Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives
Abstract
:1. The Gut Microbiome in Health and Kidney Disease
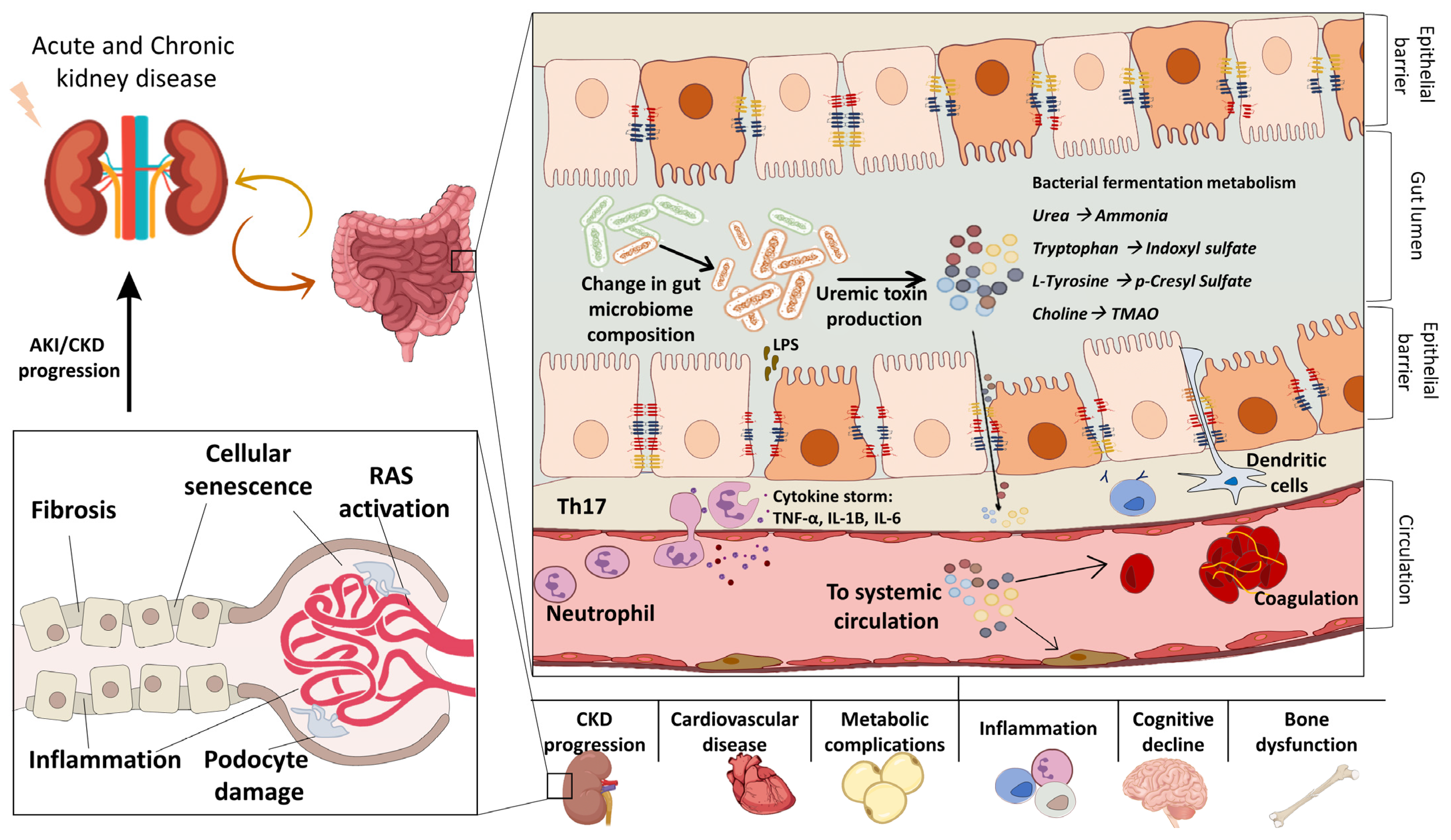
2. The Gut–Kidney Axis
2.1. Uremic Toxins in CKD
2.2. Uremic Toxins in AKI
2.3. Uremic Toxins in Kidney Transplantation
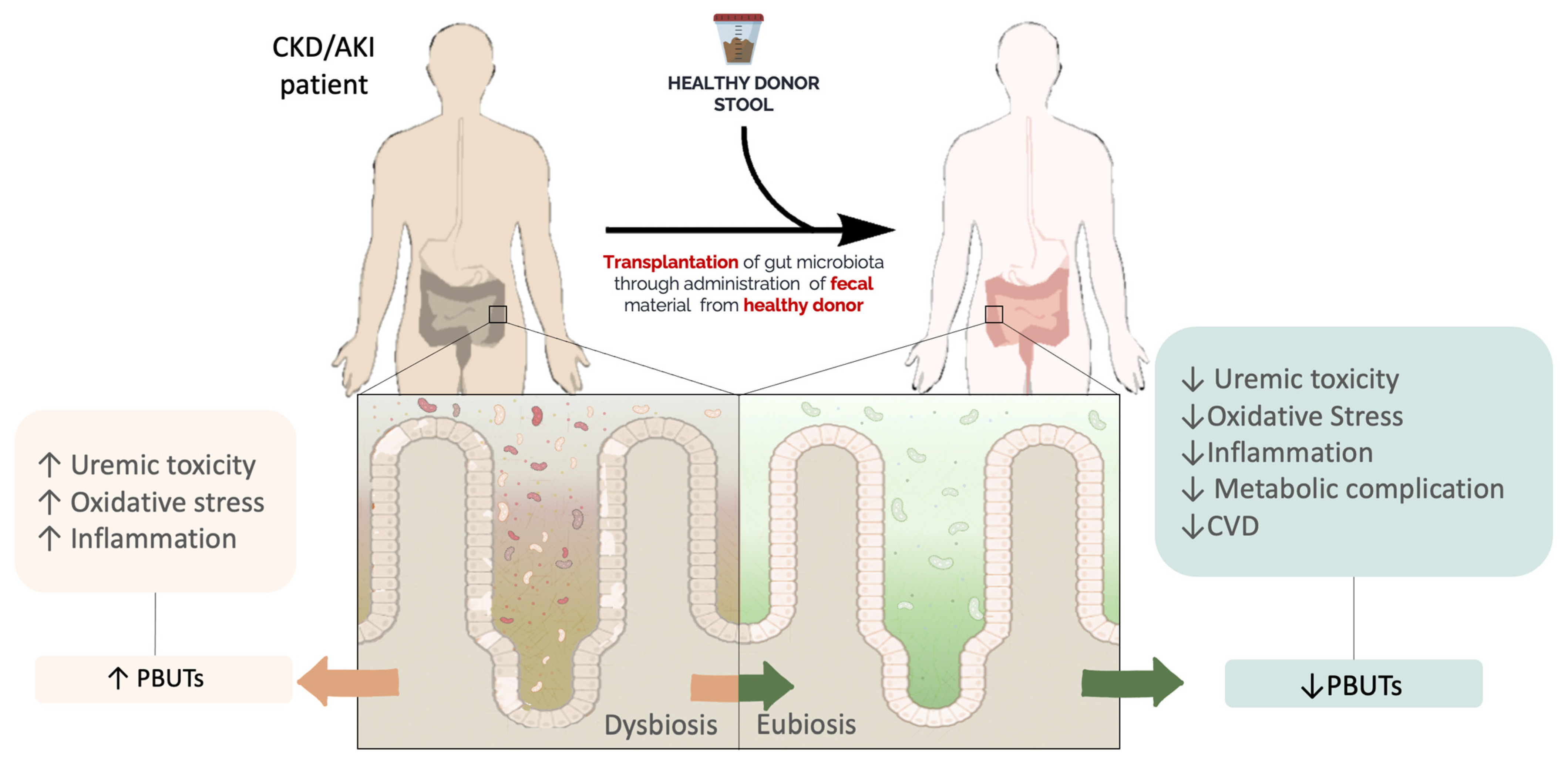
3. FMT
FMT Procedure
4. FMT and Kidney Disease
| Author, Year, Ref | AIM | FMT Modality | Experimental Design for FMT Procedure | Main Findings | Microbiome Evaluation | Effect on Uremic Toxins |
|---|---|---|---|---|---|---|
| Uchiyama et al., 2020 [85] | Explore the role of uremic dysbiosis in CKD-associated IR and sarcopenia. | Donor: CKD mice treated with adenine diet (0.2%). Recipient: healthy germ-free mice. | FMT was performed once in healthy germ-free mice from control mice or CKD mice. | Uremic flora induced sarcopenia, IR, and intestinal permeability in recipient mice. | Decrease in Bacilli, Lactobacillales, and Lactonifactor. Increase in Erysipelotrichi, Erysipelotrichales Allobaculum, Clostridium, and Alistipes. | ↑ IS ↑ PHS ↑ HA ↑ IL-6 |
| Li et al., 2020 [24] | Explore the role of gut microbiota in diabetic nephropathy. | Donors: DN mice (STZ-treated) grouped by severe or moderate proteinuria. Recipient: Antibiotic-treated mice. | Study 1: mice were treated with antibiotics and then with FMT from severe/mild proteinuria group, daily, for 3 days. After, FMT mice were treated with STZ. Study 2: STZ was administered before FMT in antibiotic-treated mice. FMT was performed from severe/mild proteinuria group, daily, for 3 days. | Alterations in gut microbiome modulated the kidney function of DN models. The author suggests that Allobaculum and Anaerosporobacter may worsen renal function, while Blautia may be a protective factor in DN. | Firmicutes were more abundant in mice treated with fecal content of mild proteinuria. Allobaculum increased in the recipients transplanted with severe proteinuria flora. Blautia increased in mice that received the microbiome from the mild proteinuria mice. | FMT from severe proteinuria mice: ↑ TMAO ↑ LPS ↓ SCFAS FMT from moderate proteinuria mice: ↓ TMAO ↓ LPS ↑ SCFAS |
| Wang et al., 2020 [14] | Explore the relationships between gut flora and renal failure. | Donor: ESRD patients or healthy donors. Recipient: CKD mice (adenine treated) and CKD rats (5/6 nephrectomy treated with antibiotics). | CKD mice: 200 ul (0.1 g/mL) of pooled stool was gavaged for 3 days. CKD rats: 1 mL (0.1 g/mL) of pooled stool was gavaged daily for 3 weeks. | FMT from ESRD patients increased uremic toxins levels and aggravated kidney injury. FMT from healthy donors lowered serum creatinine, urea, and several uremic toxins. | E. lenta was found to increase the production of HA and PAG. Fusobacterium nucleatum increased the production of indole and phenol. | Mice/rats receiving ESRD stool: ↑ IS ↑ pCS ↑ PAG ↑ PhS ice/rats receiving healthy donor stool: ↓ TGF-β1 |
| Yang et al., 2020 [27] | Explore the link between kidney and gut microbiota during AKI. | Donor: sham-operated mice or IRI mice models. Recipient: germ-free mice. | FMT was performed via gastric gavage on day 0 and day 10. | After renal IRI, gut microbiota modulated inflammation and severity of kidney injury. | NA | ↓ TNF-α ↓ IFN-γ |
| Liu et al., 2022 [135] | Explore the role of FMT on CKD. | Donor: sham-operated rats. Recipients: 1/2 nephrectomy rats treated with an antibiotic cocktail. | FMT was performed daily for 21 days. | FMT improved kidney function and oxidative stress. | FMT restored the proportion of Lactobacillus johnsonii and Lactobacillus intestinalis. | ↓ IS ↓ PCS ↓ PhS ↓ Phenylacetyl glycine ↓ TMAO |
| Barba et al., 2020 [136] | Explore the role of FMT on the metabolic complication and uremic toxins level. | Donor: healthy mice. Recipient: CKD mice induced with adenine diet (0.25%). | FMT was performed once a week for a total of three weeks by oral gavage. | FMT improved glucose intolerance, and IR. | FMT induced a significant amelioration α-diversity and restored the abundance of Oscillospira and Desulfovibrio. | ↓ PCS ↓ PCG |
| Hu et al., 2020 [137] | Investigate the role of microbiome on diabetic nephropathy. | Donor: healthy rats. Recipient: DN rats (STZ-treated rat). | FMT was performed once a day for 3 days. | FMT improved tubulointerstitial injury and inflammation and reduced both triglycerides and serum acetate levels. | FMT restored the proportion of Prevotellaceae, Ruminococcaceae, and Lactobacillaceae. | ↓ IL-6 |
| Lu et al., 2021 [138] | Investigate the role of gut microbiota in diabetic nephropathy. | Donor: healthy rats. Recipient: STZ-induced rat model of DKD. | Total of 200 uL of the suspended fecal microbiota was administered in diabetic rats by oral gavage. | FMT improved renal injury, reduced the serum acetate levels, and restored renal insulin signaling via Akt phosphorylation. | NA | NA |
| Bastos et al., 2022 [139] | Investigate the efficacy of FMT in a model of type DKD using BTBRob/ob mice. | Donor: BTBR wild-type mice. Recipient: BTBRob/ob mice: homozygous for the leptin gene knockout. | FMT was administered via rectal delivery. | FMT improved body weight and glomerular hypertension and reversed IR and colon permeability. | The treatment enriched the abundance of Odoribacteraceae. | ↓ TNF-α |
| Lauriero et al., 2021 [140] | Explore the link between gut flora and IgA nephropathy outcome. | Donors: healthy controls or non-progressor IgAN or progressor IgAN patients. Recipient: antibiotic-treated humanized IgAN mice. | FMT was performed for five days. | FMT modulated renal phenotype together with BAFF levels, IR, and inflammation. | FMT modulated the proportion of Bacteroidetes, Bacteroides spp., and Actinobacteria, and increased colonization of Firmicutes. | ↓ indole ↓ pCS ↓KC |
| Emal et al., 2017 [141] | Investigate the role of gut microbiota in kidney disease. | Donor: untreated mouse. Recipient: AKI mouse (antibiotic-treated before I/R injury). | FMT was performed for three days via oral gavage. | FMT modulated the expression of macrophage influx and the expression of chemokines receptors. | NA | NA |
