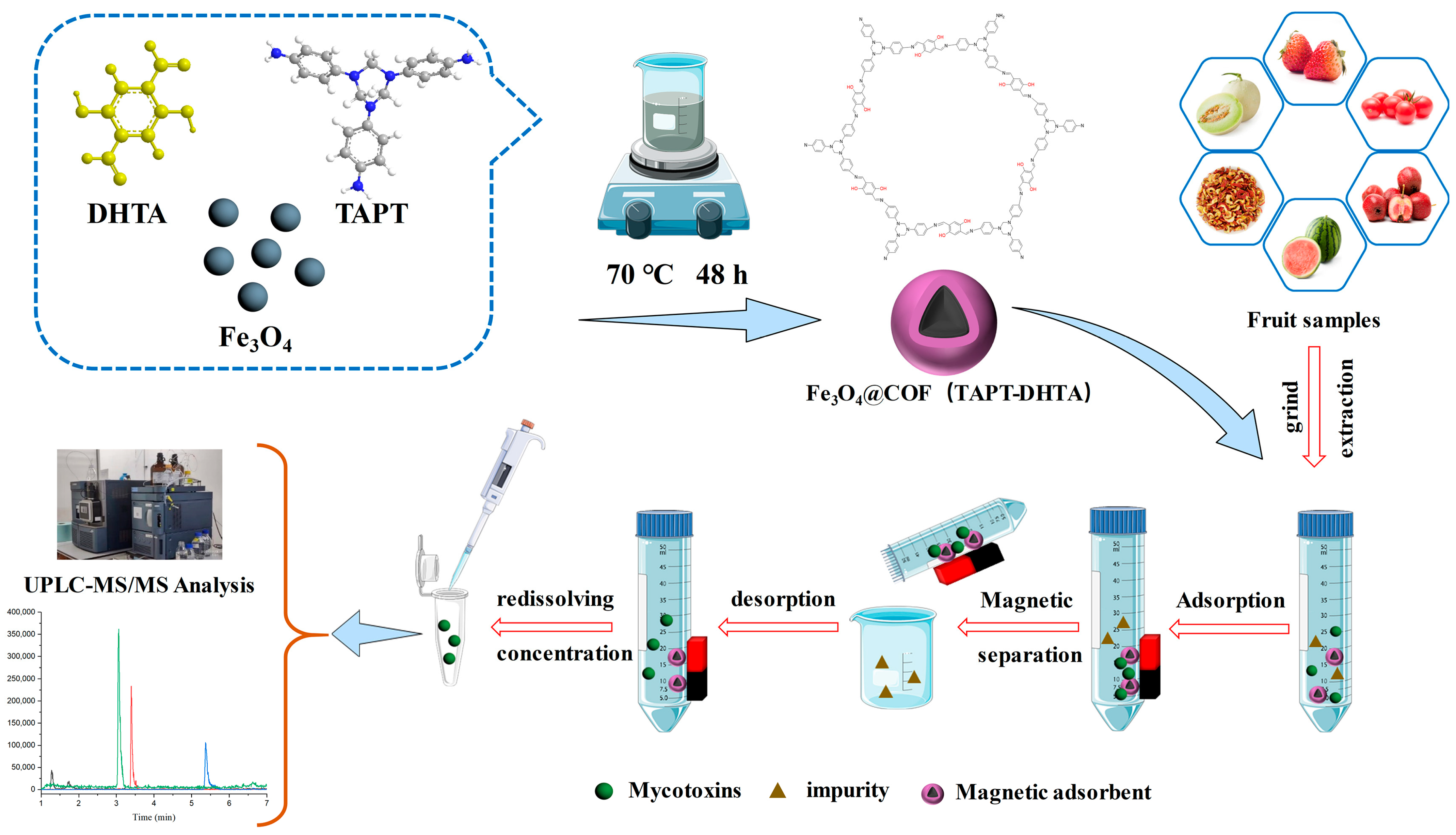
Fe3O4@COF(TAPT–DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC–MS/MS
Abstract
:1. Introduction
2. Results and Discussion
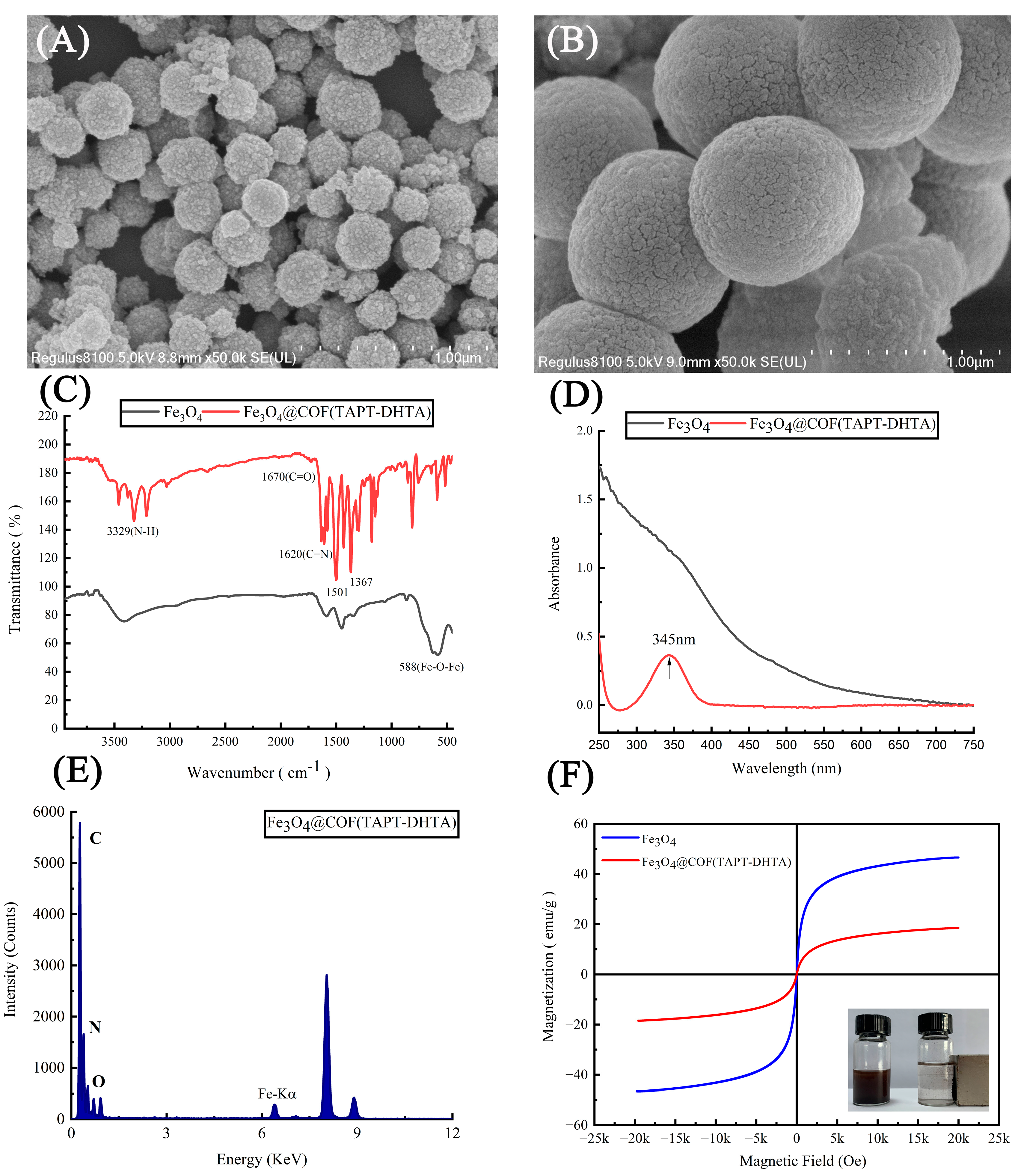
2.1. Characterization of Fe3O4@COF(TAPT–DHTA)
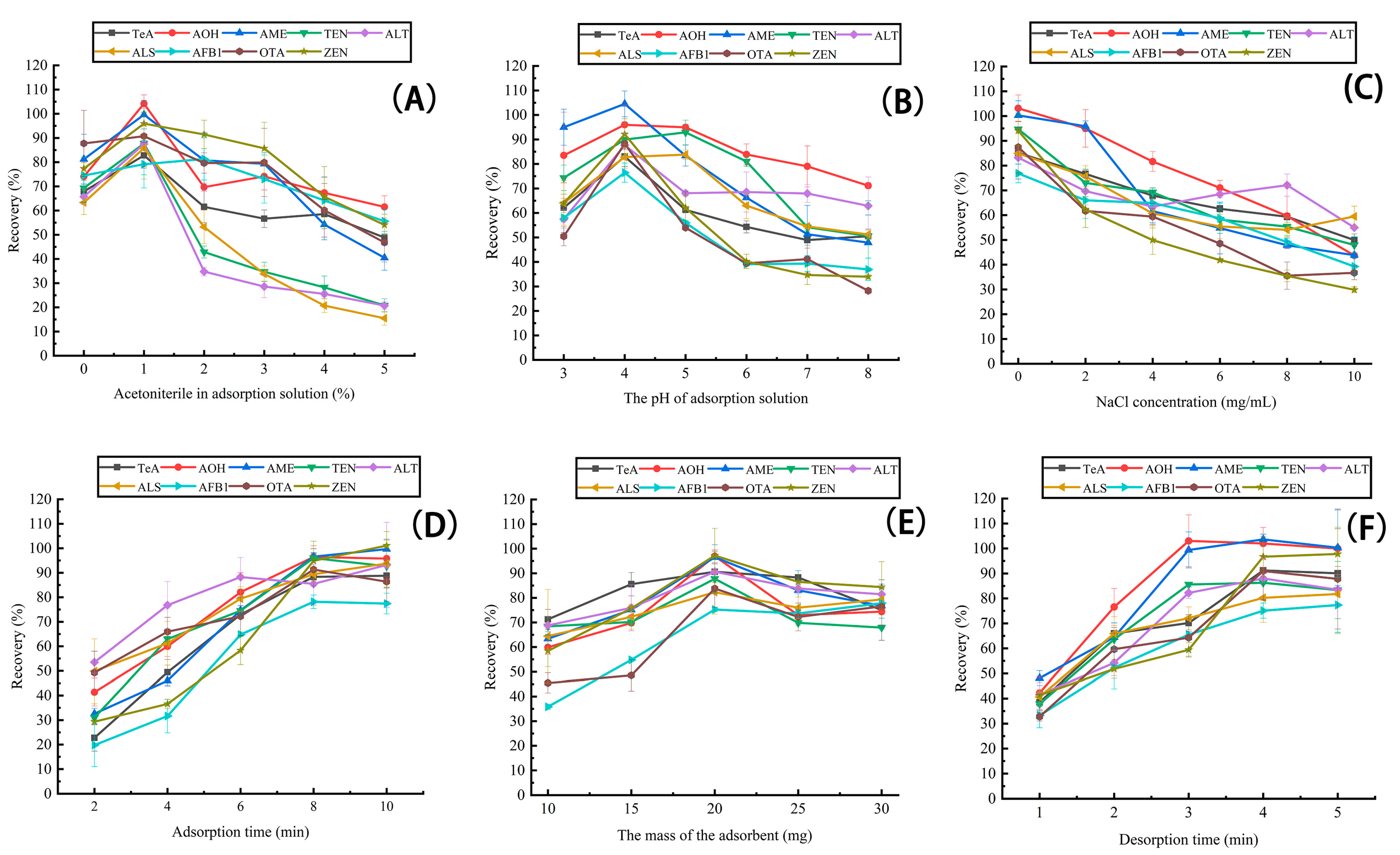
2.2. Optimization of MSPE Conditions
2.2.1. MSPE Adsorption Solution
2.2.2. The Amount of Fe3O4@COF(TAPT–DHTA)
2.2.3. Elution Solvent and Time
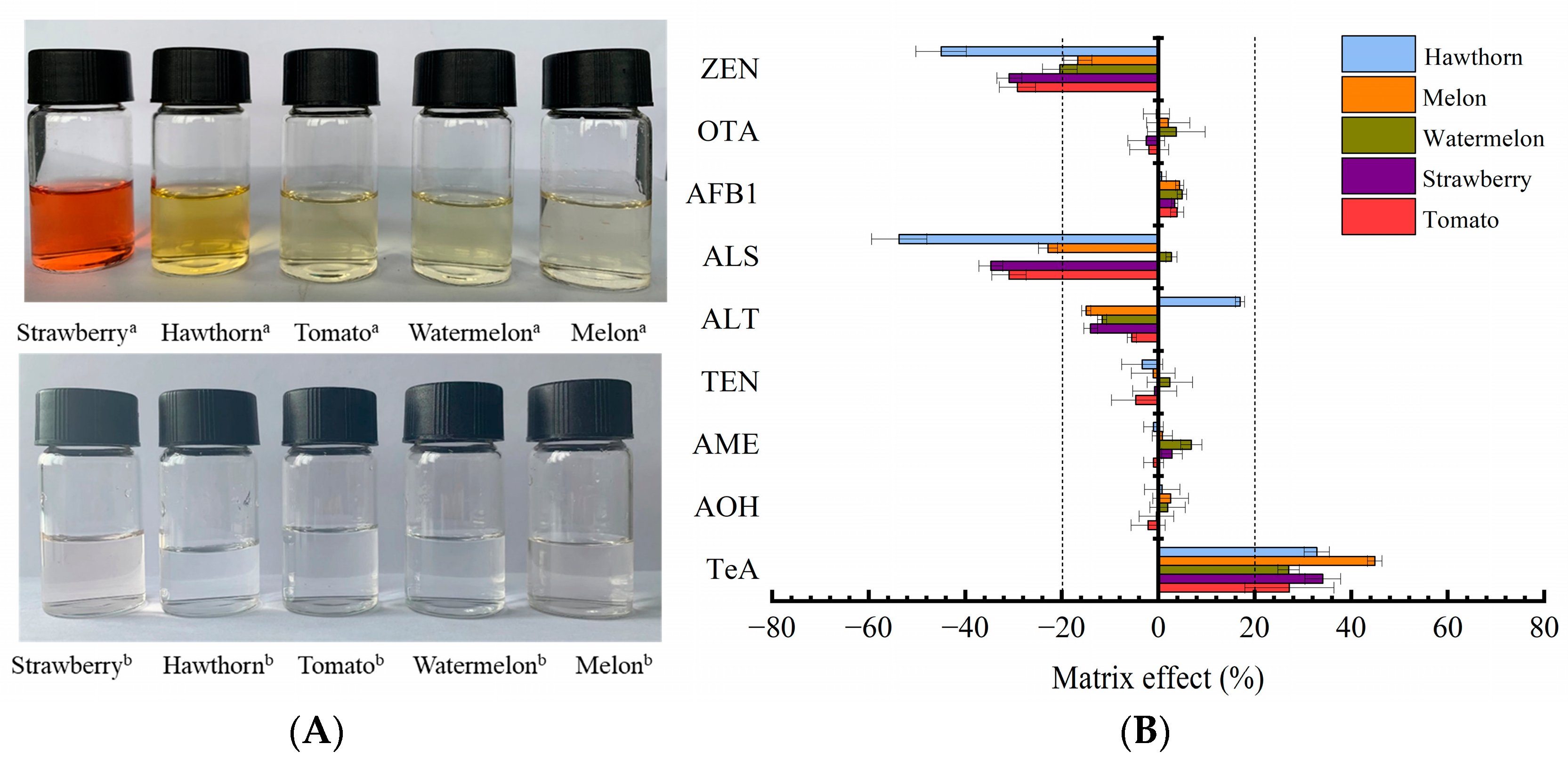
2.3. Method Validation
2.4. Method Application
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Apparatus and Characterization
4.3. Preparation of Fe3O4@COF(TAPT–DHTA)
4.4. Sample Preparation
4.5. UHPLC–MS/MS Analysis
4.6. Method Validation
