Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Objectives, Registration and Protocol
2.2. Information Sources
2.3. Search Strategy
2.4. Data Synthesis, Risk of Bias Assessment and Critical Appraisal
2.5. Statistical Analysis and Effect Measures
3. Results
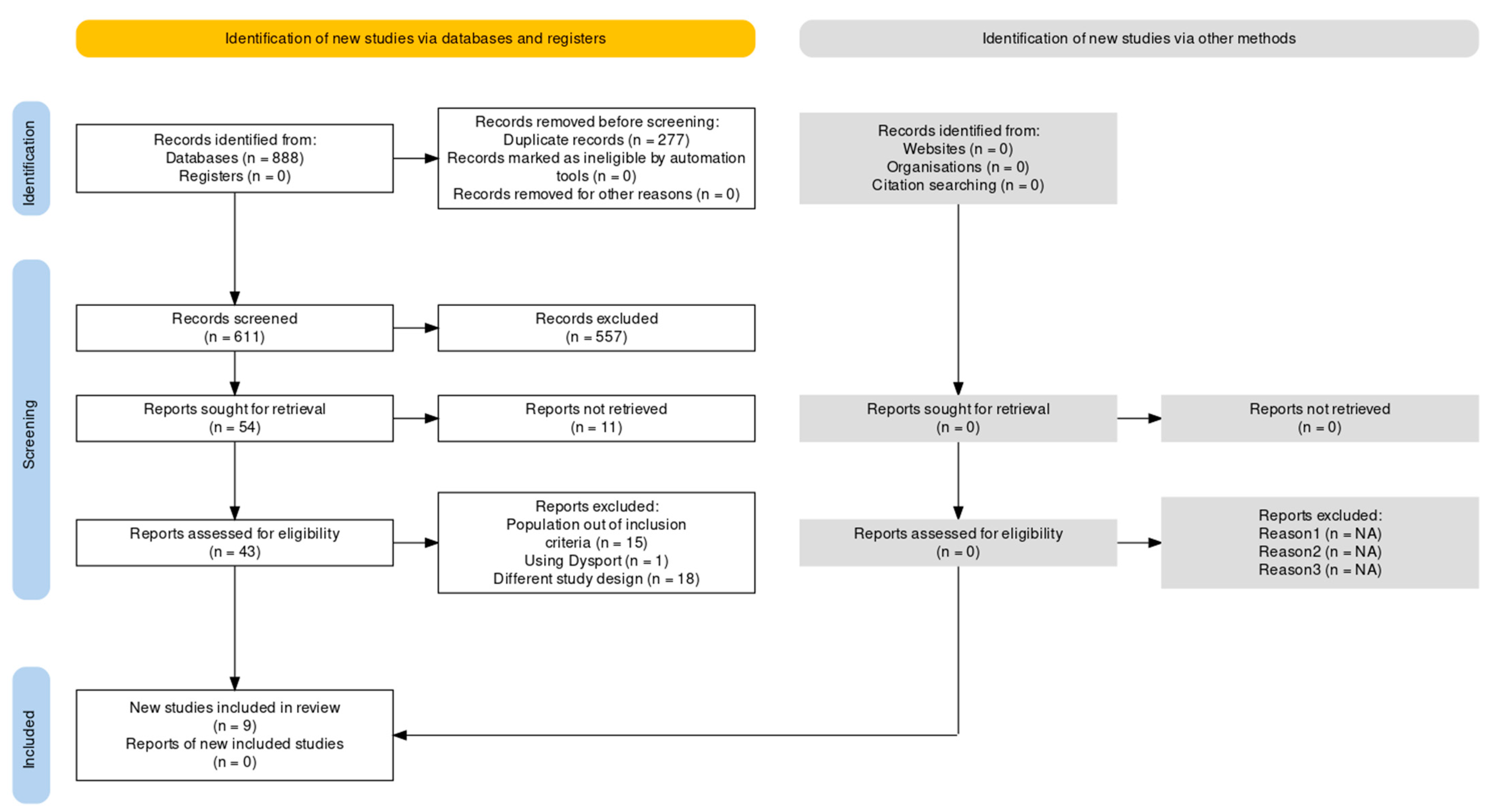
3.1. Screening and Study Selection
3.2. Qualitative Synthesis
3.3. Onabotulinumtoxin A versus Placebo
3.4. Onabotulinumtoxin A versus Topiramate
3.5. Onabotulinumtoxin A versus Non-Pharmacological Comparators
3.6. Critical Appraisal
3.7. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiner, T.J.; Stovner, L.J.; Vos, T.; Jensen, R.; Katsarava, Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J. Headache Pain 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [CrossRef]
- Schulman, E.A.; Lake, A.E.; Goadsby, P.J.; Peterlin, B.L.; Siegel, S.E.; Markley, H.G.; Lipton, R.B. Defining Refractory Migraine and Refractory Chronic Migraine: Proposed Criteria From the Refractory Headache Special Interest Section of the American Headache Society. Headache 2008, 48, 778–782. [Google Scholar] [CrossRef]
- Lambru, G.; Hill, B.; Murphy, M.; Tylova, I.; Andreou, A.P. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain 2020, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Lampl, C.; van den Brink, A.M.; Caponnetto, V.; Braschinsky, M.; Ducros, A.; Little, P.; Pozo-Rosich, P.; Reuter, U.; de la Torre, E.R.; et al. Refractory Study, Burden and attitude to resistant and refractory migraine: A survey from the European Headache Federation with the endorsement of the European Migraine & Headache Alliance. J. Headache Pain 2021, 22, 39. [Google Scholar] [PubMed]
- Merikangas, K.R. Contributions of Epidemiology to Our Understanding of Migraine. Headache 2013, 53, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; De Francesco, A.E.; Esposito, S.; Zito, M.; Morrone, L.A.; Bagetta, G.; Tonin, P.; et al. Pattern of triptans use: A retrospective prescription study in Calabria, Italy. Neural Regen Res. 2020, 15, 1340–1343. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.; Tonin, P.; Bagetta, G. Eptinezumab for the treatment of migraine. Drugs Today 2019, 55, 695–703. [Google Scholar] [CrossRef]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Nicotera, P.; Bagetta, G. Role of CGRP pathway polymorphisms in migraine: A systematic review and impact on CGRP mAbs migraine therapy. J. Headache Pain 2021, 22, 87. [Google Scholar] [CrossRef]
- Herrero, S.; Guerrero, A.L.; Ruiz, M.; Pedraza, M.I.; Mulero, P.; Barón, J.; Irene, I.; De la Cruz, C.; Peñas, M.L. Migraine in the elderly: Clinical characteristics in a series of 71 cases. J. Headache Pain 2013, 14, 152. [Google Scholar] [CrossRef]
