Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells
Abstract
:1. Introduction
2. Results
2.1. ZEA’s Effects on Piglet SCs’ Viability
2.2. Piglet SCs Ca2+ Levels in Response to ZEA
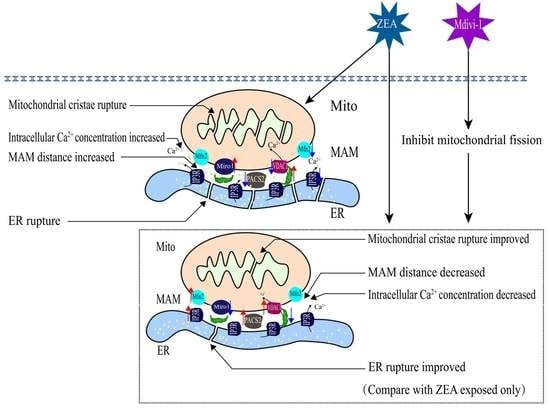
2.3. Piglet SCs’ MAM Structures under ZEA Treatment
2.4. Correlation of Mito and ER of Piglet SCs in Response to ZEA
2.5. Piglet SC Gene Expression in Response to ZEA
2.6. Piglet SC Protein Expression in Response to ZEA
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Chemicals
5.2. Cell Culture and Treatments
5.3. Cell-Viability Assay
5.4. Determination of Ca2+
5.5. MAM-Structure Determination
5.6. Assay for Mito-ER Correlation
5.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
5.8. Western Blotting (WB)
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Jin, Y.; Guo, Q.; Wang, X.; Luo, S.; Yang, W.; Li, J.; Chen, Y. Immunoaffinity cleanup and isotope dilution-based liquid chromatography tandem mass spectrometry for the determination of six major mycotoxins in feed and feedstuff. Toxins 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Gao, L.; Li, F.; Cui, j.; Yang, X.; Liu, Y.; Chen, S.; Chen, B. Effects of zearalenone on ovarian development of prepubertal gilts through growth hormone axis. Front. Vet. Sci. 2022, 9, 950063. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, B.; Li, X.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, Z. Zearalenone promotes cell proliferation or causes cell death. Toxins 2018, 10, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Liao, T.; Bao, Z.; Zhou, C.; Luo, H.; Lu, C.; Pan, M.; Wang, X. Role of AMPK in the expression of tight junction proteins in heat-treated porcine Sertoli cells. Theriogenology 2018, 121, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Nie, S.; Huang, D.; Xie, M. Effects of nonylphenol exposure on expression of cell receptors and secretory function in mouse sertoli TM4 cells. Environ. Toxicol. Pharmacol. 2014, 37, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, H.; Han, S.; Zhang, H.; Hu, J. Zearalenone induces oxidative stress and autophagy in goat sertoli cells. Ecotoxicol. Environ. Saf. 2023, 252, 114571. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Ma, L.; Chen, J.; Xu, J.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112737. [Google Scholar] [CrossRef]
- Bernard, F. Alteration of mitochondrial DNA homeostasis in drug-induced liver injury. Food Chem. Toxicol. 2020, 135, 110916. [Google Scholar] [CrossRef]
- Konietzny, A.; Wegmann, S.; Mikhaylova, M. The endoplasmic reticulum puts a new spin on synaptic tagging. Trends Neurosci. 2022, 46, 32–44. [Google Scholar] [CrossRef]
- Filadi, R.; Theurey, P.; Pizzo, P. The endoplasmic reticulum-mitochondria coupling in health and disease: Molecules, functions and significance. Cell Calcium 2017, 62, 1–15. [Google Scholar] [CrossRef]
- Légiot, A.; Céré, C.; Dupoiron, T.; Kaabouni, M.; Camougrand, N.; Manon, S. Mitochondria-associated membranes (MAMs) are involved in Bax mitochondrial localization and cytochrome c release. Microb. Cell 2019, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Missiroli, S.; Patergnani, S.; Caroccia, N.; Pedriali, G.; Perrone, M. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018, 9, 329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-associated endoplasmic reticulum membranes in cardiovascular diseases. Front. Cell Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Liu, J.; Xie, F.; Su, B.; Wang, X.; Yoon, Y. Abnormalities of mitochondrial dynamics in neurodegenerative diseases. Antioxidants 2017, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Del Dotto, V.; Mishra, P.; Vidoni, S.; Fogazza, M.; Maresca, A.; Caporali, L.; McCaffery, J.M.; Cappelletti, M.; Baruffini, E.; Lenaers, G.; et al. OPA1 isoforms in the hierarchical organization of mitochondrial functions. Cell Rep. 2017, 19, 2557–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manczak, M.; Kandimalla, R.; Yin, X.; Reddy, P.H. Mitochondrial division inhibitor 1 reduces dynamin-related protein 1 and mitochondrial fission activity. Hum. Mol. Genet. 2019, 28, 177–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishihara, N.; Nomura, M.; Jofuku, A. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat. Cell Biol. 2009, 11, 958–966. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer. Int. J. Oncol. 2019, 54, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Hai, S.; Ma, L.; Qiu, Z.; Han, M.; Wang, C.; Feng, S.; Zhao, C.; Wang, X. Effects of mitochondrial regulation on endoplamic reticulum pathway appotosis of sertoli cells induced by zearalenone and deoxynivalenol in piglets. Chin. J. Anim. Nutr. 2022, 35, 1261–1269. Available online: https://kns.cnki.net/kcms/detail//11.5461.S.20221222.0929.001.html (accessed on 1 December 2022).