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drusch, S.; Ragab, W. Mycotoxins in Fruits, Fruit Juices, and Dried Fruits. J. Food Prot. 2003, 66, 1514–1527. [Google Scholar] [CrossRef]
- Zhao, K.; Shao, B.; Yang, D.J.; Li, F.Q. Natural occurrence of four Alternaria mycotoxins in tomato- and citrus-based foods in China. J. Agric. Food Chem. 2015, 63, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Oueslati, S.; Manes, J. Evaluation of alternaria mycotoxins in strawberries: Quantification and storage condition. Food Addit. Contam. 2016, 33, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.J.; Guo, W.B.; Zhao, Z.H.; Zhang, Z.Q.; Nie, D.X.; Tangni, E.K.; Han, Z. Production of alternaria toxins in yellow peach (Amygdalus persica) upon artificial inoculation with Alternaria alternate. Toxins 2021, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Yu, J.H. Toxicity, and analysis of major mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Chen, A.Q.; Mao, X.; Sun, Q.H.; Wei, Z.X.; Li, J.; You, Y.L.; Zhao, J.Q.; Jiang, G.B.; Wu, Y.N.; Wang, L.P.; et al. Alternaria mycotoxins: An overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Fan, K.; Guo, W.B.; Huang, Q.W.; Meng, J.J.; Yao, Q.; Nie, D.X.; Han, Z.; Zhao, Z.H. Assessment of human exposure to five alternaria mycotoxins in China by biomonitoring approach. Toxins 2021, 13, 762. [Google Scholar] [CrossRef]
- GB 2761-2017; National Food Safety Standard-Maximum Levels of Mycotoxins in Foods. National Standard of People’s Republic of China: Beijing, China, 2017.