- Bayer, A.; Tadd, W. Unjustified exclusion of elderly people from studies submitted to research ethics committee for approval: Descriptive study. BMJ 2000, 321, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Mantovani, E.; Tamburin, S.; Sandrini, G.; Corasaniti, M.T.; Bagetta, G.; Tonin, P. Opioids in Post-stroke Pain: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 587050. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Vulnera, M.; Piro, B.; Bossio, R.B.; Morrone, L.A.; Sandrini, G.; Tamburin, S.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Pattern of treatment of behavioural and psychological symptoms of dementia and pain: Evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. Eur. J. Clin. Pharmacol. 2021, 77, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Garreffa, M.R.; Esposito, S.; Bagetta, G.; Naturale, M.D.; Corasaniti, M.T. Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regen Res. 2018, 13, 1619–1621. [Google Scholar] [CrossRef]
- Scuteri, D.; Piro, B.; Morrone, L.A.; Corasaniti, M.T.; Vulnera, M.; Bagetta, G. The need for better access to pain treatment: Learning from drug consumption trends in the USA. Funct. Neurol. 2017, 22, 229–230. [Google Scholar] [CrossRef]
- Scuteri, D.; Berliocchi, L.; Rombolà, L.; Morrone, L.A.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Effects of Aging on Formalin-Induced Pain Behavior and Analgesic Activity of Gabapentin in C57BL/6 Mice. Front. Pharmacol. 2020, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Matamala-Gomez, M.; Bottiroli, S.; Corasaniti, M.T.; De Icco, R.; Bagetta, G.; Tonin, P. Pain Assessment and Treatment in Dementia at the Time of Coronavirus Disease COVID-19. Front. Neurol. 2020, 11, 890. [Google Scholar] [CrossRef]
- Scuteri, D.; Contrada, M.; Tonin, P.; Corasaniti, M.T.; Nicotera, P.; Bagetta, G. Dementia and COVID-19: A Case Report and Literature Review on Pain Management. Pharmaceuticals 2022, 15, 199. [Google Scholar] [CrossRef]
- Meng, J.; Ovsepian, S.V.; Wang, J.; Pickering, M.; Sasse, A.; Aoki, K.R.; Lawrence, G.W.; Dolly, J.O. Activation of TRPV1 Mediates Calcitonin Gene-Related Peptide Release, Which Excites Trigeminal Sensory Neurons and Is Attenuated by a Retargeted Botulinum Toxin with Anti-Nociceptive Potential. J. Neurosci. 2009, 29, 4981–4992. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Ramón, C.; Martínez-Camblor, P.; Serrano-Pertierra, E.; Larrosa, D.; Pascual, J. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain 2015, 156, 820–824. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Dodick, D.W.; Turkel, C.C.; DeGryse, M.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.-C.; Brin, M.F. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Results from the Double-Blind, Randomized, Placebo-Controlled Phases of the PREEMPT Clinical Program. Headache J. Head Face Pain 2010, 50, 921–936. [Google Scholar] [CrossRef]
