Efficacy of Botulinum Toxin Type A for Prevention of Post-Mastectomy Scar in Transmen: A Prospective, Randomized Study
Abstract
:1. Introduction
2. Results
2.1. VSS Results of BoNT-A Treatment
2.2. POSAS Results of incoBoNT-A Treatment
2.3. Scar Color Assessment by Colorimeter
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Subjects
5.2. Randomization and Blinding
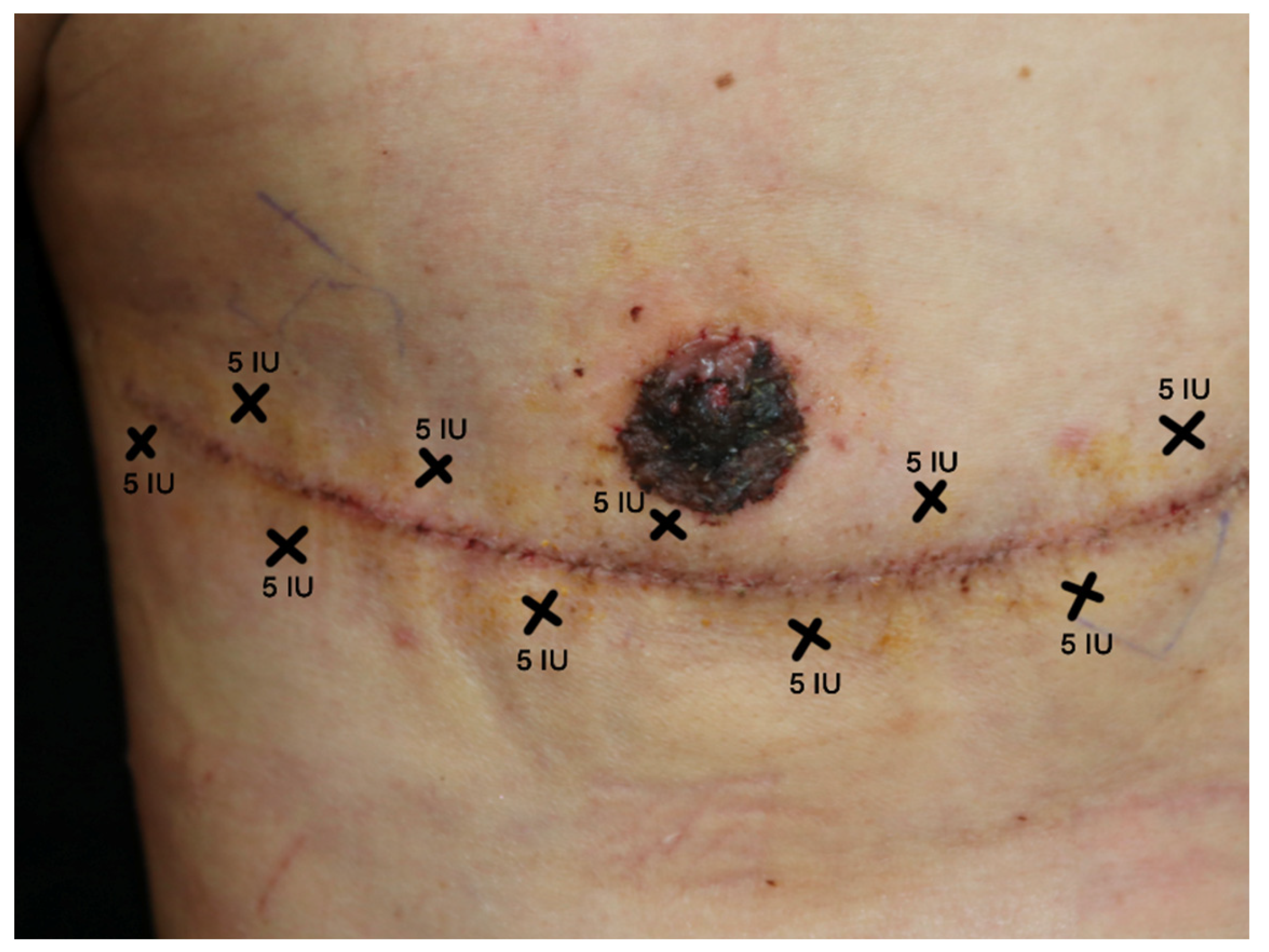
5.3. Treatment Protocol
5.4. Outcome Measurement
- L*: Represents the lightness of the color, where L* = 0 indicates black and L* = 100 indicates white.