| Nakade et al., 2018 [142] | Explore the pathophysiologic role of microbiota associated with D–amino acids AKI. | Donor: healthy B6 mouse. Recipient: germ-free B6 AKI mouse. | FMT was performed for 12 weeks before I/R injury via rectal route. | FMT protected against tubular injury in AKI mouse. | NA | NA |
| Case reports: | ||||||
| Zhao et al., 2021 [143] | Case report of FMT treatment in two female patients with IgA nephropathy. | Donor: male healthy donor. Recipient: female patients with IgAN with intense GI discomfort. | FMT was performed 40 times (200 mL/day for 5 days/week) and then a further 57 times over 5 months. | FMT lowered the 24 h urinary protein and improved the protein loss. | Case 1: FMT reversed α and β diversity. Case 2: FMT decreased Verrucomicrobia. | NA |
| Zhou et al., 2021 [144] | Case report of FMT treatment in an adult patient with membranous nephropathy (MN). | Donor: male healthy donor. Recipient: patient with MN. | FMT was performed 2 times: on day 0 and after 28 days. | FMT decreased urea and creatine levels and reversed the symptoms of edema and diarrhea. | NA | NA |
| Zhi et al., 2022 [145] | Evaluate the effect of oral FMT (encapsulated FMT) on Focal Segmental Glomerulosclerosis. | Donor: healthy donor. Recipient: CKD patient. | Twenty FMT capsules were administered once a week for three weeks via oral capsule. | FMT ameliorated urinary proteinuria and hyperlipidemia (triglyceride and cholesterol levels). A reduced levels of proinflammatory mediators was observed in the first three month after therapy. | FMT treatment restored the balance of Prevotella coprii and Bacteroides uniformiis. | ↓ IL-5 ↓ IL-4 ↓ IL-1β |
| FMT with pre-stimulated donors: | ||||||
| Cai et al., 2020 [146] | Determine the role of gut microbiome and resveratrol on diabetic nephropathy. | Donor: resveratrol-treated mice or control db/m. Recipient: animal model of DN (db/db mice). | FMT was performed by oral gavage daily for 7 days. | FMT improved renal dysfunction, intestinal permeability, and inflammation. | FMT increased Proteobacteria, Alistipes, Turicibacter, Odoribacter, and Rikenellagenus and reduced the abundance of Firmicutes, Tenericutes, Deferribacteres, and Enterococci. | ↓ TNF-α, ↓ IFN-γ, ↓ IL-6, ↓ IL-1β |
| Han et al., 2021 [147] | Explore the protective effect of microbiome and Astragulis membranaceus on CKD. | Donor: CKD mice treated with Astragalus membranaceus. Recipient: CKD mice (Cyclosporin A-treated). | FMT was performed for 6 weeks. | FMT ameliorated kidney function (glomerular dysfunction, renal tubules vacuolization, and fibrosis). | FMT reversed the proportion of Akkermansia and Lactobacillus. | NA |
Effects of FMT on Kidney Transplantation
5. FMT: A Mixed Blessing for Kidney Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kato, K.; Murakami, H.; Hosomi, K.; Tanisawa, K.; Nakagata, T.; Ohno, H.; Konishi, K.; Kawashima, H.; Chen, Y.-A.; et al. Comprehensive Analysis of Gut Microbiota of a Healthy Population and Covariates Affecting Microbial Variation in Two Large Japanese Cohorts. BMC Microbiol. 2021, 21, 151. [Google Scholar] [CrossRef] [PubMed]
- King, C.H.; Desai, H.; Sylvetsky, A.C.; LoTempio, J.; Ayanyan, S.; Carrie, J.; Crandall, K.A.; Fochtman, B.C.; Gasparyan, L.; Gulzar, N.; et al. Baseline Human Gut Microbiota Profile in Healthy People and Standard Reporting Template. PLoS ONE 2019, 14, e0206484. [Google Scholar] [CrossRef] [Green Version]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and Disease Markers Correlate with Gut Microbiome Composition across Thousands of People. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmas, V.; Pisanu, S.; Madau, V.; Casula, E.; Deledda, A.; Cusano, R.; Uva, P.; Loviselli, A.; Velluzzi, F.; Manzin, A. Gut Microbiota Markers and Dietary Habits Associated with Extreme Longevity in Healthy Sardinian Centenarians. Nutrients 2022, 14, 2436. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J. Reduction in Diversity of the Colonic Mucosa Associated Bacterial Microflora in Patients with Active Inflammatory Bowel Disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, P.M.; Adebayo, A.S.; Bowyer, R.C.E.; Freidin, M.B.; Finckh, A.; Strowig, T.; Lesker, T.R.; Alpizar-Rodriguez, D.; Gilbert, B.; Kirkham, B.; et al. Associations between Gut Microbiota and Genetic Risk for Rheumatoid Arthritis in the Absence of Disease: A Cross-Sectional Study. Lancet Rheumatol. 2020, 2, e418–e427. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the Gut Microbiome in Chronic Diseases: A Narrative Review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut Microbiome and Kidney Disease: A Bidirectional Relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Wu, I.-W.; Gao, S.-S.; Chou, H.-C.; Yang, H.-Y.; Chang, L.-C.; Kuo, Y.-L.; Dinh, M.C.V.; Chung, W.-H.; Yang, C.-W.; Lai, H.-C.; et al. Integrative Metagenomic and Metabolomic Analyses Reveal Severity-Specific Signatures of Gut Microbiota in Chronic Kidney Disease. Theranostics 2020, 10, 5398–5411. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant Gut Microbiota Alters Host Metabolome and Impacts Renal Failure in Humans and Rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chen, D.-Q.; Liu, J.-R.; Zhang, J.; Vaziri, N.D.; Zhuang, S.; Chen, H.; Feng, Y.-L.; Guo, Y.; Zhao, Y.-Y. Unilateral Ureteral Obstruction Causes Gut Microbial Dysbiosis and Metabolome Disorders Contributing to Tubulointerstitial Fibrosis. Exp. Mol. Med. 2019, 51, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.; Jose, A.; Alonzo-Palma, N.; Malik, T.; Shankaranarayanan, D.; Regunathan-Shenk, R.; Raj, D.S. Butyrate Producing Microbiota Are Reduced in Chronic Kidney Diseases. Sci. Rep. 2021, 11, 23530. [Google Scholar] [CrossRef]
- Hu, J.; Zhong, X.; Yan, J.; Zhou, D.; Qin, D.; Xiao, X.; Zheng, Y.; Liu, Y. High-Throughput Sequencing Analysis of Intestinal Flora Changes in ESRD and CKD Patients. BMC Nephrol. 2020, 21, 12. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Wang, F.; Jiang, H.; Liu, H.; Wei, M.; Wang, Z.; Xie, L. Gut Bacterial Translocation May Aggravate Microinflammation in Hemodialysis Patients. Dig. Dis. Sci. 2014, 59, 2109–2117. [Google Scholar] [CrossRef]
- Han, S.; Shang, L.; Lu, Y.; Wang, Y. Gut Microbiome Characteristics in IgA Nephropathy: Qualitative and Quantitative Analysis from Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 904401. [Google Scholar] [CrossRef]
