Cyanotoxin Analysis of Air Samples from the Great Salt Lake
Abstract
:1. Introduction
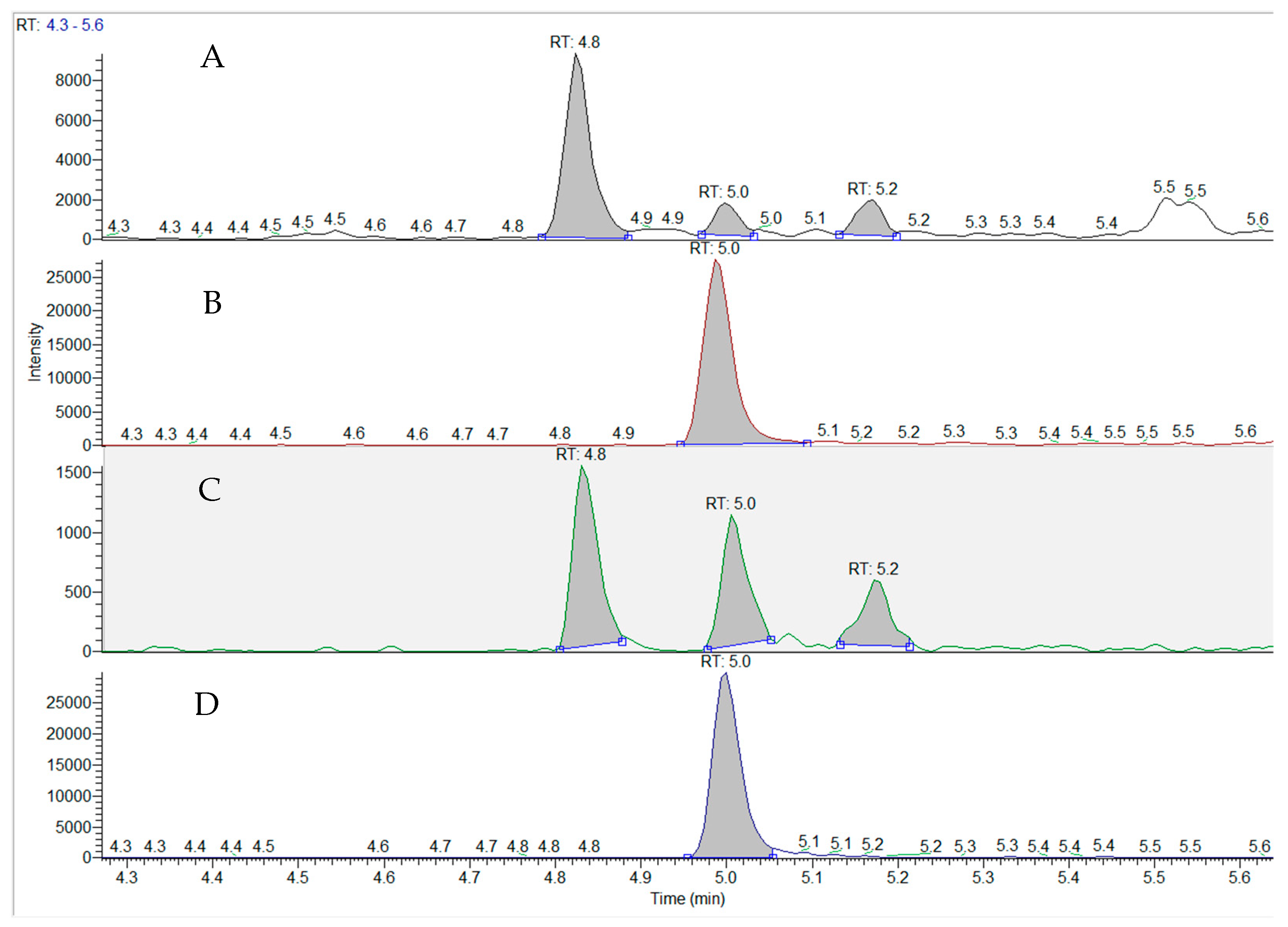
2. Results
3. Discussion
4. Materials and Methods
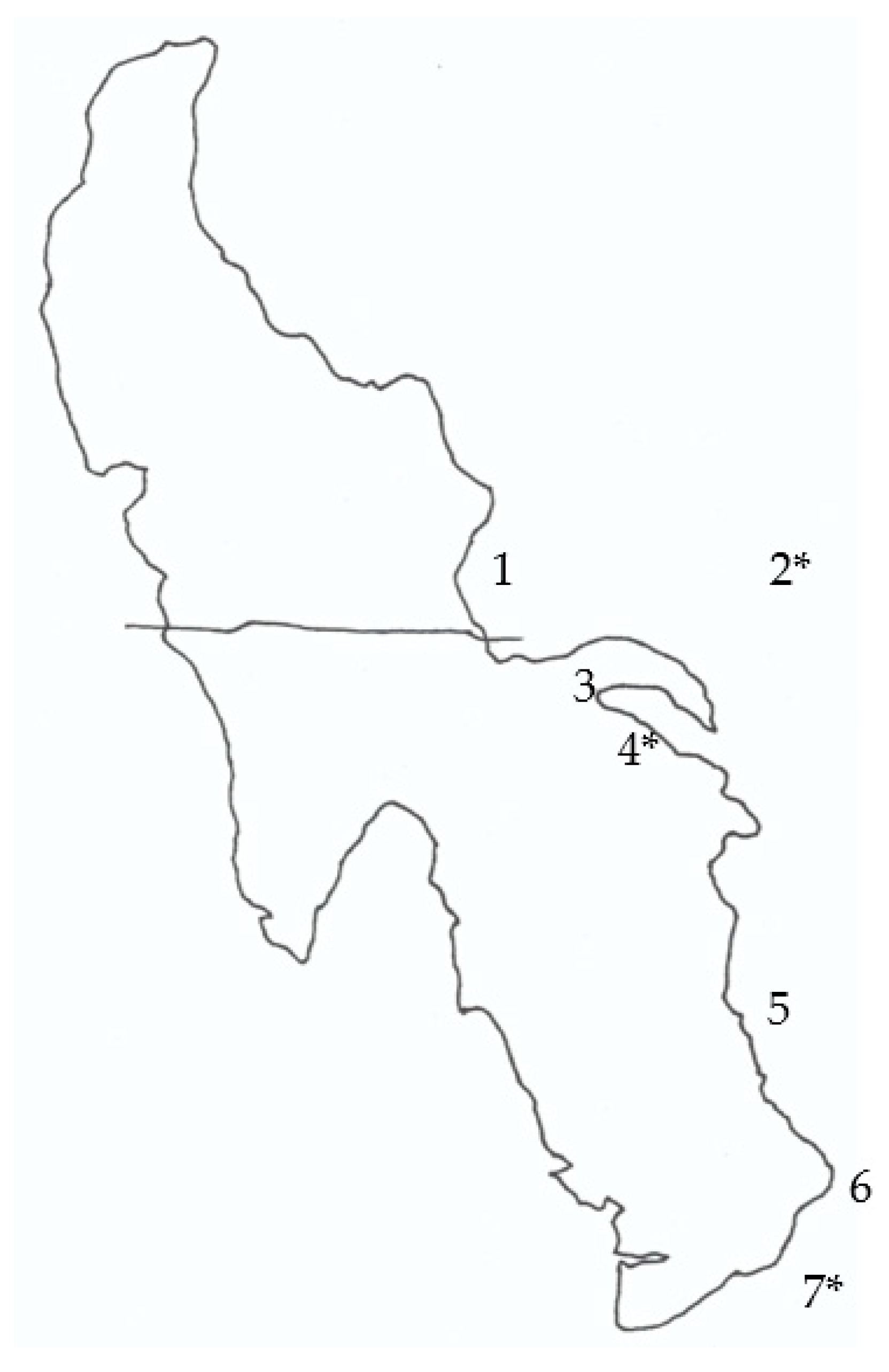
4.1. Sampling Sites
4.2. Air and Ground Sampling
4.3. Water and Lakebed Sampling
4.4. Laboratory Processing of Samples
4.5. Extraction of Cyanotoxins from Great Salt Exposed Lakebed Samples
4.6. Cyanotoxin Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 1–14. [Google Scholar] [CrossRef]
- Larson, C.A.; Belovsky, G.E. Salinity and nutrients influence species richness and evenness of phytoplankton communities in microcosm experiments from Great Salt Lake, Utah, USA. J. Plankton Res. 2013, 35, 1154–1166. [Google Scholar] [CrossRef]
- Oren, A. Cyanobacteria in hypersaline environments: Biodiversity and physiological properties. Biodivers. Conserv. 2015, 24, 781–798. [Google Scholar] [CrossRef]
- Baxter, B.K.; Zalar, P. The extremophiles of Great Salt Lake: Complex microbiology in a dynamic hypersaline ecosystem. In Model Ecosystems in Extreme Environments; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 57–99. [Google Scholar]
- Baxter, B.K. Great Salt Lake microbiology: A historical perspective. Int. Microbiol. 2018, 21, 79–95. [Google Scholar] [CrossRef]
- Marcarelli, A.M.; Wurtsbaugh, W.A.; Griset, O. Salinity controls phytoplankton response to nutrient enrichment in the Great Salt Lake, Utah, USA. Can. J. Fish. Aquat. Sci. 2006, 63, 2236–2248. [Google Scholar] [CrossRef]