- EC. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2007, 364, 5–25. [Google Scholar]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.Z.; Shi, L.; Feng, X.Y. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef]
- Van de Perre, E.; Deschuyffeleer, N.; Jacxsens, L.; Vekeman, F.; Van Der Hauwaert, W.; Asam, S.; Rychlik, M.; Devlieghere, F.; De Meulenaer, B. Screening of moulds and mycotoxins in tomatoes, bell peppers, onions, soft red fruits and derived tomato products. Food Control 2014, 37, 165–170. [Google Scholar] [CrossRef]
- Gao, S.Q.; Wu, Y.Q.; Xie, S.Y.; Shao, Z.C.; Bao, X.M.; Yan, Y.M.; Wu, Y.Y.; Wang, J.X.; Zhang, Z.E. Determination of aflatoxins in milk sample with ionic liquid modified magnetic zeolitic imidazolate frameworks. J. Chromatogr. B 2019, 1128, 121778. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Montone, C.M.; Chiozzi, R.Z.; Lagana, A. A rapid magnetic solid phase extraction method followed by Liquid Chromatography-Tandem Mass spectrometry analysis for the determination of mycotoxins in cereals. Toxins 2017, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.T.; Zhang, J.; Yang, Y.J.; Yin, J.; Li, H.; Xing, Y.; Shao, B. Development of a simple and rapid LC-MS/MS method for the simultaneous quantification of five Alternaria mycotoxins in human urine. J. Chromatogr. B 2020, 1144, 122096. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Tolgyesi, A.; Bouten, K.; Robouch, P.; Emons, H.; Stroka, J. Determination of alternaria toxins in tomato, wheat, and sunflower seeds by SPE and LC-MS/MS-A method validation through a collaborative trial. J. AOAC Int. 2022, 105, 80–94. [Google Scholar] [CrossRef]
- De Berardis, S.; De Paola, E.L.; Montevecchi, G.; Garbini, D.; Masino, F.; Antonelli, A.; Melucci, D. Determination of four alternaria alternata mycotoxins by QuEChERS approach coupled with liquid chromatography-tandem mass spectrometry in tomato-based and fruit-based products. Food Res. Int. 2018, 106, 677–685. [Google Scholar] [CrossRef]
- Targuma, S.; Njobeh, P.B.; Ndungu, P.G. Current applications of magnetic nanomaterials for extraction of mycotoxins, pesticides, and pharmaceuticals in food commodities. Molecules 2021, 26, 4284. [Google Scholar] [CrossRef]
- Moreno, V.; Zougagh, M.; Rios, A. Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nano C18SiO2 composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Mikrochim. Acta 2016, 183, 871–880. [Google Scholar] [CrossRef]
- Ma, K.X.; Zhang, M.X.; Miao, S.C.; Gu, X.Y.; Li, N.; Cui, S.H.; Yang, J. Magnetic solid-phase extraction of pyrethroid pesticides in environmental water samples with CoFe2O4-embedded porous graphitic carbon nanocomposites. J. Sep. Sci. 2018, 41, 3441–3448. [Google Scholar] [CrossRef]
- Jia, Y.Q.; Wang, Y.H.; Yan, M.; Wang, Q.; Xu, H.J.; Wang, X.S.; Zhou, H.Y.; Hao, Y.L.; Wang, M.M. Fabrication of iron oxide@MOF-808 as a sorbent for magnetic solid phase extraction of benzoylurea insecticides in tea beverages and juice samples. J. Chromatogr. A 2020, 1615, 460766. [Google Scholar] [CrossRef]
- Lin, X.P.; Wang, X.Q.; Wang, J.; Yuan, Y.W.; Di, S.S.; Wang, Z.W.; Xu, H.; Zhao, H.Y.; Zhao, C.S.; Ding, W.; et al. Magnetic covalent organic framework as a solid-phase extraction absorbent for sensitive determination of trace organophosphorus pesticides in fatty milk. J. Chromatogr. A 2020, 1627, 461387. [Google Scholar] [CrossRef]