- De Angelis, M.; Montemurno, E.; Piccolo, M.; Vannini, L.; Lauriero, G.; Maranzano, V.; Gozzi, G.; Serrazanetti, D.; Dalfino, G.; Gobbetti, M.; et al. Microbiota and Metabolome Associated with Immunoglobulin A Nephropathy (IgAN). PLoS ONE 2014, 9, e99006. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the Gut–Kidney Axis among Biopsy-Proven Diabetic Nephropathy, Type 2 Diabetes Mellitus and Healthy Controls: An Analysis of the Gut Microbiota Composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, B.; Sha, T.; Li, X. Changes in the Intestinal Microbiota in Patients with Stage 5 Chronic Kidney Disease on a Low-Protein Diet and the Effects of Human to Rat Fecal Microbiota Transplantation. Med. Sci. Monit. 2020, 26, e921557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, X.; Gao, Y.; Lv, C.; Gao, Z.; Liu, Y.; Wang, Y.; Li, S.; Wang, Z. The Potential Role of the Gut Microbiota in Modulating Renal Function in Experimental Diabetic Nephropathy Murine Models Established in Same Environment. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165764. [Google Scholar] [CrossRef]
- Lei, J.; Xie, Y.; Sheng, J.; Song, J. Intestinal Microbiota Dysbiosis in Acute Kidney Injury: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Ren. Fail. 2022, 44, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Popkov, V.A.; Klimenko, N.S.; Tyakht, A.V.; Baydakova, G.V.; Frolova, O.Y.; Zorova, L.D.; Pevzner, I.B.; Zorov, D.B.; Plotnikov, E.Y. Microbiome-Metabolome Signature of Acute Kidney Injury. Metabolites 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kim, C.J.; Go, Y.S.; Lee, H.Y.; Kim, M.-G.; Oh, S.W.; Cho, W.Y.; Im, S.-H.; Jo, S.K. Intestinal Microbiota Control Acute Kidney Injury Severity by Immune Modulation. Kidney Int. 2020, 98, 932–946. [Google Scholar] [CrossRef]
- Swarte, J.C.; Douwes, R.M.; Hu, S.; Vich Vila, A.; Eisenga, M.F.; van Londen, M.; Gomes-Neto, A.W.; Weersma, R.K.; Harmsen, H.J.M.; Bakker, S.J.L. Characteristics and Dysbiosis of the Gut Microbiome in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut Microbial Community Structure and Complications After Kidney Transplantation. Transplantation 2014, 98, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, B.R.; Rocchetti, M.T.; de Angelis, M.; Cosola, C.; Marzocco, S.; di Micco, L.; di Bari, I.; Accetturo, M.; Vacca, M.; Gobbetti, M.; et al. Nutritional Therapy Modulates Intestinal Microbiota and Reduces Serum Levels of Total and Free Indoxyl Sulfate and P-Cresyl Sulfate in Chronic Kidney Disease (Medika Study). J. Clin. Med. 2019, 8, 1424. [Google Scholar] [CrossRef]
- Bakhtiary, M.; Morvaridzadeh, M.; Agah, S.; Rahimlou, M.; Christopher, E.; Zadro, J.R.; Heshmati, J. Effect of Probiotic, Prebiotic, and Synbiotic Supplementation on Cardiometabolic and Oxidative Stress Parameters in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Clin. Ther. 2021, 43, e71–e96. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Liu, C.K.; Miao, S.; Fielding, R.; Katzel, L.I.; Giffuni, J.; Well, A.; Seliger, S.L. Effect of Long-Term Exercise Training on Physical Performance and Cardiorespiratory Function in Adults With CKD: A Randomized Controlled Trial. Am. J. Kidney Dis. 2023, 81, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Brookes, E.M.; Power, D.A. Elevated Serum Urea-to-Creatinine Ratio Is Associated with Adverse Inpatient Clinical Outcomes in Non-End Stage Chronic Kidney Disease. Sci. Rep. 2022, 12, 20827. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of Urea in Intestinal Barrier Dysfunction and Disruption of Epithelial Tight Junction in Chronic Kidney Disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of Colonic Epithelial Tight Junction in Uremia: A Likely Cause of CKD-Associated Inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-K.; Lim, P.-S.; Jin, J.-S.; Wu, M.-Y.; Chen, C.-H. Impaired Gut Epithelial Tight Junction Expression in Hemodialysis Patients Complicated with Intradialytic Hypotension. Biomed. Res. Int. 2018, 2018, 2670312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, H.-J.; Yen, C.-H.; Wu, I.-W.; Hsu, K.-H.; Chen, C.-K.; Sun, C.-Y.; Chou, C.-C.; Chen, C.-Y.; Tsai, C.-J.; Wu, M.-S.; et al. The Association of Uremic Toxins and Inflammation in Hemodialysis Patients. PLoS ONE 2014, 9, e102691. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G.; de Smet, R.; Lameire, N. New Insights in Uremic Toxins. Kidney Int. 2003, 63, S6–S10. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Kazama, J.J.; Wakamatsu, T.; Takahashi, Y.; Kaneko, Y.; Goto, S.; Narita, I. Removal of Uremic Toxins by Renal Replacement Therapies: A Review of Current Progress and Future Perspectives. Ren. Replace. Ther. 2016, 2, 43. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between Two Sulfate-Conjugated Uremic Toxins, p -Cresyl Sulfate and Indoxyl Sulfate, during Binding with Human Serum Albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Faria, J.; Stefens, M.F.J.; Stamatialis, D.; Verhaar, M.C.; Gerritsen, K.G.F.; Masereeuw, R. Drugs Commonly Applied to Kidney Patients May Compromise Renal Tubular Uremic Toxins Excretion. Toxins 2020, 12, 391. [Google Scholar] [CrossRef]
- Lesaffer, G.; de Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R. Intradialytic Removal of Protein-Bound Uraemic Toxins: Role of Solute Characteristics and of Dialyser Membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-Bound Uremic Toxins in Hemodialysis Patients Measured by Liquid Chromatography/Tandem Mass Spectrometry and Their Effects on Endothelial ROS Production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef]