- a*: Indicates redness and its position between magenta and green. Values on this scale range from a* = −60 (green) to a* = 60 (magenta).
- b*: Indicates yellowness and its position between yellow and blue. Values on this scale range from b* = −60 (blue) to b* = 60 (yellow).
5.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, S.; Diamond, M.; Green, J.; Karasic, D.; Reed, T.; Whittle, S.; Wylie, K. Transgender people: Health at the margins of society. Lancet 2016, 388, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Reisner, S.L.; Poteat, T.; Keatley, J.; Cabral, M.; Mothopeng, T.; Dunham, E.; Holland, C.E.; Max, R.; Baral, S.D. Global health burden and needs of transgender populations: A review. Lancet 2016, 388, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.; Bockting, W.; Botzer, M.; Cohen-Kettenis, P.; DeCuypere, G.; Feldman, J.; Fraser, L.; Green, J.; Knudson, G.; Meyer, W.J.; et al. Standards of Care for the Health of Transsexual, Transgender, and Gender-Nonconforming People, Version 7. Int. J. Transgenderism 2012, 13, 165–232. [Google Scholar] [CrossRef]
- Lane, M.; Ives, G.C.; Sluiter, E.C.; Waljee, J.F.; Yao, T.H.; Hu, H.M.; Kuzon, W.M. Trends in Gender-affirming Surgery in Insured Patients in the United States. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1738. [Google Scholar] [CrossRef] [PubMed]
- Canner, J.K.; Harfouch, O.; Kodadek, L.M.; Pelaez, D.; Coon, D.; Offodile, A.C., 2nd; Haider, A.H.; Lau, B.D. Temporal Trends in Gender-Affirming Surgery Among Transgender Patients in the United States. JAMA Surg. 2018, 153, 609–616. [Google Scholar] [CrossRef] [PubMed]
- van de Grift, T.C.; Kreukels, B.P.; Elfering, L.; Özer, M.; Bouman, M.B.; Buncamper, M.E.; Smit, J.M.; Mullender, M.G. Body Image in Transmen: Multidimensional Measurement and the Effects of Mastectomy. J. Sex. Med. 2016, 13, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, M.; Salonen, K.; Helminen, M.; Karhunen-Enckell, U. Chest-wall contouring surgery in female-to-male transgender patients: A one-center retrospective analysis of applied surgical techniques and results. Scand. J. Surg. 2017, 106, 74–79. [Google Scholar] [CrossRef]
- Cregten-Escobar, P.; Bouman, M.B.; Buncamper, M.E.; Mullender, M.G. Subcutaneous mastectomy in female-to-male transsexuals: A retrospective cohort-analysis of 202 patients. J. Sex. Med. 2012, 9, 3148–3153. [Google Scholar] [CrossRef]
- Kühn, S.; Keval, S.; Sader, R.; Küenzlen, L.; Kiehlmann, M.; Djedovic, G.; Bozkurt, A.; Rieger, U.M. Mastectomy in female-to-male transgender patients: A single-center 24-year retrospective analysis. Arch. Plast. Surg. 2019, 46, 433–440. [Google Scholar] [CrossRef]
- Berman, B.; Maderal, A.; Raphael, B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol. Surg. 2017, 43, S3–S18. [Google Scholar] [CrossRef]