- Brock, T.D. Halophilic-blue-green algae. Arch. Microbiol. 1976, 107, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Cirés, S.; Casero, M.C.; Quesada, A. Toxicity at the edge of life: A review on cyanobacterial toxins from extreme environments. Mar. Drugs 2017, 15, 233. [Google Scholar] [CrossRef]
- Roney, H.C.; Booth, G.M.; Cox, P.A. Competitive exclusion of cyanobacterial species in the Great Salt Lake. Extremophiles 2009, 13, 355–361. [Google Scholar] [CrossRef]
- Barrett, K.L.; Belovsky, G.E. Invertebrates and phytoplankton of Great Salt Lake: Is salinity the driving factor? In Great Salt Lake Biology: A Terminal Lake in a Time of Change; Baxter, B., Butler, J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 145–173. [Google Scholar]
- Lindsay, M.R.; Dunham, E.C.; Boyd, E.S. Microbialites of Great Salt Lake. In Great Salt Lake Biology: A Terminal Lake in a Time of Change; Baxter, B., Butler, J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 87–118. [Google Scholar]
- Pack, D.A. Two ciliata of Great Salt Lake. Biol. Bull. 1919, 36, 273–282. [Google Scholar] [CrossRef]
- Cox, P.A. Introduction: The evolutionary mystery of gamete dimorphism. In The Evolution of Anisogamy; Togashi, T., Cox, P.A., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 1–16. [Google Scholar]
- Javor, B. Great Salt Lake. In Hypersaline Environments; Brock/Springer Series in Contemporary Biosciuenve; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar] [CrossRef]
- Stephens, D.W. A summary of biological investigations concerning the Great Salt Lake, Utah (1861–1973). Great Basin Nat. 1974, 34, 221–229. [Google Scholar]
