, A.A.; Hudel, M.; Ash, D.; et al. Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance. Front. Physiol. 2022, 12, 793251. [Google Scholar] [CrossRef]
- Rubins, J.B.; Duane, P.G.; Clawson, D.; Charboneau, D.; Young, J.; Niewoehner, D.E. Toxicity of Pneumolysin to Pulmonary Alveolar Epithelial Cells. Infect. Immun. 1993, 61, 1352–1358. [Google Scholar] [CrossRef]
- Rayner, C.F.; Jackson, A.D.; Rutman, A.; Dewar, A.; Mitchell, T.J.; Andrew, P.W.; Cole, P.J.; Wilson, R. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect. Immun. 1995, 63, 442–447. [Google Scholar] [CrossRef]
- Rao, R. Oxidative-Stress induced disruption of epithelial and endothelial tight junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Zahlten, J.; Kim, Y.J.; Doehn, J.M.; Pribyl, T.; Hocke, A.C.; García, P.; Hammerschmidt, S.; Suttorp, N.; Hippenstiel, S.; Hübner, R.H. Streptococcus pneumoniae-induced oxidative stress in lung epitheliacells depends on pneumococcal autolysis and is reversible by resveratrol. J. Infect. Dis. 2015, 211, 1822–1830. [Google Scholar] [CrossRef]
- Peter, A.; Fatykhova, D.; Kershaw, O.; Gruber, A.D.; Rueckert, J.; Neudecker, J.; Toennies, M.; Bauer, T.T.; Schneider, P.; Schimek, M.; et al. Localization and pneumococcal alteration of junction proteins in the human alveolar-capillary compartment. Histochem. Cell Biol. 2017, 147, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Spellerberg, B.; Cundell, D.R.; Sandros, J.; Pearce, B.J.; Idanpaan-Heikkila, I.; Rosenow, C.; Masure, H.R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 1996, 19, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Cleland, W.W. Dithiothreitol, a new protective reagent for SH groups. Biochemistry 1964, 3, 480–482. [Google Scholar] [CrossRef]
- Ohkura, K.; Nagamune, H.; Kourai, H. Structural Analysis of Human Specific Cytolysin Intermedilysin Aiming Application to Cancer Immunotherapy. Anticancer Res. 2004, 24, 3343–3354. [Google Scholar]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Ansari, A.; Siddiqui, Z.; Ashraf, J.M.; Abdullah, S.M.S.; et al. Protein oxidation: An overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. Sch. 2017, 24, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.L.; Carroll, K.S. The Redox Biochemistry of Protein Sulfenylation and Sulfinylation. J. Biol. Chem. 2013, 288, 26480–26488. [Google Scholar] [CrossRef] [PubMed]
- Maestro, B.; Sanz, J.M. Choline, Binding Proteins from Streptococcus pneumoniae: A Dual Role as Enzybiotics and Targets for the Design of New Antimicrobials. Antibiotics 2016, 5, 21. [Google Scholar] [CrossRef]
- Greene, N.G.; Narciso, A.R.; Filipe, S.R.; Camilli, A. Peptidoglycan Branched Stem Peptides Contribute to Streptococcus pneumoniae Virulence by Inhibiting Pneumolysin Release. PLoS Pathog. 2015, 11, e1004996. [Google Scholar] [CrossRef]
- Kaur, R.; Surendran, N.; Ochs, M.; Pichicheroa, M.E. Human Antibodies to PhtD, PcpA, and Ply Reduce Adherence to Human Lung Epithelial Cells and Murine Nasopharyngeal Colonization by Streptococcus pneumoniae. Infect. Immun. 2014, 82, 5069–5075. [Google Scholar] [CrossRef]
- Grousd, J.A.; Rich, H.E.; Alcorn, J.F. Host-Pathogen Interactions in Gram-Positive Bacterial Pneumonia. Clin. Microbiol. Rev. 2019, 32, e00107-18. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Rafikov, R.; Haigh, S.; Zhi, W.B.; Kumar, S.; Doulias, P.T.; Rafikova, O.; Pillich, H.; Chakraborty, T.; et al. RhoA S-nitrosylation as a regulatory mechanism influencing endothelial barrier function in response to G+-bacterial toxins. Biochem. Pharmacol. 2017, 127, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Stevenson Keller, T.C.; Kramer, C.M.; Luckstadt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krot, M.M. Red blood cell function and dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.C.; Dabbs, R.C.; Oswalt, K.L.; Brown, L.R.; Rosch, J.W.; Seo, K.S.; Donaldson, J.R.; McDaniel, L.S.; Thornton, J.A. Pyruvate oxidase of Streptococcus pneumoniae contributes to pneumolysin release. BMC Microbiol. 2016, 16, 271. [Google Scholar] [CrossRef]
- Benisty, R.; Cohen, A.Y.; Feldman, A.; Cohen, Z.; Porat, N. Endogenous H2O2 produced by Streptococcus pneumoniae controls FabF activity. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2010, 1801, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Filipe, S.R.; Tomasz, A. Inhibition of the expression of penicillin resistance in Streptococcus pneumoniae by inactivation of cell wall muropeptide branching genes. Proc. Natl. Acad. Sci. USA 2000, 97, 4891–4896. [Google Scholar] [CrossRef]
- Khan, M.N.; Sharma, S.K.; Filkins, L.M.; Pichichero, M.E. PcpA of Streptococcus pneumoniae mediates adherence tonasopharyngeal and lung epithelial cells and elicits functional antibodies in humans. Microbes Infect. 2012, 14, 1102–1110. [Google Scholar] [CrossRef]