- Bock, O.; Schmid-Ott, G.; Malewski, P.; Mrowietz, U. Quality of life of patients with keloid and hypertrophic scarring. Arch. Dermatol. Res. 2006, 297, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Téot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Zimbler, M.S.; Holds, J.B.; Kokoska, M.S.; Glaser, D.A.; Prendiville, S.; Hollenbeak, C.S.; Thomas, J.R. Effect of botulinum toxin pretreatment on laser resurfacing results: A prospective, randomized, blinded trial. Arch. Facial Plast. Surg. 2001, 3, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Gassner, H.G.; Sherris, D.A.; Otley, C.C. Treatment of facial wounds with botulinum toxin A improves cosmetic outcome in primates. Plast. Reconstr. Surg. 2000, 105, 1948–1953. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Y.; Zhang, D.; Liu, Y.; Li, Y. Local Injection of Botulinum Toxin Type A to Prevent Postoperative Scar. J. Craniofacial Surg. 2020, 31, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Peitzmeier, S.; Gardner, I.; Weinand, J.; Corbet, A.; Acevedo, K. Health impact of chest binding among transgender adults: A community-engaged, cross-sectional study. Cult. Health Sex. 2017, 19, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Elliot, D.; Cory-Pearce, R.; Rees, G.M. The behaviour of presternal scars in a fair-skinned population. Ann. R. Coll. Surg. Engl. 1985, 67, 238–240. [Google Scholar] [PubMed]
- Mahdavian Delavary, B.; van der Veer, W.M.; Ferreira, J.A.; Niessen, F.B. Formation of hypertrophic scars: Evolution and susceptibility. J. Plast. Surg. Hand Surg. 2012, 46, 95–101. [Google Scholar] [CrossRef]
- Ziade, M.; Domergue, S.; Batifol, D.; Jreige, R.; Sebbane, M.; Goudot, P.; Yachouh, J. Use of botulinum toxin type A to improve treatment of facial wounds: A prospective randomised study. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 209–214. [Google Scholar] [CrossRef]
- Lee, B.J.; Jeong, J.H.; Wang, S.G.; Lee, J.C.; Goh, E.K.; Kim, H.W. Effect of botulinum toxin type a on a rat surgical wound model. Clin. Exp. Otorhinolaryngol. 2009, 2, 20–27. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.J.; Lee, J.W.; Jeong, H.S.; Suh, I.S. Clinical trial to evaluate the efficacy of botulinum toxin type A injection for reducing scars in patients with forehead laceration: A double-blinded, randomized controlled study. Medicine 2019, 98, e16952. [Google Scholar] [CrossRef]
- Grando, S.A.; Zachary, C.B. The non-neuronal and nonmuscular effects of botulinum toxin: An opportunity for a deadly molecule to treat disease in the skin and beyond. Br. J. Dermatol. 2018, 178, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Oh, E.J.; Chung, J.Y.; Park, J.W.; Cho, B.C.; Chung, H.Y. The effects of botulinum toxin A on the survival of a random cutaneous flap. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Li, X. Efficacy and Safety of Botulinum Toxin Type A in Preventing Postoperative Scars and Improving the Cosmetic Appearance of Scars: A Systematic Review and Meta-Analysis. J. Cutan. Med. Surg. 2020, 24, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.J.; Fung, E.; Rigby, M.H.; Burke, E.; Hart, R.D.; Trites Jonathan, R.B.; Gassner, H.G.; Taylor, S.M. The Use of Botulinum Toxin Type A in the Healing of Thyroidectomy Wounds: A Randomized, Prospective, Placebo-Controlled Study. Plast. Reconstr. Surg. 2019, 143, 375e–381e. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Yang, J.; Liu, J.Q.; Xie, S.T.; Zhang, Y.J.; Zhang, W.; Zhang, J.L.; Zheng, Z.; Hu, D.H. A Randomized, Placebo-Controlled, Double-Blind, Prospective Clinical Trial of Botulinum Toxin Type A in Prevention of Hypertrophic Scar Development in Median Sternotomy Wound. Aesthetic Plast. Surg. 2018, 42, 1364–1369. [Google Scholar] [CrossRef]