- Savage, A.; Knott, B. Artemia parthenogenetica in Lake Hayward, Western Australia. II. Feeding biology in a shallow, seasonally stratified, hypersaline lake. Int. J. Salt Lake Res. 1998, 7, 13–24. [Google Scholar] [CrossRef]
- Caudell, J.N.; Conover, M.R. Energy content and digestibility of brine shrimp (Artemia franciscana) and other prey items of eared grebes (Podiceps nigricollis) on the Great Salt Lake, Utah. Biol. Conserv. 2006, 130, 251–254. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A. Biostromes, brine flies, birds and the bioaccumulation of selenium in Great Salt Lake, Utah. Nat. Resour. Environ. Issues 2009, 15, 2. [Google Scholar]
- Conover, M.R.; Bell, M.E. Importance of Great Salt Lake to pelagic birds: Eared grebes, phalaropes, gulls, ducks, and white pelicans. In Great Salt Lake Biology: A Terminal Lake in a Time of Change; Baxter, B., Butler, J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 239–262. [Google Scholar]
- Roberts, A.J. Avian diets in a saline ecosystem: Great Salt Lake, Utah, USA. Hum. Wildl. Interact 2013, 7, 158–168. [Google Scholar]
- Abbott, B.W.; Baxter, B.K.; Busche, K.; de Freitas, L.; Frie, R.; Gomez, T. Emergency Measures Needed to Rescue Great Salt Lake from Ongoing Collapse. 2023. Available online: https://www.researchgate.net/profile/Benjamin-Abbott/publication/366876763_Emergency_measures_needed_to_rescue_Great_Salt_Lake_from_ongoing_collapse/links/63b67aaaa03100368a53d6b9/Emergency-measures-needed-to-rescue-Great-Salt-Lake-from-ongoing-collapse.pdf (accessed on 9 November 2023).