- Wen, L.; Liu, L.; Wang, X.; Wang, M.L.; Lin, J.M.; Zhao, R.S. Spherical mesoporous covalent organic framework as a solid-phase extraction adsorbent for the ultrasensitive determination of sulfonamides in food and water samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020, 1625, 461275. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Du, J.J.; Wu, D.; Liu, J.C.; Li, N.; Sun, Z.W.; Li, G.L.; Wu, Y.N. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC. Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Emmerling, S.T.; Schuldt, R.; Bette, S.; Yao, L.; Dinnebier, R.E.; Kastner, J.; Lotsch, B.V. Interlayer interactions as design tool for Large-Pore COFs. J. Am. Chem. Soc. 2021, 143, 15711–15722. [Google Scholar] [CrossRef]
- Vardhan, H.; Hou, L.X.; Yee, E.; Nafady, A.; Al-Abdrabalnabi, M.A.; Al-Enizi, A.M.; Pan, Y.X.; Yang, Z.Y.; Ma, S.Q. Vanadium docked covalent-organic frameworks: An effective heterogeneous catalyst for modified mannich-type reaction. ACS Sustain. Chem. Eng. 2019, 7, 4878–4888. [Google Scholar] [CrossRef]
- Xu, G.J.; Hou, L.F.; Li, B.Y.; Wang, X.L.; Liu, L.; Li, N.; Wang, M.L.; Zhao, R.S. Facile preparation of hydroxyl bearing covalent organic frameworks for analysis of phenoxy carboxylic acid pesticide residue in plant-derived food. Food Chem. 2021, 345, 128749. [Google Scholar] [CrossRef]
- Li, N.; Wu, D.; Li, X.T.; Zhou, X.X.; Fan, G.S.; Li, G.L.; Wu, Y.N. Effective enrichment and detection of plant growth regulators in fruits and vegetables using a novel magnetic covalent organic framework material as the adsorbents. Food Chem. 2020, 306, 125455. [Google Scholar] [CrossRef]
- Wang, Y.F.; Mu, G.D.; Wang, X.J.; Zhang, F.; Li, Y.L.; Lu, D.J.; Chen, F.M.; Yang, M.L.; He, M.Y.; Liu, T. Fast construction of core-shell structured magnetic covalent organic framework as sorbent for solid-phase extraction of zearalenone and its derivatives prior to their determination by UHPLC-MS/MS. Mikrochim. Acta 2021, 188, 246. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.M.; Wang, Y.W.; Qin, Y.T.; Yan, X.L.; Li, Y.; Chen, L.G. Two-Dimensional Imine-Linked covalent organic frameworks as a platform for selective oxidation of olefins. ACS Appl. Mater. Interfaces 2017, 9, 22856–22863. [Google Scholar] [CrossRef]
- Song, B.; Wei, H.; Wang, Z.Q.; Zhang, X.; Smet, M.; Dehaen, W. Supramolecular Nanofibers by Self-Organization of Bola-amphiphiles through a Combination of Hydrogen Bonding and π-π Stacking Interactions. Adv. Mater. 2007, 19, 416–420. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Liu, F.J.; He, W.Z.; Qin, Q.M.; Hu, D.Q.; Wu, A.B.; Jiang, W.B.; Wang, C. Screening of multi-mycotoxins in fruits by ultra-performance liquid chromatography coupled to ion mobility quadrupole time-of-flight mass spectrometry. Food Chem. 2022, 368, 130858. [Google Scholar] [CrossRef]
- Tang, Y.; Mu, L.; Cheng, J.X.; Du, Z.X.; Yang, Y.Y. Determination of multi-class mycotoxins in apples and tomatoes by combined use of QuEChERS method and Ultra-High-Performance Liquid Chromatography Tandem mass spectrometry. Food Anal. Method 2020, 13, 1381–1390. [Google Scholar] [CrossRef]
- SN-T 4295-2015; Determination of Alternaria Toxins Residues in Fruit and Vegetables for Export-LC-MS/MS Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2015.
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Runowski, M.; Lis, S. Synthesis, surface modification/decoration of luminescent–magnetic core/shell nanomaterials, based on the lanthanide doped fluorides (Fe3O4/SiO2/NH2/PAA/LnF3). J. Lumin. 2016, 170, 484–490. [Google Scholar] [CrossRef]
- Yin, S.J.; Zhao, C.P.; Jiang, H.; Lu, M.; Wang, Y.; Chen, H.; Yang, F.Q. Preparation of amino-functionalized covalent organic framework modified Fe3O4 nanoparticles for the selective enrichment of flavonoid glycosides. Microchem. J. 2021, 164, 105990. [Google Scholar] [CrossRef]