- Basile, C.; Libutti, P.; di Turo, A.L.; Casino, F.G.; Vernaglione, L.; Tundo, S.; Maselli, P.; de Nicolo, E.V.; Ceci, E.; Teutonico, A.; et al. Removal of Uraemic Retention Solutes in Standard Bicarbonate Haemodialysis and Long-Hour Slow-Flow Bicarbonate Haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 1296–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlak, D.; Tankiewicz, A.; Mysliwiec, P.; Buczko, W. Tryptophan Metabolism via the Kynurenine Pathway in Experimental Chronic Renal Failure. Nephron 2002, 90, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; de Maré, A.; de Leger, W.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and P-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Mozar, A.; Louvet, L.; Godin, C.; Mentaverri, R.; Brazier, M.; Kamel, S.; Massy, Z.A. Indoxyl Sulphate Inhibits Osteoclast Differentiation and Function. Nephrol. Dial. Transplant. 2012, 27, 2176–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Enhances p53-TGF-β1-Smad3 Pathway in Proximal Tubular Cells. Am. J. Nephrol. 2013, 37, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The Cardiovascular Effect of the Uremic Solute Indole-3 Acetic Acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallée, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic Uremic Solutes Increase Tissue Factor Production in Endothelial Cells by the Aryl Hydrocarbon Receptor Pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-T.; Wu, P.-H.; Lee, H.-H.; Mubanga, M.; Chen, C.-S.; Kuo, M.-C.; Chiu, Y.-W.; Kuo, P.-L.; Hwang, S.-J. Indole-3 Acetic Acid Increased Risk of Impaired Cognitive Function in Patients Receiving Hemodialysis. Neurotoxicology 2019, 73, 85–91. [Google Scholar] [CrossRef]
- Pawlak, K.; Domaniewski, T.; Mysliwiec, M.; Pawlak, D. The Kynurenines Are Associated with Oxidative Stress, Inflammation and the Prevalence of Cardiovascular Disease in Patients with End-Stage Renal Disease. Atherosclerosis 2009, 204, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Karu, N.; McKercher, C.; Nichols, D.S.; Davies, N.; Shellie, R.A.; Hilder, E.F.; Jose, M.D. Tryptophan Metabolism, Its Relation to Inflammation and Stress Markers and Association with Psychological and Cognitive Functioning: Tasmanian Chronic Kidney Disease Pilot Study. BMC Nephrol. 2016, 17, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Wang, X.; Liu, X.; Wang, L.; Ren, F.; Wang, X.; Leng, X. Hippuric Acid Promotes Renal Fibrosis by Disrupting Redox Homeostasis via Facilitation of NRF2–KEAP1–CUL3 Interactions in Chronic Kidney Disease. Antioxidants 2020, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wei, R.; Wang, Y.; Su, T.; Li, P.; Chen, X. The Uremic Toxin Hippurate Promotes Endothelial Dysfunction via the Activation of Drp1-Mediated Mitochondrial Fission. Redox Biol. 2018, 16, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Vanholder, R.; van Biesen, W.; Pletinck, A.; Schepers, E.; Neirynck, N.; Speeckaert, M.; de Bacquer, D.; Verbeke, F. Free p-Cresyl Sulfate Shows the Highest Association with Cardiovascular Outcome in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2021, 36, 998–1005. [Google Scholar] [CrossRef]
- Poveda, J.; Sanchez-Niño, M.D.; Glorieux, G.; Sanz, A.B.; Egido, J.; Vanholder, R.; Ortiz, A. P-Cresyl Sulphate Has pro-Inflammatory and Cytotoxic Actions on Human Proximal Tubular Epithelial Cells. Nephrol. Dial. Transplant. 2014, 29, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y.; et al. P-Cresyl Sulfate Promotes Insulin Resistance Associated with CKD. J. Am. Soc. Nephrol. 2013, 24, 88–99. [Google Scholar] [CrossRef] [Green Version]
- Verbeke, F.; Vanholder, R.; van Biesen, W.; Glorieux, G. Contribution of Hypoalbuminemia and Anemia to the Prognostic Value of Plasma P-Cresyl Sulfate and p-Cresyl Glucuronide for Cardiovascular Outcome in Chronic Kidney Disease. J. Pers. Med. 2022, 12, 1239. [Google Scholar] [CrossRef]
- Kim, R.B.; Morse, B.L.; Djurdjev, O.; Tang, M.; Muirhead, N.; Barrett, B.; Holmes, D.T.; Madore, F.; Clase, C.M.; Rigatto, C.; et al. Advanced Chronic Kidney Disease Populations Have Elevated Trimethylamine N-Oxide Levels Associated with Increased Cardiovascular Events. Kidney Int. 2016, 89, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Gruppen, E.G.; Garcia, E.; Connelly, M.A.; Jeyarajah, E.J.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.F. TMAO Is Associated with Mortality: Impact of Modestly Impaired Renal Function. Sci. Rep. 2017, 7, 13781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Zheng, B.; Liu, N.; Liu, J.; Liu, W.; Huang, X.; Zeng, X.; Chen, L.; Li, Z.; Ouyang, D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats With Diabetic Kidney Disease. Front. Physiol. 2021, 12, 682482. [Google Scholar] [CrossRef]
- Claro, L.; Moreno-Amaral, A.; Gadotti, A.; Dolenga, C.; Nakao, L.; Azevedo, M.; de Noronha, L.; Olandoski, M.; de Moraes, T.; Stinghen, A.; et al. The Impact of Uremic Toxicity Induced Inflammatory Response on the Cardiovascular Burden in Chronic Kidney Disease. Toxins 2018, 10, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.J.; et al. Protein-Bound Uremic Toxins, Inflammation and Oxidative Stress: A Cross-Sectional Study in Stage 3–4 Chronic Kidney Disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Huang, S.-Y.; Wu, C.-C.; Hsu, C.-F. P-Cresylsulfate, the Protein-Bound Uremic Toxin, Increased Endothelial Permeability Partly Mediated by Src-Induced Phosphorylation of VE-Cadherin. Toxins 2020, 12, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xie, Y.; Yang, B.; Huang, S.; Zhang, Y.; Jia, Z.; Ding, G.; Zhang, A. MicroRNA-214 Targets COX-2 to Antagonize Indoxyl Sulfate (IS)-Induced Endothelial Cell Apoptosis. Apoptosis 2020, 25, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The Uremic Solute Indoxyl Sulfate Induces Oxidative Stress in Endothelial Cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef]
- Yisireyili, M.; Shimizu, H.; Saito, S.; Enomoto, A.; Nishijima, F.; Niwa, T. Indoxyl Sulfate Promotes Cardiac Fibrosis with Enhanced Oxidative Stress in Hypertensive Rats. Life Sci. 2013, 92, 1180–1185. [Google Scholar] [CrossRef]