- Melaram, R.; Newton, A.R.; Chaffin, J. Microcystin contamination and toxicity: Implications for agriculture and public health. Toxins 2022, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Shingai, Q.K.; Wilkinson, G.M. Microcystin as a biogeochemical cycle: Pools, fluxes, and fates of the cyanotoxin in inland waters. Limnol. Oceanogr. Lett. 2023, 8, 406–418. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Manivel, N.; Sarvalingam, B.; Seenivasan, B.; Srinivasan, H.; Davoodbasha, M.; Yang, F. State-of-the-art review on the ecotoxicology, health hazards, and economic loss of the impact of microcystins and their ultrastructural cellular changes. Aquat. Toxicol. 2023, 256, 106417. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Ülger, T.G.; Göktaş, B.; Öztürk, Ş.; Karataş, D.Ö.; Beyzi, E. Cyanotoxin genotoxicity: A review. Toxin Rev. 2022, 41, 699–712. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Plata-Calzado, C.; Prieto, A.I.; Cameán, A.M.; Jos, A. Toxic Effects Produced by Anatoxin-a under Laboratory Conditions: A Review. Toxins 2022, 14, 861. [Google Scholar] [CrossRef]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 86, 139–209. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, T.; Moffitt, M.; Kellman, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, M.; Łakomiak, A.; Woźny, M.; Brzuzan, P. Neurotoxicity of cyanobacterial toxins. Environ. Biotechnol. 2014, 10, 26–43. [Google Scholar] [CrossRef]
- Falfushynska, H.; Kasianchuk, N.; Siemens, E.; Henao, E.; Rzymski, P. A review of common cyanotoxins and their effects on fish. Toxics 2023, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.F.; Van Hassel, W.H.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and food contamination in developing countries: Review of their types, toxicity, analysis, occurrence and mitigation strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, M.; Testai, E.; Tazart, Z.; Scardala, S.; Codd, G.A. Co-Occurrence of Taste and Odor Compounds and Cyanotoxins in Cyanobacterial Blooms: Emerging Risks to Human Health? Microorganisms 2023, 11, 872. [Google Scholar] [CrossRef]
- Lima, S.T.; Fallon, T.R.; Cordoza, J.L.; Chekan, J.R.; Delbaje, E.; Hopiavuori, A.R.; Alvarenga, D.O.; Wood, S.M.; Luhavaya, H.; Baumgartner, J.T.; et al. Biosynthesis of guanitoxin enables global environmental detection in freshwater cyanobacteria. J. Am. Chem. Soc. 2022, 144, 9372–9379. [Google Scholar] [CrossRef]
- Fiore, M.F.; de Lima, S.T.; Carmichael, W.W.; McKinnie, S.M.; Chekan, J.R.; Moore, B.S. Guanitoxin, re-naming a cyanobacterial organophosphate toxin. Harmful Algae 2020, 92, 101737. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Parsons, W.H.; Du Bois, J. Saxitoxin. Angew. Chem. Int. Ed. 2014, 53, 5760–5784. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Moustafa, A.; Loram, J.E.; Hackett, J.D.; Anderson, D.M.; Plumley, F.G.; Bhattacharya, D. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 2009, 4, e5758. [Google Scholar] [CrossRef] [PubMed]