- Abedini, R.; Mehdizade, R.N.; Haddady, A.S.; Rahmati, J.; Teymourpour, A.; Nasimi, M. Botulinum Toxin Type A Injection for Mammoplasty and Abdominoplasty Scar Management: A Split-Scar Double-Blinded Randomized Controlled Study. Aesthetic Plast. Surg. 2020, 44, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Chen, M.; Zhou, L. MiR-17 targets PTEN and facilitates glial scar formation after spinal cord injuries via the PI3K/Akt/mTOR pathway. Brain Res. Bull. 2017, 128, 68–75. [Google Scholar] [CrossRef]
- Zhang, X.; Lan, D.; Ning, S.; Jia, H.; Yu, S. Botulinum toxin type A prevents the phenotypic transformation of fibroblasts induced by TGF-b1 via the PTEN/PI3K/Akt signaling pathway. Int. J. Mol. Med. 2019, 44, 661–671. [Google Scholar]
- Gou, L.; Chen, L.; Bi, S.; Chai, L.; Wang, Z.; Cao, C.; Tao, L.; Li, S. PTEN inhibits proliferation and functions of hypertrophic scar fibroblasts. Mol. Cell. Biochem. 2012, 361, 161–168. [Google Scholar]
- Nien, M.S.; Cheng, W.P.; Feng, J.; Cui, Y.Y. The molecular mechanism of GADD153 in apoptosis of keloid fibroblasts exposed to botulinum toxin type A. J. Cell. Mol. Med. 2021, 25, 9402–9410. [Google Scholar] [CrossRef] [PubMed]
- Butzelaar, L.; Soykan, E.A.; Galindo, G.F.; Beelen, R.H.; Ulrich, M.M.; Niessen, F.B.; van der Molen, A.B.M. Going into surgery: Risk factors for hypertrophic scarring. Wound Repair. Regen. 2015, 23, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, H.J.; Kim, Y.W.; Cheon, Y.W. The Efficacy and Safety of Early Postoperative Botulinum Toxin A Injection for Facial Scars. Aesthetic Plast. Surg. 2018, 42, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.M.; Francis, C.S.; Motamed, A.; Karatsonyi, A.L.; Hammoudeh, J.A.; Sanchez-Lara, P.A.; Reinisch, J.F.; Urata, M.M. Hypertrophic scarring in cleft lip repair: A comparison of incidence among ethnic groups. Clin. Epidemiol. 2012, 4, 187–191. [Google Scholar] [PubMed]
- Marston, A.P.; Costello, M.S.; Farhood, Z.; Brandstetter, K.A.; Murphey, A.W.; Nguyen, S.A.; Discolo, C.M.; Patel, K.G. Association of Pediatric Patient Demographic Factors and Scar Anatomic Features With Scar Outcomes After Surgical Repair of Cleft Lip. JAMA Facial Plast. Surg. 2019, 21, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.M. Management of the aging face in the Asian patient. Facial Plast. Surg. Clin. N. Am. 2007, 15, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zou, Y.; Chang, S.J.; Qiu, Y.; Chen, H.; Gang, M.; Jin, Y.; Lin, X. Effects of Botulinum Toxin on Improving Facial Surgical Scars: A Prospective, Split-Scar, Double-Blind, Randomized Controlled Trial. Plast. Reconstr. Surg. 2018, 141, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.; Wallace, C.G.; Hsiao, Y.C.; Chang, C.J.; Chen, P.K. Botulinum toxin to improve results in cleft lip repair: A double-blinded, randomized, vehicle-controlled clinical trial. PLoS ONE 2014, 9, e115690. [Google Scholar] [CrossRef]
- Zelken, J.; Yang, S.Y.; Chang, C.S.; Chang, C.J.; Yang, J.Y.; Chuang, S.S.; Chen, H.C.; Hsiao, Y.C. Donor Site Aesthetic Enhancement with Preoperative Botulinum Toxin in Forehead Flap Nasal Reconstruction. Ann. Plast. Surg. 2016, 77, 535–538. [Google Scholar] [CrossRef]