- Rodrigues, G.G.C.; Dellê, H.; Brito, R.B.O.; Cardoso, V.O.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Cunha, R.S.; Stinghen, A.E.M.; Dalboni, M.A.; Barreto, F.C. Indoxyl Sulfate Contributes to Uremic Sarcopenia by Inducing Apoptosis in Myoblasts. Arch. Med. Res. 2020, 51, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Zhang, H.; Liu, T.; Zhang, H.; Teng, J.; Ji, J.; Ding, X. Indoxyl Sulfate Enhance the Hypermethylation of Klotho and Promote the Process of Vascular Calcification in Chronic Kidney Disease. Int. J. Biol. Sci. 2016, 12, 1236–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Nie, L.; Huang, Y.; Zhang, J.; Xiao, T.; Guan, X.; Zhao, J. Amelioration of Uremic Toxin Indoxyl Sulfate-Induced Endothelial Cell Dysfunction by Klotho Protein. Toxicol. Lett. 2012, 215, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Fourdinier, O.; Glorieux, G.; Brigant, B.; Diouf, M.; Pletinck, A.; Vanholder, R.; Choukroun, G.; Verbeke, F.; Massy, Z.A.; Metzinger-Le Meuth, V.; et al. Syndecan-1 and Free Indoxyl Sulfate Levels Are Associated with MiR-126 in Chronic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 10549. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Tsai, T.-C.; Chang, C.-H.; Chang, K.-T.; Ko, P.-H.; Lai, L.-C. Indoxyl Sulfate Elevated Lnc-SLC15A1-1 Upregulating CXCL10/CXCL8 Expression in High-Glucose Endothelial Cells by Sponging MicroRNAs. Toxins 2021, 13, 873. [Google Scholar] [CrossRef]
- Ito, S.; Osaka, M.; Higuchi, Y.; Nishijima, F.; Ishii, H.; Yoshida, M. Indoxyl Sulfate Induces Leukocyte-Endothelial Interactions through Up-Regulation of E-Selectin. J. Biol. Chem. 2010, 285, 38869–38875. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yan, J.; Wang, M.; Lv, J.; Yan, F.; Chen, J. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation by Regulation of β-Catenin and YAP Pathways. J. Mol. Histol. 2021, 52, 197–205. [Google Scholar] [CrossRef]
- Wang, W.-J.; Cheng, M.-H.; Sun, M.-F.; Hsu, S.-F.; Weng, C.-S. Indoxyl Sulfate Induces Renin Release and Apoptosis of Kidney Mesangial Cells. J. Toxicol. Sci. 2014, 39, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, H.; Zhang, C.; Zhou, Y. Indoxyl Sulfate Reduces Ito,f by Activating ROS/MAPK and NF-ΚB Signaling Pathways. JCI Insight 2022, 7, e145475. [Google Scholar] [CrossRef] [PubMed]
- Edamatsu, T.; Fujieda, A.; Itoh, Y. Phenyl Sulfate, Indoxyl Sulfate and p-Cresyl Sulfate Decrease Glutathione Level to Render Cells Vulnerable to Oxidative Stress in Renal Tubular Cells. PLoS ONE 2018, 13, e0193342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. P-Cresyl Sulfate Causes Renal Tubular Cell Damage by Inducing Oxidative Stress by Activation of NADPH Oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Bolati, D.; Shimizu, H.; Yisireyili, M.; Nishijima, F.; Niwa, T. Indoxyl Sulfate, a Uremic Toxin, Downregulates Renal Expression of Nrf2 through Activation of NF-ΚB. BMC Nephrol. 2013, 14, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salyers, Z.R.; Coleman, M.; Balestrieri, N.P.; Ryan, T.E. Indoxyl Sulfate Impairs Angiogenesis via Chronic Aryl Hydrocarbon Receptor Activation. Am. J. Physiol. Cell Physiol. 2021, 320, C240–C249. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Huang, J.; Luo, C.; Ye, H.; Ling, X.; Wu, Q.; Shen, W.; Zhou, L. Klotho Retards Renal Fibrosis through Targeting Mitochondrial Dysfunction and Cellular Senescence in Renal Tubular Cells. Physiol. Rep. 2021, 9, e14696. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Suppression of Klotho Expression by Protein-Bound Uremic Toxins Is Associated with Increased DNA Methyltransferase Expression and DNA Hypermethylation. Kidney Int. 2012, 81, 640–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Yoshifuji, A.; Kimura, I.; et al. Contribution of Uremic Dysbiosis to Insulin Resistance and Sarcopenia. Nephrol. Dial. Transplant. 2020, 35, 1501–1517. [Google Scholar] [CrossRef]
- McCaleb, M.L.; Izzo, M.S.; Lockwood, D.H. Characterization and Partial Purification of a Factor from Uremic Human Serum That Induces Insulin Resistance. J. Clin. Investig. 1985, 75, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockler-Pinto, M.B.; Saldanha, J.F.; Yi, D.; Mafra, D.; Fouque, D.; Soulage, C.O. The Uremic Toxin Indoxyl Sulfate Exacerbates Reactive Oxygen Species Production and Inflammation in 3T3-L1 Adipose Cells. Free Radic. Res. 2016, 50, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Afsar, B.; Elsurer, R.; Covic, A.; Johnson, R.J.; Kanbay, M. Relationship between Uric Acid and Subtle Cognitive Dysfunction in Chronic Kidney Disease. Am. J. Nephrol. 2011, 34, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Vannorsdall, T.D.; Jinnah, H.A.; Gordon, B.; Kraut, M.; Schretlen, D.J. Cerebral Ischemia Mediates the Effect of Serum Uric Acid on Cognitive Function. Stroke 2008, 39, 3418–3420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Zhou, Z.; Miura, H.; Papapetropoulos, A.; McCarthy, E.T.; Sharma, R.; Savin, V.J.; Lianos, E.A. ADMA Injures the Glomerular Filtration Barrier: Role of Nitric Oxide and Superoxide. Am. J. Physiol. Ren. Physiol. 2009, 296, F1386–F1395. [Google Scholar] [CrossRef]
- Ohtsuki, S.; Asaba, H.; Takanaga, H.; Deguchi, T.; Hosoya, K.; Otagiri, M.; Terasaki, T. Role of Blood-Brain Barrier Organic Anion Transporter 3 (OAT3) in the Efflux of Indoxyl Sulfate, a Uremic Toxin: Its Involvement in Neurotransmitter Metabolite Clearance from the Brain. J. Neurochem. 2002, 83, 57–66. [Google Scholar] [CrossRef]
- Watanabe, K.; Sato, E.; Mishima, E.; Watanabe, M.; Abe, T.; Takahashi, N.; Nakayama, M. Effect of Uremic Toxins on Hippocampal Cell Damage: Analysis in Vitro and in Rat Model of Chronic Kidney Disease. Heliyon 2021, 7, e06221. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, S.-C.; Yeh, E.-L.; Lin, P.-C.; Tsai, S.-F.; Huang, Y.-C. Indoxyl Sulfate, Homocysteine, and Antioxidant Capacities in Patients at Different Stages of Chronic Kidney Disease. Nutr. Res. Pract. 2022, 16, 464. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska-Rosołowska, A.; Sroka, N.; Kakareko, K.; Rosołowski, M.; Zbroch, E.; Hryszko, T. The Links between Microbiome and Uremic Toxins in Acute Kidney Injury: Beyond Gut Feeling—A Systematic Review. Toxins 2020, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Kurella Tamura, M.; Tam, K.; Vittinghoff, E.; Raj, D.; Sozio, S.M.; Rosas, S.E.; Makos, G.; Lora, C.; He, J.; Go, A.S.; et al. Inflammatory Markers and Risk for Cognitive Decline in Chronic Kidney Disease: The CRIC Study. Kidney Int. Rep. 2017, 2, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood?Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an Endogenous Oxidative Stress Generator, Causes Neuronal Cell Death with Apoptotic Features and Region Selectivity. J. Neurochem. 2002, 70, 299–307. [Google Scholar] [CrossRef]