- Stewart, I.; Schluter, P.J.; Shaw, G.R. Cyanobacterial lipopolysaccharides and human health–a review. Environ. Health 2006, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Chatziefthimiou, A.D.; Souza, N.R.; Cox, P.A. Desert dust as a vector for cyanobacterial toxins. In The Arabian Seas: Biodiversity, Environmental Challenges and Conservation Measures; Jawad, L.A., Ed.; Springer Nature: Berlin/Heidelberg, Germany, 2021; pp. 161–178. [Google Scholar]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral Scler. 2009, 10 (Suppl. 2), 109–117. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Chatziefthimiou, A.D.; Banack, S.A.; Cox, P.A.; Metcalf, J.S. Desert crust microorganisms, their environment, and human health. J. Arid. Env. 2015, 112, 127–133. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Richer, R.; Cox, P.A.; Codd, G.A. Cyanotoxins in desert environments may present a risk to human health. Sci. Total Env. 2012, 421, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Richer, R.; Anchassi, D.; El-Assaad, I.; El-Matbouly, M.; Ali, F.; Makki, I.; Metcalf, J.S. Variation in the coverage of biological soil crusts in the State of Qatar. J. Arid. Env. 2012, 78, 187–190. [Google Scholar] [CrossRef]
- Scott, A.F.; Black, F.J. Mercury bioaccumulation and biomagnification in Great Salt Lake ecosystems. In Great Salt Lake Biology: A Terminal Lake in a Time of Change; Baxter, B., Butler, J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 435–461. [Google Scholar]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin, Beta-N-methylamino-L-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Jones, E.F.; Wurtsbaugh, W.A. The Great Salt Lake’s monimolimnion and its importance for mercury bioaccumulation in brine shrimp (Artemia franciscana). Limnol. Ocean. 2014, 59, 141–155. [Google Scholar] [CrossRef]
- Rush, T.; Liu, X.; Lobner, D. Synergistic toxicity of the environmental neurotoxins methylmercury and β-N-methylamino-L-alanine. Neuroreport 2012, 23, 216–219. [Google Scholar] [CrossRef]
- Turner, P.C.; Gammie, A.J.; Hollinrake, K.; Codd, G.A. Pneumonia associated with contact with cyanobacteria. BMJ Br. Med. J. 1990, 300, 1440. [Google Scholar] [CrossRef] [PubMed]
- Pouria, S.; de Andrade, A.; Barbosa, J.; Cavalcanti, R.L.; Barreto, V.T.S.; Ward, C.J.; Preiser, W.; Poon, G.K.; Neild, G.H.; Codd, G.A. Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 1998, 352, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.; Antunes, M.B.; de Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.T.; et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Azevedo, S.M.; An, J.S.; Molica, R.J.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. N. Engl. J. Med. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Davis, D.A.; Cox, P.A.; Banack, S.A.; Lecusay, P.D.; Garamszegi, S.P.; Hagan, M.J.; Powell, J.T.; Metcalf, J.S.; Palmour, R.M.; Beierschmitt, A.; et al. L-serine reduces spinal cord pathology in a vervet model of preclinical ALS/MND. J. Neuropathol. Exp. Neurol. 2020, 79, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.E.; Adhikari, S.; Halden, R.U. Systematic and state-of the science review of the role of environmental factors in Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease. Sci. Total Environ. 2022, 817, 152504. [Google Scholar] [CrossRef] [PubMed]
- Longinetti, E.; Pupillo, E.; Belometti, C.; Bianchi, E.; Poloni, M.; Fang, F.; Beghi, E. Geographical clusters of amyotrophic lateral sclerosis and the Bradford Hill criteria. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 329–343. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Co-occurrence of cyanobacteria and cyanotoxins with other environmental health hazards: Impacts and implications. Toxins 2020, 12, 629. [Google Scholar] [CrossRef]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Regional Screening Levels (RSLs)-Generic Tables. 2019. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 9 November 2023).