- Duncan Jonathan, A.L.; Bond, J.S.; Mason, T.; Ludlow, A.; Cridland, P.; O’Kane, S.; Ferguson Mark, W.J. Visual analogue scale scoring and ranking: A suitable and sensitive method for assessing scar quality? Plast. Reconstr. Surg. 2006, 118, 909–918. [Google Scholar] [CrossRef]
- van de Kar, A.L.; Corion, L.U.; Smeulders, M.J.; Draaijers, L.J.; van der Horst, C.M.; van Zuijlen, P.P. Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast. Reconstr. Surg. 2005, 116, 514–522. [Google Scholar] [CrossRef]
- Andreassi, L.; Flori, L. Practical applications of cutaneous colorimetry. Clin. Dermatol. 1995, 13, 369–373. [Google Scholar] [CrossRef]
- Shriver, M.D.; Parra, E.J. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. Am. J. Phys. Anthropol. 2000, 112, 17–27. [Google Scholar] [CrossRef]





| Characteristics | n (%) |
|---|---|
| Age, years, mean (SD) | 30.4 (2.0) |
| Comorbidities | |
| Yes | 1 (6.7) |
| No | 14 (93.3) |
| History of keloid/hypertrophic scar in other sites of the body | |
| Yes | 1 (6.7) |
| No | 14 (93.3) |
| Family history of keloid/hypertrophic scar or abnormal wound healing | |
| Yes | 0 (0.0) |
| No | 15 (100.0) |
| Current medication | |
| Yes | 1 (6.7) |
| No | 14 (93.3) |
| Alcohol drinking | |
| Yes | 6 (40.0) |
| No | 9 (60.0) |
| Smoking | |
| Yes | 7 (46.7) |
| No | 8 (53.3) |
| Concurrent hormonal taking | |
| Yes | 0 (0.0) |
| No | 15 (100.0) |
| Fitzpatrick’s phototype | |
| Type 3 | 6 (40.0) |
| Type 4 | 9 (60.0) |
| Colorimeter on the normal skin area (mean ± SD) | |
| L* | 61.2 (±2.6) |
| a* | 12.6 (±2.5) |
| b* | 10.4 (±2.1) |
| Assessment Tools (Score Range) | incoBoNT-A Group | Control Group | p Value |
|---|---|---|---|
| n = 15 | n = 15 | ||
| Mean (SD) | Mean (SD) | ||
| Vancouver scar scale (0–13) | 8.07 (0.96) | 8.47 (0.52) | 0.170 |
| Patient and observer scar assessment scale (POSAS) | |||
| Observer scar assessment scale (OSAS) | |||
| Summary (0–60) | 19.73 (4.89) | 20.93 (3.39) | 0.440 |
| Overall (0–10) | 6.20 (1.01) | 6.60 (0.51) | 0.180 |
| Patient scar assessment scale (PSAS) | |||
| Summary (0–60) | 29.27 (5.34) | 31.80 (5.00) | 0.191 |
| Overall (0–10) | 8.13 (1.92) | 8.13 (1.92) | 1.000 |
| Colorimeter on surgical scar area | |||
| L* (0–100) | 54.07 (6.73) | 51.22 (4.94) | 0.200 |
| a* (−60–60) | 14.18 (0.56) | 14.45 (0.68) | 0.250 |
| b* (−60–60) | 16.82 (6.81) | 16.49 (0.70) | 0.850 |
| Assessment Tools | incoBoNT-A | Control | Coef. (95% CI) | p Value |
|---|---|---|---|---|
| Mean (SE) | Mean (SE) | |||
| Vancouver Scar Scale (VSS) | ||||
| 2 weeks | 6.33 (0.25) | 6.87 (0.25) | −0.53 (−1.21, 0.15) | 0.124 |
| 1 month | 5.87 (0.25) | 6.40 (0.25) | −0.53 (−1.21, 0.15) | 0.124 |
| 3 months | 6.89 (0.26) | 7.36 (0.26) | −0.47 (−1.20, 0.26) | 0.208 |
| 6 months | 7.43 (0.26) | 8.82 (0.26) | −1.39 (−2.12, −0.66) | <0.001 * |
| Patient and Observer Scar Assessment Scale (POSAS) | ||||
| Observer Scar Assessment Scale (OSAS) | ||||
| Summary score | ||||
| 2 weeks | 13.47 (0.83) | 14.13 (0.83) | −0.67 (−2.97, 1.64) | 0.571 |
| 1 month | 12.60 (0.83) | 13.40 (0.83) | −0.80 (−3.10, 1.50) | 0.496 |
| 3 months | 11.46 (0.89) | 13.77 (0.89) | −2.31 (−4.78, 0.17) | 0.068 |