- Reyes-Ocampo, J.; Ramírez-Ortega, D.; Vázquez Cervantes, G.I.; Pineda, B.; Montes de Oca Balderas, P.; González-Esquivel, D.; Sánchez-Chapul, L.; Lugo-Huitrón, R.; Silva-Adaya, D.; Ríos, C.; et al. Mitochondrial Dysfunction Related to Cell Damage Induced by 3-Hydroxykynurenine and 3-Hydroxyanthranilic Acid: Non-Dependent-Effect of Early Reactive Oxygen Species Production. Neurotoxicology 2015, 50, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smith, D.G.; Smythe, G.A.; Armati, P.J.; Brew, G.J. Expression of The Kynurenine Pathway Enzymes in Human Microglia and Macrophages. In Developments in Tryptophan and Serotonin Metabolism; Springer: Berlin/Heidelberg, Germany, 2003; pp. 105–112. [Google Scholar]
- Lee, M.-C.; Ting, K.K.; Adams, S.; Brew, B.J.; Chung, R.; Guillemin, G.J. Characterisation of the Expression of NMDA Receptors in Human Astrocytes. PLoS ONE 2010, 5, e14123. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Wang, L.; Brew, B.J. Quinolinic Acid Selectively Induces Apoptosis of Human Astrocytes: Potential Role in AIDS Dementia Complex. J. Neuroinflamm. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Gandolfo, M.T.; Ko, G.J.; Satpute, S.; Racusen, L.; Rabb, H. Early Exposure to Germs Modifies Kidney Damage and Inflammation after Experimental Ischemia-Reperfusion Injury. Am. J. Physiol. Ren. Physiol. 2009, 297, F1457–F1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, A.; Patra, A.; Mandal, S.; Roy, S.; Das, K.; Kar, S.; Nandi, D. Hypoxia: A Cause of Acute Renal Failure and Alteration of Gastrointestinal Microbial Ecology. Saudi J. Kidney Dis. Transplant. 2018, 29, 879. [Google Scholar] [CrossRef]
- Kalim, S.; Clish, C.B.; Deferio, J.J.; Ortiz, G.; Moffet, A.S.; Gerszten, R.E.; Thadhani, R.; Rhee, E.P. Cross-Sectional Examination of Metabolites and Metabolic Phenotypes in Uremia. BMC Nephrol. 2015, 16, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Hao, G.; Pan, Y.; Ma, S.; Yang, T.; Shi, P.; Zhu, Q.; Xie, Y.; Ma, S.; Zhang, Q.; et al. Serum Indoxyl Sulfate Is Associated with Mortality in Hospital-Acquired Acute Kidney Injury: A Prospective Cohort Study. BMC Nephrol. 2019, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Veldeman, L.; Vanmassenhove, J.; van Biesen, W.; Massy, Z.A.; Liabeuf, S.; Glorieux, G.; Vanholder, R. Evolution of Protein-Bound Uremic Toxins Indoxyl Sulphate and p-Cresyl Sulphate in Acute Kidney Injury. Int. Urol. Nephrol. 2019, 51, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Yao, J.; Lin, J.; Liu, J.; Dong, L.; Duan, M. Th17/Regulatory T-Cell Imbalance and Acute Kidney Injury in Patients with Sepsis. J. Clin. Med. 2022, 11, 4027. [Google Scholar] [CrossRef]
- Dong, T.; Aronsohn, A.; Gautham Reddy, K.; Te, H.S. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig. Dis. Sci. 2016, 61, 3621–3626. [Google Scholar] [CrossRef]
- Lee, J.R.; Magruder, M.; Zhang, L.; Westblade, L.F.; Satlin, M.J.; Robertson, A.; Edusei, E.; Crawford, C.; Ling, L.; Taur, Y.; et al. Gut Microbiota Dysbiosis and Diarrhea in Kidney Transplant Recipients. Am. J. Transplant. 2019, 19, 488–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winichakoon, P.; Chaiwarith, R.; Chattipakorn, N.; Chattipakorn, S.C. Impact of Gut Microbiota on Kidney Transplantation. Transplant. Rev. 2022, 36, 100668. [Google Scholar] [CrossRef]
- Ahmad, S.; Bromberg, J.S. Current Status of the Microbiome in Renal Transplantation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 570–576. [Google Scholar] [CrossRef]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; van Landschoot, M.; Eloot, S.; Rops, A.; van de Voorde, J.; de Vriese, A.; et al. Protein-Bound Uremic Toxins Stimulate Crosstalk between Leukocytes and Vessel Wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free P-Cresylsulphate Is a Predictor of Mortality in Patients at Different Stages of Chronic Kidney Disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Desjardins, L.; Massy, Z.A.; Brazier, F.; Westeel, P.F.; Mazouz, H.; Titeca-Beauport, D.; Diouf, M.; Glorieux, G.; Vanholder, R.; et al. Levels of Indoxyl Sulfate in Kidney Transplant Patients, and the Relationship With Hard Outcomes. Circ. J. 2016, 80, 722–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Bammens, B.; Claes, K.; Sprangers, B.; Naesens, M.; Kuypers, D.; Augustijns, P.; Meijers, B. The Influence of Renal Transplantation on Retained Microbial–Human Co-Metabolites. Nephrol. Dial. Transplant. 2016, 31, 1721–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Linde, E.; van Roij, C.J.M.; Meijers, B.K.I.; de Loor, H.; Kessels, R.P.C.; Wetzels, J.F.M. Cognitive Function and Uremic Toxins after Kidney Transplantation: An Exploratory Study. Kidney360 2020, 1, 1398–1406. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, X.; Nie, L.; Liu, Y.; He, T.; Xiong, J.; Xu, X.; Li, Y.; Yang, K.; Wang, Y.; et al. DNA Hypermethylation of SFRP5 Contributes to Indoxyl Sulfate-Induced Renal Fibrosis. J. Mol. Med. 2017, 95, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Korytowska, N.; Wyczałkowska-Tomasik, A.; Pączek, L.; Giebułtowicz, J. Evaluation of Salivary Indoxyl Sulfate with Proteinuria for Predicting Graft Deterioration in Kidney Transplant Recipients. Toxins 2021, 13, 571. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Molnar, M.Z.; Amaral, A.P.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Kiss, I.; Rosivall, L.; Kosa, J.; Lakatos, P.; et al. Elevated Fibroblast Growth Factor 23 Is a Risk Factor for Kidney Transplant Loss and Mortality. J. Am. Soc. Nephrol. 2011, 22, 956–966. [Google Scholar] [CrossRef]