- Mao, H.; Chen, W.; Chen, L.; Li, L. Potential role of mitochondria-associated endoplasmic reticulum membrane proteins in diseases. Biochem. Pharmacol. 2022, 199, 115011. [Google Scholar] [CrossRef]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef]
- Wang, X.; Cao, H.; Fang, Y.; Bai, H.; Chen, J.; Xing, C.; Zhuang, Y.; Guo, X.; Hu, G.; Yang, F. Activation of endoplasmic reticulum-mitochondria coupling drives copper-induced autophagy in duck renal tubular epithelial cells. Ecotoxicol. Environ. Saf. 2022, 235, 113438. [Google Scholar] [CrossRef]
- Knoell, J.; Chillappagari, S.; Knudsen, L.; Korfei, M.; Dartsch, R.; Jonigk, D.; Kuehnel, M.; Hoetzenecker, K.; Guenther, A.; Mahavadi, P. PACS2-TRPV1 axis is required for ER-mitochondrial tethering during ER stress and lung fibrosis. Cell. Mol. Life Sci. 2022, 279, 151. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, L.; Liu, C.; Yang, J.; Zhang, J.; Huang, L. PACS2 is required for ox-LDL-induced endothelial cell apoptosis by regulating mitochondria-associated ER membrane formation and mitochondrial Ca2+ elevation. Exp. Cell Res. 2019, 379, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lu, L.; Zhu, J.; Gu, X.; Liu, B.; Li, D.; Chen, G. Miro1 provides neuroprotection via the mitochondrial trafficking pathway in a rat model of traumatic brain injury. Brain Res. 2021, 1773, 147685. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Li, L.; Vanhauwaert, R.; Nguyen, K.T.; Davis, M.D.; Bu, G.; Wszolek, Z.; Wang, X. Miro1 Marks Parkinson’s Disease Subset and Miro1 Reducer Rescues Neuron Loss in Parkinson’s Models. Cell Metab. 2019, 30, 1131–1140.e7. [Google Scholar] [CrossRef]
- Xu, H.; Guan, N.; Ren, Y.; Wei, Q.; Tao, Y.; Yang, G.; Liu, X.; Bu, D.; Ying, Z.; Zhu, S. IP3R-Grp75-VDAC1-MCU calcium regulation axis antagonists protect podocytes from apoptosis and decrease proteinuria in an Adriamycin nephropathy rat model. BMC Nephrol. 2018, 19, 140. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Gong, M.; He, J.; Xie, B.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; et al. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 2022, 52, 102289. [Google Scholar] [CrossRef]
- Szabadkai, G.; Bianchi, K.; Varnai, P. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006, 175, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Xie, N.; Wang, C.; Wu, C.; Cheng, X.; Gao, Y.; Zhang, H.; Zhang, Y.; Lian, Y. Mdivi-1 Protects Epileptic Hippocampal Neurons from Apoptosis via Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress in Vitro. Neurochem. Res. 2016, 41, 1335–1342. [Google Scholar] [CrossRef]
- Dromparis, P.; Paulin, R.; Sutendra, G.; Qi, A.C.; Bonnet, S.; Michelakis, E.D. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and trigges pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ. Res. 2013, 113, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Hai, S.; Chen, J.; Ma, L.; Rahman, S.U.; Zhao, C.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Zearalenone Induces Apoptosis in Porcine Endometrial Stromal Cells through JNK Signaling Pathway Based on Endoplasmic Reticulum Stress. Toxins 2022, 14, 758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Zhu, L.; Cao, L.; Xu, W.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J. Autophagy protects PC12 cells against deoxynivalenol toxicity via the Class III PI3K/beclin 1/Bcl-2 pathway. J. Cell Physiol. 2020, 235, 7803–7815. [Google Scholar] [CrossRef] [PubMed]






| Gene | Accession Number | Primer Sequences (5′→3′) | Primer | Product/bp |
|---|---|---|---|---|
| GAPDH | 396,823 | TGACCCCTTCATTGACCTCC CCATTTGATGTTGGCGGGAT | F R | 160 |
| PACS-2 | 110,258,361 | AGCACAGTGCAGGACAC CACTATCCCAACCTTCACGA | F R | 180 |
| Grp75 | 100,521,183 | GCACGAGGAAAGCCTTAGAG GGAGAAGATGGGACGACAAA | F R | 166 |
| Mfn2 | 100,512,354 | ATGGGCATTCTCGTTGTTGGAG GCAGCTTCTCGCTGGCGTAC | F R | 175 |
| Miro1 | 100,523,466 | CACAAGCCTTCACTTGCAATA GTGTCACGTGCGGGTA | F R | 90 |
| IP3R | 397,454 | TTCCATCCTAACGGAACGAG CTCTGTAGTCAGCTCCTTGG | F R | 127 |
| VDAC1 | 397,010 | CCCACGTATGCCGATCTT GTCAGACCATATTCGGTCCA | F R | 197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Chen, C.; Hai, S.; Wang, C.; Rahman, S.U.; Huang, W.; Zhao, C.; Feng, S.; Wang, X. Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells. Toxins 2023, 15, 253. https://doi.org/10.3390/toxins15040253
Ma L, Chen C, Hai S, Wang C, Rahman SU, Huang W, Zhao C, Feng S, Wang X. Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells. Toxins. 2023; 15(4):253. https://doi.org/10.3390/toxins15040253
Chicago/Turabian StyleMa, Li, Chuangjiang Chen, Sirao Hai, Chenlong Wang, Sajid Ur Rahman, Wanyue Huang, Chang Zhao, Shibin Feng, and Xichun Wang. 2023. "Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells" Toxins 15, no. 4: 253. https://doi.org/10.3390/toxins15040253
APA StyleMa, L., Chen, C., Hai, S., Wang, C., Rahman, S. U., Huang, W., Zhao, C., Feng, S., & Wang, X. (2023). Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells. Toxins, 15(4), 253. https://doi.org/10.3390/toxins15040253