- EC. Commission Decision 2002/657/EC Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, L221, 8–36. Available online: https://www.legislation.gov.uk/eudn/2002/657 (accessed on 20 May 2022).
- Zhao, Y.; Wan, L.H.; Bai, X.L.; Liu, Y.M.; Zhang, F.P.; Liu, Y.M.; Liao, X. Quantification of mycotoxins in vegetable oil by UPLC-MS/MS after magnetic solid-phase extraction. Food Addit. Contam. 2017, 34, 1201–1210. [Google Scholar] [CrossRef]
- Xu, H.W.; Sun, J.D.; Wang, H.M.; Zhang, Y.Z.; Sun, X.L. Adsorption of aflatoxins and ochratoxins in edible vegetable oils with dopamine-coated magnetic multi-walled carbon nanotubes. Food Chem. 2021, 365, 130409. [Google Scholar] [CrossRef] [PubMed]
- Li, W.K.; Zhang, H.X.; Shi, Y.P. Simultaneous determination of aflatoxin B1 and zearalenone by magnetic nanoparticle filled amino-modified multi-walled carbon nanotubes. Anal. Methods 2018, 10, 3353–3363. [Google Scholar] [CrossRef]
- Tang, Z.T.; Han, Q.R.; Yu, G.; Liu, F.; Tan, Y.Z.; Peng, C. Fe3O4@PDA/MIL-101(Cr) as magnetic solid-phase extraction sorbent for mycotoxins in licorice prior to ultrahigh-performance liquid chromatography-tandem mass spectrometry analysis. Food Sci. Nutr. 2022, 10, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, Y.; He, W.; Hu, D.; Wu, A.; Wu, W. Development and Application of a QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Method to Quantitate Multi-Component Alternaria Toxins in Jujube. Toxins 2018, 10, 382. [Google Scholar] [CrossRef]
- Xing, L.J.; Zou, L.J.; Luo, R.F.; Wang, Y. Determination of five Alternaria toxins in wolfberry using modified QuEChERS and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2020, 311, 125975. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Mycotoxin | Formula | Linear Range (μg kg−1) | LOD a (μg kg−1) | LOQ b (μg kg−1) | R2 |
|---|---|---|---|---|---|---|
| Neat solution | TeA | y = 2477.96x + 3394.45 | 0.5–200 | 0.9973 | ||
| AOH | y = 1411.49x + 941.08 | 0.5–200 | 0.9959 | |||
| AME | y = 1817.16x + 322.29 | 0.5–200 | 0.9989 | |||
| TEN | y = 5284.07x−58.31 | 0.05–200 | 0.9969 | |||
| ALT | y = 524.42x + 638.12 | 1–200 | 0.9928 | |||
| ALS | y = 143.63x + 11.17 | 0.5–200 | 0.9982 | |||
| AFB1 | y = 13387.40x + 2131.25 | 0.05–200 | 0.9978 | |||
| OTA | y = 12142.70x + 1612.83 | 0.1–200 | 0.9998 | |||
| ZEN | y = 2592.33x − 45.41 | 0.2–200 | 0.9956 | |||
| Tomato | TeA | y = 3151.74x + 2661.33 | 0.5–200 | 0.20 | 0.50 | 0.9975 |
| AOH | y = 1381.90x + 88.99 | 0.5–200 | 0.20 | 0.50 | 0.9961 | |
| AME | y = 1802.95x + 139.73 | 0.5–200 | 0.10 | 0.50 | 0.9968 | |
| TEN | y = 5037.77x − 1339.19 | 0.05–200 | 0.01 | 0.05 | 0.9975 | |
| ALT | y = 495.76x + 2523.89 | 1–200 | 0.30 | 1.00 | 0.9973 | |
| ALS | y = 99.21x − 44.54 | 1–200 | 0.50 | 1.00 | 0.9933 | |
| AFB1 | y = 13910.30x − 3759.31 | 0.5–200 | 0.20 | 0.50 | 0.9957 | |
| OTA | y = 11913.80x − 3011.72 | 0.1–200 | 0.05 | 0.10 | 0.9969 | |
| ZEN | y = 1836.13x − 408.39 | 0.5–200 | 0.20 | 0.50 | 0.9966 | |
| Watermelon | TeA | y = 3147.83x + 3812.73 | 0.5–200 | 0.20 | 0.50 | 0.9988 |
| AOH | y = 1439.20x + 166.73 | 0.5–200 | 0.20 | 0.50 | 0.9998 | |
| AME | y = 1942.00x + 660.55 | 0.5–200 | 0.10 | 0.50 | 0.9995 | |
| TEN | y = 5411.94x−86.24 | 0.1–200 | 0.05 | 0.10 | 0.9995 | |
| ALT | y = 463.38x + 2883.52 | 1–200 | 0.30 | 1.00 | 0.9952 | |
| ALS | y = 147.57x − 105.27 | 1–200 | 0.50 | 1.00 | 0.9985 | |
| AFB1 | y = 14048.90x − 407.44 | 0.05–200 | 0.01 | 0.05 | 0.9998 | |
| OTA | y = 12596.50x − 1507.82 | 0.5–200 | 0.20 | 0.50 | 0.9991 | |
| ZEN | y = 2064.16x − 407.63 | 0.5–200 | 0.10 | 0.50 | 0.9923 | |