- Frenay, A.-R.S.; van den Berg, E.; de Borst, M.H.; Beckmann, B.; Tsikas, D.; Feelisch, M.; Navis, G.; Bakker, S.J.L.; van Goor, H. Plasma ADMA Associates with All-Cause Mortality in Renal Transplant Recipients. Amino Acids 2015, 47, 1941–1949. [Google Scholar] [CrossRef] [Green Version]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal Enema as an Adjunct in the Treatment of Pseudomembranous Enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal Microbiota Transplantation: Review and Update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Zipursky, J.S.; Sidorsky, T.I.; Freedman, C.A.; Sidorsky, M.N.; Kirkland, K.B. Patient Attitudes Toward the Use of Fecal Microbiota Transplantation in the Treatment of Recurrent Clostridium difficile Infection. Clin. Infect. Dis. 2012, 55, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Bhutiani, N.; Schucht, J.E.; Miller, K.R.; McClave, S.A. Technical Aspects of Fecal Microbial Transplantation (FMT). Curr. Gastroenterol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule- vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium difficile Infection. JAMA 2017, 318, 1985. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, B.E.; Saraiya, N.; Poeth, K.; Schwartz, R.M.; Epstein, M.E.; Honig, G. Effectiveness of Fecal-Derived Microbiota Transfer Using Orally Administered Capsules for Recurrent Clostridium difficile Infection. BMC Infect. Dis. 2015, 15, 191. [Google Scholar] [CrossRef] [Green Version]
- Varga, A.; Kocsis, B.; Sipos, D.; Kása, P.; Vigvári, S.; Pál, S.; Dembrovszky, F.; Farkas, K.; Péterfi, Z. How to Apply FMT More Effectively, Conveniently and Flexible—A Comparison of FMT Methods. Front. Cell. Infect. Microbiol. 2021, 11, 657320. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides Difficile Infection. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Chehri, M.; Christensen, A.H.; Halkjær, S.I.; Günther, S.; Petersen, A.M.; Helms, M. Case Series of Successful Treatment with Fecal Microbiota Transplant (FMT) Oral Capsules Mixed from Multiple Donors Even in Patients Previously Treated with FMT Enemas for Recurrent Clostridium difficile Infection. Medicine 2018, 97, e11706. [Google Scholar] [CrossRef]
- Youngster, I.; Russell, G.H.; Pindar, C.; Ziv-Baran, T.; Sauk, J.; Hohmann, E.L. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA 2014, 312, 1772. [Google Scholar] [CrossRef] [Green Version]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-Bound Uremic Toxin Lowering Strategies in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Nephrol. 2021, 34, 1805–1817. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Wang, X.; Liu, P.; Wang, L.; Li, Y.; Wang, X.; Ren, F. Fecal Microbiota Transplantation Restores Normal Fecal Composition and Delays Malignant Development of Mild Chronic Kidney Disease in Rats. Front. Microbiol. 2022, 13, 1037257. [Google Scholar] [CrossRef]
- Barba, C.; Soulage, C.O.; Caggiano, G.; Glorieux, G.; Fouque, D.; Koppe, L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins 2020, 12, 741. [Google Scholar] [CrossRef]
- Hu, Z.B.; Lu, J.; Chen, P.P.; Lu, C.C.; Zhang, J.X.; Li, X.Q.; Yuan, B.Y.; Huang, S.J.; Ruan, X.Z.; Liu, B.C.; et al. Dysbiosis of Intestinal Microbiota Mediates Tubulointerstitial Injury in Diabetic Nephropathy via the Disruption of Cholesterol Homeostasis. Theranostics 2020, 10, 2803–2816. [Google Scholar] [CrossRef]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 Deficiency Protects against Podocyte Insulin Resistance in Diabetic Nephropathy through the Restoration of AMPKα Activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef]
- Bastos, R.M.C.; Simplício-Filho, A.; Sávio-Silva, C.; Oliveira, L.F.V.; Cruz, G.N.F.; Sousa, E.H.; Noronha, I.L.; Mangueira, C.L.P.; Quaglierini-Ribeiro, H.; Josefi-Rocha, G.R.; et al. Fecal Microbiota Transplant in a Pre-Clinical Model of Type 2 Diabetes Mellitus, Obesity and Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 3842. [Google Scholar] [CrossRef]
- Lauriero, G.; Abbad, L.; Vacca, M.; Celano, G.; Chemouny, J.M.; Calasso, M.; Berthelot, L.; Gesualdo, L.; de Angelis, M.; Monteiro, R.C. Fecal Microbiota Transplantation Modulates Renal Phenotype in the Humanized Mouse Model of IgA Nephropathy. Front. Immunol. 2021, 12, 694787. [Google Scholar] [CrossRef]
- Emal, D.; Rampanelli, E.; Stroo, I.; Butter, L.M.; Teske, G.J.; Claessen, N.; Stokman, G.; Florquin, S.; Leemans, J.C.; Dessing, M.C. Depletion of Gut Microbiota Protects against Renal Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 1450–1461. [Google Scholar] [CrossRef] [Green Version]
- Nakade, Y.; Iwata, Y.; Furuichi, K.; Mita, M.; Hamase, K.; Konno, R.; Miyake, T.; Sakai, N.; Kitajima, S.; Toyama, T.; et al. Gut Microbiota–Derived D-Serine Protects against Acute Kidney Injury. JCI Insight 2018, 3, e97957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of Refractory IgA Nephropathy by Intensive Fecal Microbiota Transplantation: The First Case Reports. Ren. Fail. 2021, 43, 928–933. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Wu, D.; Yang, Y. Fecal Microbiota Transplantation for Membranous Nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef]
- Zhi, W.; Yuan, X.; Song, W.; Jin, G.; Li, Y. Fecal Microbiota Transplantation May Represent a Good Approach for Patients with Focal Segmental Glomerulosclerosis: A Brief Report. J. Clin. Med. 2022, 11, 6700. [Google Scholar] [CrossRef]
- Cai, T.-T.; Ye, X.-L.; Li, R.-R.; Chen, H.; Wang, Y.-Y.; Yong, H.-J.; Pan, M.-L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jiang, Y.; Li, W.; Liu, Y. Astragalus Membranaceus and Salvia Miltiorrhiza Ameliorates Cyclosporin A-Induced Chronic Nephrotoxicity through the “Gut-Kidney Axis”. J. Ethnopharmacol. 2021, 269, 113768. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Pan, P.; Chen, K.-W.; Fan, J.-X.; Li, C.-X.; Cheng, H.; Zhang, X.-Z. An Orally Delivered Microbial Cocktail for the Removal of Nitrogenous Metabolic Waste in Animal Models of Kidney Failure. Nat. Biomed. Eng. 2020, 4, 853–862. [Google Scholar] [CrossRef]
- Yu, D.H.; Ying, N.; Lian, Z.H.; Fa, Y.Q. The Alteration Human of Gut Microbiota and Metabolites before and after Renal Transplantation. Microb. Pathog. 2021, 160, 105191. [Google Scholar] [CrossRef]
- Guirong, Y.E.; Minjie, Z.; Lixin, Y.U.; Junsheng, Y.E.; Lin, Y.; Lisha, S. [Gut Microbiota in Renal Transplant Recipients, Patients with Chronic Kidney Disease and Healthy Subjects]. Nan Fang Yi Ke Da Xue Xue Bao 2018, 38, 1401–1408. [Google Scholar] [CrossRef]
- Zaza, G.; Dalla Gassa, A.; Felis, G.; Granata, S.; Torriani, S.; Lupo, A. Impact of Maintenance Immunosuppressive Therapy on the Fecal Microbiome of Renal Transplant Recipients: Comparison between an Everolimus- and a Standard Tacrolimus-Based Regimen. PLoS ONE 2017, 12, e0178228. [Google Scholar] [CrossRef]
- Wu, H.; Noordmans, G.A.; O’Brien, M.R.; Ma, J.; Zhao, C.Y.; Zhang, G.Y.; Kwan, T.K.T.; Alexander, S.I.; Chadban, S.J. Absence of MyD88 Signaling Induces Donor-Specific Kidney Allograft Tolerance. J. Am. Soc. Nephrol. 2012, 23, 1701–1716. [Google Scholar] [CrossRef]