- Null, S.E.; Wurtsbaugh, W.A. Water development, consumptive water uses, and Great Salt Lake. In Great Salt Lake Biology: A Terminal Lake in a Time of Change; Baxter, B., Butler, J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar]
- Diaz-Parga, P.; Goto, J.J.; Krishnan, V.V. On the differential roles of Mg2+, Zn2+, and Cu2+ in the equilibrium of β-N-methyl-amino-L-alanine (BMAA) and its carbamates. Neurotox. Res. 2021, 39, 6–16. [Google Scholar] [CrossRef]
- Nunn, P.B.; O’Brien, P.; Pettit, L.D.; Pyburn, S.I. Complexes of zinc, copper, and nickel with the nonprotein amino acid L-α-amino-β-methylaminopropionic acid: A naturally occurring neurotoxin. J. Inorg. Biochem. 1989, 37, 175–183. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Marcuello, C.; Lostao, A.; Calvo-Begueria, L.; Velazquez-Campoy, A.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Microcystin-LR binds iron, and iron promotes self-assembly. Environ. Sci. Technol. 2017, 51, 4841–4850. [Google Scholar] [CrossRef] [PubMed]
- Facciponte, D.N.; Bough, M.W.; Seidler, D.; Carroll, J.L.; Ashare, A.; Andrew, A.S.; Tsongalis, G.J.; Vaickus, L.J.; Henegan, P.L.; Butt, T.H.; et al. Identifying aerosolized cyanobacteria in the human respiratory tract: A proposed mechanism for cyanotoxin-associated diseases. Sci. Total Environ. 2018, 645, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.; Lewandowska, A.U.; Śliwińska-Wilczewska, S. The importance of cyanobacteria and microalgae present in aerosols to human health and the environment–Review study. Environ. Int. 2019, 131, 104964. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; McNeel, S.V.; Barber, T.; Kirkpatrick, B.; Williams, C.; Irvin, M.; Zhou, Y.; Johnson, T.B.; Nierenberg, K.; Aubel, M.; et al. Recreational exposure to microcystins during algal blooms in two California lakes. Toxicon 2010, 55, 909–921. [Google Scholar] [CrossRef]
- Nie, C.; Geng, X.; Zhang, R.; Wang, L.; Li, L.; Chen, J. Abundant Cyanobacteria in Autumn Adhering to the Heating, Ventilation, and Air-Conditioning (HVAC) in Shanghai. Microorganisms 2023, 11, 1835. [Google Scholar] [CrossRef]
- Banack, S.A. Second laboratory validation of β-N-methylamino-L-alanine, N-(2aminoethyl) glycine, and 2, 4-diaminobuytric acid by ultra-performance liquid chromatography and tandem mass spectrometry. Neurotox. Res. 2021, 39, 107–116. [Google Scholar] [CrossRef]

| Sampling Location | BMAA Filter | AEG Filter | DAB Filter | MC Impinger | BMAA Impinger | AEG Impinger | DAB Impinger |
|---|---|---|---|---|---|---|---|
| ng * | ng * | ng * | ng * | ng * | ng * | ng * | |
| Aug. 2022 | |||||||
| Site 4 | 0.14 | 3.73 | ND | 0.13 | 0.33 | 0.56 | ND |
| Site 7 | 0.14 | ND | ND | NQ | 9.68 | 0.43 | ND |