| Melon | TeA | y = 3590.11x + 3091.17 | 0.5–200 | 0.20 | 0.50 | 0.9981 |
| AOH | y = 1447.78x + 35.81 | 0.5–200 | 0.20 | 0.50 | 0.9973 | |
| AME | y = 1832.77x + 632.65 | 0.5–200 | 0.10 | 0.50 | 0.9993 | |
| TEN | y = 5228.12x−492.67 | 0.1–200 | 0.05 | 0.10 | 0.9993 | |
| ALT | y = 446.22x + 6640.54 | 1–200 | 0.30 | 1.00 | 0.9976 | |
| ALS | y = 110.85x − 74.61 | 1–200 | 0.50 | 1.00 | 0.9968 | |
| AFB1 | y = 13980.80x − 1061.58 | 0.1–200 | 0.02 | 0.10 | 0.9998 | |
| OTA | y = 12393.30x − 462.63 | 0.5–200 | 0.10 | 0.50 | 0.9994 | |
| ZEN | y = 2160.20x − 575.37 | 0.5–200 | 0.10 | 0.50 | 0.9931 | |
| Strawberry | TeA | y = 3323.10x + 3464.16 | 0.5–200 | 0.20 | 0.50 | 0.9972 |
| AOH | y = 1406.51x − 68.41 | 0.5–200 | 0.20 | 0.50 | 0.9983 | |
| AME | y = 1869.42x + 594.67 | 0.5–200 | 0.10 | 0.50 | 0.9992 | |
| TEN | y = 5245.70x − 460.86 | 0.1–200 | 0.05 | 0.10 | 0.9996 | |
| ALT | y = 450.92x + 2765.14 | 1–200 | 0.30 | 1.00 | 0.9922 | |
| ALS | y = 93.84x + 58.02 | 1–200 | 0.50 | 1.00 | 0.9940 | |
| AFB1 | y = 13843.00x − 1246.62 | 0.1–200 | 0.02 | 0.10 | 0.9996 | |
| OTA | y = 11841.30x − 470.11 | 0.5–200 | 0.10 | 0.50 | 0.9993 | |
| ZEN | y = 1792.03x − 54.64 | 0.5–200 | 0.10 | 0.50 | 0.9983 | |
| Hawthorn | TeA | y = 3292.18x + 5601.93 | 0.5–200 | 0.20 | 0.50 | 0.9958 |
| AOH | y = 1423.11x + 12363.80 | 0.5–200 | 0.20 | 0.50 | 0.9943 | |
| AME | y = 1799.34x + 365.52 | 0.5–200 | 0.20 | 0.50 | 0.9996 | |
| TEN | y = 5082.12x + 1587.85 | 0.05–200 | 0.02 | 0.05 | 0.9996 | |
| ALT | y = 419.79x + 9686.70 | 1–200 | 0.30 | 1.00 | 0.9910 | |
| ALS | y = 66.53x + 34.26 | 1–200 | 0.50 | 1.00 | 0.9918 | |
| AFB1 | y = 13483.20x − 304.85 | 0.1–200 | 0.02 | 0.10 | 0.9992 | |
| OTA | y = 12097.60x − 1068.53 | 0.5–200 | 0.10 | 0.50 | 0.9993 | |
| ZEN | y = 1426.21x − 169.96 | 0.5–200 | 0.10 | 0.50 | 0.9972 |
| Mycotoxins | Spiked Levels (μg kg−1) | Tomato | Watermelon | Melon | Strawberry | Hawthorn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery (X ± SD, %) n = 3 | Intra-Day Precision (Intra-RSD, %) n = 5 | Inter-Day Precision (Inter-RSD, %) n = 5 | Recovery (X ± SD, %) n = 3 | Intra-Day Precision (Intra-RSD, %) n = 5 | Inter-Day Precision (Inter-RSD, %) n = 5 | Recovery (X ± SD, %) n = 3 | Intra-Day Precision (Intra-RSD, %) n = 5 | Inter-Day Precision (Inter-RSD, %) n = 5 | Recovery (X ± SD, %) n = 3 | Intra-Day Precision (Intra-RSD, %) n = 5 | Inter-Day Precision (Inter-RSD, %) n = 5 | Recovery (X ± SD, %) n = 3 | Intra-Day Precision (Intra-RSD, %) n = 5 | Inter-Day Precision (Inter-RSD, %) n = 5 | ||
| TeA | 2 | 95.8 ± 0.2 | 2.4 | 8.7 | 84.4 ± 0.2 | 4.9 | 9.2 | 94.6 ± 0.2 | 6.9 | 8.2 | 99.2 ± 0.2 | 5.0 | 10.6 | 96.3 ± 0.2 | 6.6 | 11.4 |
| 50 | 90.8 ± 1.5 | 3.1 | 6.2 | 79.2 ± 0.4 | 5.4 | 3.7 | 84.5 ± 1.4 | 7.5 | 11.1 | 78.5 ± 1.4 | 4.2 | 11.7 | 96.4 ± 2.0 | 3.8 | 8.8 | |
| 100 | 85.6 ± 3.1 | 2.6 | 6.1 | 74.8 ± 3.7 | 4.9 | 8.6 | 81.1 ± 3.8 | 5.4 | 7.8 | 80.4 ± 5.7 | 7.6 | 10.7 | 88.7 ± 6.2 | 3.9 | 11.7 | |
| AOH | 2 | 82.0 ± 0.1 | 3.8 | 4.3 | 88.3 ± 0.1 | 4.9 | 6.0 | 93.1 ± 0.2 | 3.8 | 12.4 | 82.0 ± 0.1 | 3.2 | 3.7 | 103.6 ± 0.1 | 2.1 | 9.64 |
| 50 | 84.5 ± 1.3 | 4.3 | 12.9 | 92.4 ± 2.2 | 5.4 | 9.7 | 100.9 ± 4.5 | 3.4 | 7.4 | 101.9 ± 4.3 | 5.2 | 8.6 | 95.9 ± 4.3 | 6.9 | 8.7 | |
| 100 | 111.7 ± 3.4 | 3.8 | 5.1 | 94.2 ± 2.3 | 4.2 | 4.0 | 82.5 ± 4.9 | 7.7 | 4.6 | 83.2 ± 4.6 | 4.8 | 9.2 | 76.3 ± 4.5 | 4.3 | 9.7 | |
| AME | 2 | 98.1 ± 0.2 | 3.1 | 7.5 | 89.1 ± 0.2 | 2.3 | 8.4 | 104.4 ± 0.2 | 3.9 | 5.3 | 87.7 ± 0.1 | 3.4 | 7.0 | 109.9 ± 0.2 | 6.2 | 5.81 |
| 50 | 82.5 ± 1.1 | 2.8 | 8.4 | 78.4 ± 1.3 | 3.0 | 5.7 | 91.2 ± 2.6 | 4.3 | 9.6 | 96.5 ± 1.6 | 4.5 | 10.8 | 107.9 ± 1.3 | 5.2 | 8.3 | |