- Magruder, M.; Edusei, E.; Zhang, L.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Malha, L.; Sze, C.; Lubetzky, M.; Dadhania, D.M.; et al. Gut Commensal Microbiota and Decreased Risk for Enterobacteriaceae Bacteriuria and Urinary Tract Infection. Gut Microbes 2020, 12, 1805281. [Google Scholar] [CrossRef]
- Carron, C.; Pais de Barros, J.-P.; Gaiffe, E.; Deckert, V.; Adda-Rezig, H.; Roubiou, C.; Laheurte, C.; Masson, D.; Simula-Faivre, D.; Louvat, P.; et al. End-Stage Renal Disease-Associated Gut Bacterial Translocation: Evolution and Impact on Chronic Inflammation and Acute Rejection After Renal Transplantation. Front. Immunol. 2019, 10, 1630. [Google Scholar] [CrossRef] [Green Version]
- Stripling, J.; Kumar, R.; Baddley, J.W.; Nellore, A.; Dixon, P.; Howard, D.; Ptacek, T.; Lefkowitz, E.J.; Tallaj, J.A.; Benjamin, W.H.; et al. Loss of Vancomycin-Resistant Enterococcus Fecal Dominance in an Organ Transplant Patient With Clostridium difficile Colitis After Fecal Microbiota Transplant. Open Forum Infect. Dis. 2015, 2, ofv078. [Google Scholar] [CrossRef] [Green Version]
- Tariq, R.; Pardi, D.S.; Tosh, P.K.; Walker, R.C.; Razonable, R.R.; Khanna, S. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection Reduces Recurrent Urinary Tract Infection Frequency. Clin. Infect. Dis. 2017, 65, 1745–1747. [Google Scholar] [CrossRef]
- Koppe, L.; Croze, M.L.; Monteiro, E.B.; Benoit, B.; Bres, E.; Guebre-Egziabher, F.; Daleprane, J.B.; Fouque, D.; Soulage, C.O. The Protein-Bound Uremic Toxin p-Cresyl-Sulfate Promotes Intracellular ROS Production and Lipid Peroxidation in 3T3-L1 Adipose Cells. Biochimie 2021, 189, 137–143. [Google Scholar] [CrossRef]
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Tlaskalova-Hogenova, H.; Cermakova, M.; Tomasova, P.; Sediva, B.; Kuzma, M.; Bulant, J.; Bilej, M.; et al. Microbiota, Microbial Metabolites, and Barrier Function in A Patient with Anorexia Nervosa after Fecal Microbiota Transplantation. Microorganisms 2019, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Matute, P.; Íñiguez, M.; de Toro, M.; Recio-Fernández, E.; Oteo, J.A. Autologous Fecal Transplantation from a Lean State Potentiates Caloric Restriction Effects on Body Weight and Adiposity in Obese Mice. Sci. Rep. 2020, 10, 9388. [Google Scholar] [CrossRef]
- Hu, X.-F.; Zhang, W.-Y.; Wen, Q.; Chen, W.-J.; Wang, Z.-M.; Chen, J.; Zhu, F.; Liu, K.; Cheng, L.-X.; Yang, J.; et al. Fecal Microbiota Transplantation Alleviates Myocardial Damage in Myocarditis by Restoring the Microbiota Composition. Pharmacol. Res. 2019, 139, 412–421. [Google Scholar] [CrossRef]
- Burrello, C.; Garavaglia, F.; Cribiù, F.M.; Ercoli, G.; Lopez, G.; Troisi, J.; Colucci, A.; Guglietta, S.; Carloni, S.; Guglielmetti, S.; et al. Therapeutic Faecal Microbiota Transplantation Controls Intestinal Inflammation through IL10 Secretion by Immune Cells. Nat. Commun. 2018, 9, 5184. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ren, R.; Sun, G.; Peng, L.; Tian, Y.; Yang, Y. Pilot Study of Cytokine Changes Evaluation after Fecal Microbiota Transplantation in Patients with Ulcerative Colitis. Int. Immunopharmacol. 2020, 85, 106661. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, J.; Li, J.; Zhao, Z.; Guo, H.; Zhang, Y.; Cheng, S.; Sun, J.; Pan, H.; Fan, S.; et al. Intestinal Microbiota Impact Sepsis Associated Encephalopathy via the Vagus Nerve. Neurosci. Lett. 2018, 662, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Karbach, S.H.; Schönfelder, T.; Brandão, I.; Wilms, E.; Hörmann, N.; Jäckel, S.; Schüler, R.; Finger, S.; Knorr, M.; Lagrange, J.; et al. Gut Microbiota Promote Angiotensin II–Induced Arterial Hypertension and Vascular Dysfunction. J. Am. Heart Assoc. 2016, 5, e003698. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut Microbiota Dependent Trimethylamine N-Oxide Aggravates Angiotensin II–Induced Hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Chang, S.-C.; Wu, M.-S. Uremic Toxins Induce Kidney Fibrosis by Activating Intrarenal Renin–Angiotensin–Aldosterone System Associated Epithelial-to-Mesenchymal Transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [PubMed]
| Name | Class | Precursor | Characteristics | Conventional HD Reduction Rate (%) | Effect of PBUTs | Ref |
|---|---|---|---|---|---|---|
| Indoxyl sulfate | Indoles | Tryptophan | PBUT | 30% | CVD, vascular injury, bone disease, and nephrotoxicity | [46,47,48,49] |
| Indole3 Acetic Acid | Indoles | Tryptophan | PBUT | 40% | Cardiovascular dysfunction, endothelial damage, and cognitive impairment | [50,51,52] |
| Kynurenine, kynurenic acid, quinolinic acid | Kinurenine pathway | Tryptophan | PBUT | 20% | Cardiovascular dysfunction and cognitive impairment | [53,54] |
| Hippuric Acid | Hippurates | Benzoic acid | PBUT | 60–70% | Renal fibrosis and endothelial dysfunction | [55,56] |
| P-cresyl sulfate | Phenols | Tyrosine | PBUT | 30% | Cardiovascular damage, renal tubular injury, and insulin resistance | [57,58,59] |
| P-cresyl glucuronide | Phenols | Tyrosine | PBUT | 70% | Vascular damage | [60] |
| TMAO | Amine oxide | Choline, betaine, carnitine | Water-soluble compound | 80% | CVD and renal inflammation | [61,62,63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caggiano, G.; Stasi, A.; Franzin, R.; Fiorentino, M.; Cimmarusti, M.T.; Deleonardis, A.; Palieri, R.; Pontrelli, P.; Gesualdo, L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins 2023, 15, 115. https://doi.org/10.3390/toxins15020115
Caggiano G, Stasi A, Franzin R, Fiorentino M, Cimmarusti MT, Deleonardis A, Palieri R, Pontrelli P, Gesualdo L. Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives. Toxins. 2023; 15(2):115. https://doi.org/10.3390/toxins15020115
Chicago/Turabian StyleCaggiano, Gianvito, Alessandra Stasi, Rossana Franzin, Marco Fiorentino, Maria Teresa Cimmarusti, Annamaria Deleonardis, Rita Palieri, Paola Pontrelli, and Loreto Gesualdo. 2023. "Fecal Microbiota Transplantation in Reducing Uremic Toxins Accumulation in Kidney Disease: Current Understanding and Future Perspectives" Toxins 15, no. 2: 115. https://doi.org/10.3390/toxins15020115