| Sept. 2022 | |||||||
| Site 2 | ND | ND | ND | ND | ND | 2.38 | 0.92 |
| Site 4 | ND | ND | ND | ND | ND | 1.82 | 0.58 |
| Site 7 | ND | ND | ND | ND | ND | 0.84 | 0.52 |
| Oct. 2022 | |||||||
| Site 2 | ND | ND | ND | ND | ND | 1.08 | 0.40 |
| Site 4 | ND | ND | ND | ND | ND | 0.98 | 0.30 |
| Site 7 | ND | ND | ND | ND | 0.35 | 1.21 | 0.40 |
| Free (ng/mg) | Hydrolysed Free (ng/mg) | Hydrolysed (ng/mg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site/Time | Cyano | BMAA | AEG | DAB | BMAA | AEG | DAB | BMAA | AEG | DAB |
| Aug. 2022 | ||||||||||
| 1 | + | ND | ND | 0.212 | 0.004 | 0.007 | 2.214 | ND | 0.973 | 1.248 |
| 3 | + | ND | 0.217 | 0.217 | 0.002 | 0.004 | 2.333 | ND | 0.251 | 1.120 |
| 4 | + | 0.005 | 0.134 | 0.580 | ND | ND | 3.693 | ND | 1.913 | 4.925 |
| 5 | + | NQ | ND | 0.074 | 0.002 | 0.004 | 1.724 | ND | 0.119 | 1.498 |
| 6 | − | ND | ND | 0.001 | ND | ND | 0.026 | ND | ND | ND |
| 7 | + | ND | ND | 0.017 | ND | ND | 1.347 | ND | ND | 0.003 |
| Sept. 2022 | ||||||||||
| 1 | + | ND | ND | 0.170 | ND | ND | 2.394 | ND | ND | 6.272 |
| 2 | − | ND | 0.843 | 0.032 | ND | ND | 0.187 | ND | ND | 2.696 |
| 3 | + | ND | 0.032 | 1.489 | ND | ND | 10.181 | ND | 0.061 | 4.218 |
| 4 | + | ND | 0.900 | 0.176 | ND | ND | 1.772 | ND | 0.882 | 1.135 |
| 5 | + | ND | ND | 0.031 | ND | ND | 0.924 | ND | ND | 0.001 |
| 6 | − | ND | NQ | 0.010 | ND | ND | 0.138 | ND | ND | 0.201 |
| 7 | − | 0.009 | ND | 0.003 | ND | ND | 0.219 | ND | ND | 0.002 |
| Oct. 2022 | ||||||||||
| 1 | + | ND | ND | 0.062 | ND | ND | 1.747 | ND | ND | 0.047 |
| 2 | − | ND | ND | ND | ND | ND | 0.148 | ND | NQ | 0.078 |
| 3 | + | ND | ND | 0.065 | ND | ND | 3.544 | ND | NQ | 1.382 |
| 4 | + | ND | ND | 0.646 | ND | ND | 0.077 | ND | ND | 2.525 |
| 5 | − | ND | ND | 0.002 | ND | ND | 0.513 | ND | ND | ND |
| 6 | − | ND | ND | ND | ND | ND | 0.098 | ND | NQ | 0.052 |
| 7 | − | ND | ND | 0.009 | ND | ND | 1.507 | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metcalf, J.S.; Banack, S.A.; Cox, P.A. Cyanotoxin Analysis of Air Samples from the Great Salt Lake. Toxins 2023, 15, 659. https://doi.org/10.3390/toxins15110659
Metcalf JS, Banack SA, Cox PA. Cyanotoxin Analysis of Air Samples from the Great Salt Lake. Toxins. 2023; 15(11):659. https://doi.org/10.3390/toxins15110659
Chicago/Turabian StyleMetcalf, James S., Sandra Anne Banack, and Paul Alan Cox. 2023. "Cyanotoxin Analysis of Air Samples from the Great Salt Lake" Toxins 15, no. 11: 659. https://doi.org/10.3390/toxins15110659
APA StyleMetcalf, J. S., Banack, S. A., & Cox, P. A. (2023). Cyanotoxin Analysis of Air Samples from the Great Salt Lake. Toxins, 15(11), 659. https://doi.org/10.3390/toxins15110659