- Selva, L.; Ciruela, P.; Blanchette, K.; Del Amo, E.; Pallares, R.; Orihuela, C.J.; Muñoz-Almagro, C. Prevalence and Clonal Distribution of pcpA psrP and Pilus-1 Among Pediatric Isolates of Streptococcus Pneumoniae. PLoS ONE 2012, 7, e41587. [Google Scholar] [CrossRef]
- Johnston, J.W.; Briles, D.E.; Myers, L.E.; Hollingshead, S.K. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 2006, 74, 1171–1180. [Google Scholar] [CrossRef]
- Pluvinage, B.; Chitayat, S.; Ficko-Blean, E.; Abbott, D.W.; Kunjachen, J.M.; Grodin, J.; Spencer, H.L.; Smith, S.P.; Boraston, B.A. Analysis of StrH, the Surface-Attached exo-β-d-N-Acetylglucosaminidase from Streptococcus pneumoniae. J. Mol. Biol. 2013, 2, 334–349. [Google Scholar] [CrossRef]
- King, S.J.; Hippe, K.R.; Weiser, J.N. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 2006, 59, 961–974. [Google Scholar] [CrossRef]
- Ramos-Sevillano, E.; Moscoso, M.; García, P.; García, E.; Yuste, J. Nasopharyngeal colonization and invasive disease are enhanced by the cell wall hydrolases LytB and LytC of Streptococcus pneumoniae. PLoS ONE 2011, 6, e23626. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Sridhar, S.; Rick, F.G.; Gorshkov, B.; Umapathy, N.S.; Yang, G.; Oseghale, A.; Verin, A.D.; Chakraborty, T.; Matthay, M.A.; et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc. Natl. Acad. Sci. USA 2012, 109, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Lock, R.A.; Hansman, D.J. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 1983, 40, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Price, K.E.; Camilli, A. Pneumolysin Localizes to the Cell Wall of Streptococcus pneumonia. J. Bacteriol. 2009, 191, 2163–2168. [Google Scholar] [CrossRef]
- Mraheil, M.A.; Billion, A.; Mohamed, W.; Mukherjee, K.; Kuenne, C.; Pischimarov, J.; Krawitz, C.; Retey, J.; Hartsch, T.; Chakraborty, T.; et al. The intracellular sRNA transcriptome of Listeria monocytogenes during growth in macrophages. Nucleic Acids Res. 2011, 39, 4235–4248. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. Computer Software. 2022. Available online: https://github.com/raivokolde/pheatmap (accessed on 9 August 2023).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Wickham, H. reshape2: Flexibly Reshape Data: A Reboot of the Reshape Package. R Package Version 1.4.4. Computer Software. 2020. Available online: https://github.com/cran/reshape2 (accessed on 9 August 2023).
- Slowikowski, K. ggrepel: Repulsive Text and Label Geoms for “ggplot2”. R Package Version 0.9-10. Computer Software. 2021. Available online: https://github.com/slowkow/ggrepel (accessed on 9 August 2023).
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labelling. R Package Version 1.18.0. Computer Software. 2023. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 9 August 2023).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R Package Version 1.1-3. Computer Software. 2022. Available online: xxxhttps://github.com/cran/RColorBrewer (accessed on 9 August 2023).
- Linlin, Y. ggvenn: Draw Venn Diagram by ‘ggplot2’. R Package Version 0.1.9. Computer Software. 2021. Available online: https://github.com/yanlinlin82/ggvenn (accessed on 9 August 2023).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Arni Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data. R Package Version 3.1.3. Computer Software. 2022. Available online: https://github.com/cran/gplots (accessed on 9 August 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. forcats: Tools for Working with Categorical Variables (Factors). R Package Version 0.5. 1. Computer Software. 2021. Available online: https://github.com/tidyverse/forcats (accessed on 9 August 2023).
- Mailund, T. Manipulating data frames: Dplyr. In R 4 Data Science Quick Reference: A Pocket Guide to APIs, Libraries, and Packages; Apress: Berkeley, CA, USA, 2019; pp. 109–160. [Google Scholar]
- Schauberger, P.; Walker, A.; Braglia, L. Openxlsx: Read, Write and Edit xlsx Files. R Package Version 4.2.5.2. Computer Software. 2020. Available online: https://github.com/ycphs/openxlsx (accessed on 9 August 2023).
- Stephens, M. False discovery rates: A new deal. Biostatistics 2017, 18, 275–294. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazant, J.; Ott, B.; Hudel, M.; Hain, T.; Lucas, R.; Mraheil, M.A. Impact of Endogenous Pneumococcal Hydrogen Peroxide on the Activity and Release of Pneumolysin. Toxins 2023, 15, 593. https://doi.org/10.3390/toxins15100593
Bazant J, Ott B, Hudel M, Hain T, Lucas R, Mraheil MA. Impact of Endogenous Pneumococcal Hydrogen Peroxide on the Activity and Release of Pneumolysin. Toxins. 2023; 15(10):593. https://doi.org/10.3390/toxins15100593
Chicago/Turabian StyleBazant, Jasmin, Benjamin Ott, Martina Hudel, Torsten Hain, Rudolf Lucas, and Mobarak Abu Mraheil. 2023. "Impact of Endogenous Pneumococcal Hydrogen Peroxide on the Activity and Release of Pneumolysin" Toxins 15, no. 10: 593. https://doi.org/10.3390/toxins15100593