| 100 | 78.2 ± 3.9 | 5.4 | 8.4 | 81.5 ± 4.8 | 3.0 | 4.7 | 88.8 ± 2.9 | 4.1 | 5.4 | 91.7 ± 5.6 | 3.8 | 10.1 | 98.9 ± 3.1 | 5.4 | 5.2 | |
| TEN | 2 | 89.7 ± 0.1 | 4.4 | 7.9 | 75.2 ± 0.1 | 5.7 | 2.2 | 82.8 ± 0.1 | 5.2 | 5.8 | 92.9 ± 0.1 | 7.2 | 11.9 | 88.1 ± 0.1 | 6.7 | 6.7 |
| 50 | 91.5 ± 0.7 | 5.2 | 5.1 | 83.7 ± 1.5 | 6.4 | 11.7 | 96.6 ± 1.6 | 5.5 | 6.2 | 91.9 ± 1.3 | 6.7 | 9.3 | 92.0 ± 1.3 | 5.6 | 9.7 | |
| 100 | 75.4 ± 1.8 | 7.3 | 3.9 | 90.1 ± 2.3 | 6.2 | 4.3 | 75.7 ± 4.3 | 3.9 | 9.4 | 79.8 ± 4.5 | 5.8 | 9.5 | 82.5 ± 2.3 | 6.1 | 4.7 | |
| ALT | 2 | 86.1 ± 0.1 | 7.9 | 7.2 | 80.4 ± 0.2 | 8.6 | 7.6 | 81.1 ± 0.1 | 3.3 | 9.1 | 99.9 ± 0.2 | 4.0 | 12.7 | 96.8 ± 0.2 | 4.6 | 10.7 |
| 50 | 90.8 ± 1.4 | 6.2 | 4.4 | 81.5 ± 1.4 | 7.5 | 11.8 | 75.6 ± 1.1 | 5.5 | 9.3 | 78.5 ± 1.4 | 7.2 | 11.7 | 99.4 ± 2.0 | 5.3 | 9.3 | |
| 100 | 85.6 ± 3.1 | 6.1 | 11.5 | 76.9 ± 2.7 | 7.5 | 6.2 | 75.6 ± 2.4 | 6.8 | 5.2 | 74.9 ± 1.7 | 8.1 | 3.8 | 77.6 ± 2.2 | 4.6 | 4.8 | |
| ALS | 2 | 93.0 ± 0.1 | 3.6 | 5.9 | 85.7 ± 0.2 | 4.3 | 3.0 | 87.8 ± 0.1 | 2.7 | 4.2 | 83.6 ± 0.2 | 8.6 | 6.6 | 84.7 ± 0.1 | 4.8 | 11.0 |
| 50 | 86.8 ± 1.9 | 4.1 | 6.8 | 90.3 ± 1.3 | 2.2 | 4.2 | 88.2 ± 1.7 | 7.5 | 10.4 | 88.0 ± 1.8 | 3.0 | 11.4 | 88.4 ± 1.3 | 5.1 | 8.9 | |
| 100 | 88.71 ± 5.84 | 4.7 | 6.1 | 83.1 ± 4.0 | 5.4 | 6.1 | 91.3 ± 5.3 | 5.7 | 9.1 | 90.7 ± 4.9 | 3.8 | 8.9 | 89.9 ± 5.6 | 4.4 | 4.2 | |
| AFB1 | 2 | 81.1 ± 0.1 | 2.9 | 8.4 | 81.3 ± 0.1 | 7.9 | 10.6 | 96.6 ± 0.1 | 6.1 | 5.1 | 102.1 ± 0.2 | 7.1 | 8.7 | 97.1 ± 0.1 | 3.6 | 8.4 |
| 50 | 83.6 ± 0.9 | 3.9 | 7.6 | 79.0 ± 1.8 | 4.4 | 8.9 | 79.6 ± 1.9 | 8.6 | 9.5 | 85.4 ± 1.7 | 5.9 | 10.9 | 85.3 ± 1.7 | 5.8 | 8.9 | |
| 100 | 77.2 ± 2.6 | 4.6 | 6.1 | 82.5 ± 5.9 | 5.4 | 11.5 | 74.3 ± 4.7 | 5.5 | 8.9 | 80.6 ± 2.9 | 6.3 | 5.9 | 77.4 ± 5.1 | 5.0 | 10.9 | |
| OTA | 2 | 108.1 ± 0.1 | 6.2 | 5.4 | 100.6 ± 0.1 | 4.5 | 3.5 | 89.8 ± 0.2 | 6.7 | 12.3 | 75.6 ± 0.1 | 5.5 | 8.5 | 90.6 ± 0.2 | 3.3 | 9.2 |
| 50 | 111.7 ± 1.3 | 5.7 | 7.5 | 97.3 ± 1.6 | 7.4 | 11.0 | 99.6 ± 1.8 | 4.2 | 11.9 | 93.6 ± 1.7 | 3.8 | 11.80 | 98.5 ± 1.6 | 6.9 | 11.0 | |
| 100 | 84.1 ± 4.4 | 6.0 | 8.7 | 76.3 ± 5.6 | 6.9 | 12.3 | 75.7 ± 1.9 | 7.7 | 4.2 | 83.1 ± 2.5 | 2.1 | 4.9 | 83.8 ± 1.9 | 7.8 | 3.8 | |
| ZEN | 2 | 78.8 ± 0.2 | 4.3 | 9.6 | 91.2 ± 0.1 | 5.2 | 5.0 | 107.5 ± 0.1 | 3.8 | 4.6 | 84.8 ± 0.2 | 7.2 | 9.9 | 78.2 ± 0.4 | 6.2 | 3.2 |
| 50 | 99.7 ± 1.5 | 7.1 | 10.0 | 84.8 ± 1.6 | 9.0 | 8.4 | 101.7 ± 1.5 | 4.2 | 9.9 | 76.6 ± 1.8 | 6.7 | 11.2 | 79.2 ± 0.7 | 5.0 | 5.8 | |
| 100 | 85.3 ± 2.8 | 6.2 | 5.4 | 91.2 ± 4.0 | 4.8 | 7.4 | 87.1 ± 2.3 | 3.8 | 4.4 | 82.0 ± 4.9 | 5.7 | 3.8 | 87.1 ± 3.3 | 5.9 | 6.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, Q.; Guo, W.; Guo, D.; Han, Z.; Nie, D. Fe3O4@COF(TAPT–DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC–MS/MS. Toxins 2023, 15, 117. https://doi.org/10.3390/toxins15020117
Wang J, Huang Q, Guo W, Guo D, Han Z, Nie D. Fe3O4@COF(TAPT–DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC–MS/MS. Toxins. 2023; 15(2):117. https://doi.org/10.3390/toxins15020117
Chicago/Turabian StyleWang, Jie, Qingwen Huang, Wenbo Guo, Dakai Guo, Zheng Han, and Dongxia Nie. 2023. "Fe3O4@COF(TAPT–DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC–MS/MS" Toxins 15, no. 2: 117. https://doi.org/10.3390/toxins15020117
APA StyleWang, J., Huang, Q., Guo, W., Guo, D., Han, Z., & Nie, D. (2023). Fe3O4@COF(TAPT–DHTA) Nanocomposites as Magnetic Solid-Phase Extraction Adsorbents for Simultaneous Determination of 9 Mycotoxins in Fruits by UHPLC–MS/MS. Toxins, 15(2), 117. https://doi.org/10.3390/toxins15020117