| 6 months | 19.00 (0.89) | 21.15 (0.89) | −2.15 (−4.63, 0.32) | 0.088 |
| Overall score | ||||
| 2 weeks | 4.00 (0.21) | 4.53 (0.21) | −0.53 (−1.12, 0.05) | 0.073 |
| 1 month | 3.27 (0.21) | 3.67 (0.21) | −0.40 (−0.98, 0.18) | 0.179 |
| 3 months | 2.48 (0.23) | 3.86 (0.23) | −1.38 (−2.01, −0.75) | <0.001 * |
| 6 months | 4.48 (0.23) | 5.01 (0.23) | −0.54 (−1.16, 0.09) | 0.093 |
| Patient Scar Assessment Scale (PSAS) | ||||
| Summary score | ||||
| 2 weeks | 13.67 (1.27) | 16.07 (1.27) | −2.40 (−5.92, 1.11) | 0.181 |
| 1 month | 11.27 (1.27) | 11.13 (1.27) | 0.13 (−3.38, 3.65) | 0.941 |
| 3 months | 12.54 (1.36) | 12.54 (1.36) | 6.64 × 10−13 (−3.78, 3.78) | 1.000 |
| 6 months | 25.31 (1.36) | 21.62 (1.36) | 3.69 (−0.08, 7.47) | 0.055 |
| Overall score | ||||
| 2 weeks | 3.80 (0.34) | 3.80 (0.34) | −4.00 × 10−15 (0.95, 0.95) | 1.000 |
| 1 month | 2.47 (0.34) | 3.13 (0.34) | −0.67 (−1.61, 0.28) | 0.168 |
| 3 months | 1.54 (0.37) | 2.46 (0.37) | −0.92 (−1.94, 0.09) | 0.076 |
| 6 months | 4.85 (0.37) | 4.38 (0.37) | 0.46 (−0.56, 1.48) | 0.374 |
| Scar color assessment by Colorimeter | ||||
| L* (lightness) | ||||
| 2 weeks | 57.30 (0.94) | 55.45 (0.94) | 1.85 (−0.74, 4.44) | 0.162 |
| 1 month | 58.68 (0.94) | 56.11 (0.94) | 2.56 (−0.03, 5.16) | 0.052 |
| 3 months | 58.85 (1.00) | 56.44 (1.00) | 2.41 (−0.36, 5.18) | 0.088 |
| 6 months | 56.73 (1.00) | 55.64 (1.00) | 1.09 (−1.68, 3.86) | 0.441 |
| a* (redness) | ||||
| 2 weeks | 16.33 (0.48) | 15.34 (0.48) | 0.99 (−0.33, 2.31) | 0.143 |
| 1 month | 16.31 (0.48) | 15.49 (0.48) | 0.82 (−0.50, 2.14) | 0.224 |
| 3 months | 15.21 (0.50) | 17.18 (0.50) | −1.97 (0.59, 3.34) | 0.005 * |
| 6 months | 13.36 (0.50) | 15.70 (0.50) | −2.33 (−3.71, −0.96) | 0.001 * |
| b* (yellowness) | ||||
| 2 weeks | 15.31 (0.90) | 17.54 (0.90) | −2.22 (−4.71, 0.26) | 0.079 |
| 1 month | 11.98 (0.90) | 11.47 (0.90) | 0.51 (−1.97, 2.99) | 0.686 |
| 3 months | 9.52 (0.96) | 12.42 (0.96) | −2.90 (−5.55, −0.25) | 0.032 * |
| 6 months | 14.93 (0.96) | 15.20 (0.96) | 0.27 (−2.38, 2.92) | 0.843 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winayanuwattikun, W.; Vachiramon, V.; Rattananukrom, T.; Palakornkitti, P.; Sitpahul, N. Efficacy of Botulinum Toxin Type A for Prevention of Post-Mastectomy Scar in Transmen: A Prospective, Randomized Study. Toxins 2023, 15, 636. https://doi.org/10.3390/toxins15110636
Winayanuwattikun W, Vachiramon V, Rattananukrom T, Palakornkitti P, Sitpahul N. Efficacy of Botulinum Toxin Type A for Prevention of Post-Mastectomy Scar in Transmen: A Prospective, Randomized Study. Toxins. 2023; 15(11):636. https://doi.org/10.3390/toxins15110636
Chicago/Turabian StyleWinayanuwattikun, Waranaree, Vasanop Vachiramon, Teerapong Rattananukrom, Pasita Palakornkitti, and Ngamcherd Sitpahul. 2023. "Efficacy of Botulinum Toxin Type A for Prevention of Post-Mastectomy Scar in Transmen: A Prospective, Randomized Study" Toxins 15, no. 11: 636. https://doi.org/10.3390/toxins15110636
APA StyleWinayanuwattikun, W., Vachiramon, V., Rattananukrom, T., Palakornkitti, P., & Sitpahul, N. (2023). Efficacy of Botulinum Toxin Type A for Prevention of Post-Mastectomy Scar in Transmen: A Prospective, Randomized Study. Toxins, 15(11), 636. https://doi.org/10.3390/toxins15110636