- Aurora, S.; Dodick, D.; Turkel, C.; DeGryse, R.; Silberstein, S.; Lipton, R.; Diener, H.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; E DeGryse, R.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; De Icco, R.; Tassorelli, C.; Smania, N.; Tamburin, S. Botulinum neurotoxin type A for the treatment of pain: Not just in migraine and trigeminal neuralgia. J. Headache Pain 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- De Icco, R.; Perrotta, A.; Berra, E.; Allena, M.; Alfonsi, E.; Tamburin, S.; Serrao, M.; Sandrini, G.; Tassorelli, C. OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity. Toxins 2019, 11, 359. [Google Scholar] [CrossRef]
- Sandrini, G.; Perrotta, A.; Tassorelli, C.; Torelli, P.; Brighina, F.; Sances, G.; Nappi, G. Botulinum toxin type-A in the prophylactic treatment of medication-overuse headache: A multicenter, double-blind, randomized, placebo-controlled, parallel group study. J. Headache Pain 2011, 12, 427–433. [Google Scholar] [CrossRef]
- Dolly, O. Synaptic Transmission: Inhibition of Neurotransmitter Release by Botulinum Toxins. Headache 2003, 43 (Suppl. S1), 16–24. [Google Scholar] [CrossRef]
- Welch, M.J.; Purkiss, J.R.; Foster, K.A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000, 38, 245–258. [Google Scholar] [CrossRef]
- Aoki, K. Review of a Proposed Mechanism for the Antinociceptive Action of Botulinum Toxin Type A. Neurotoxicology 2005, 26, 785–793. [Google Scholar] [CrossRef]
- Burstein, R.; Zhang, X.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014, 34, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Luvisetto, S. Botulinum Neurotoxins beyond Neurons: Interplay with Glial Cells. Toxins 2022, 14, 704. [Google Scholar] [CrossRef] [PubMed]
- Herd, C.P.; Tomlinson, C.L.; Rick, C.; Scotton, W.J.; Edwards, J.; Ives, N.; Clarke, C.E.; Sinclair, A. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst. Rev. 2018, 6, CD011616. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Liberati, M.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Ryan, R.; RCCaCRG Group. Cochrane Consumers and Communication Review Group: Data Synthesis and Analysis. Available online: http://cccrg.cochrane.org (accessed on 13 March 2019).
- Hultcrantz, M.; Rind, D.; Akl, E.A.; Treweek, S.; Mustafa, R.A.; Iorio, A.; Alper, B.S.; Meerpohl, J.J.; Murad, M.H.; Ansari, M.T.; et al. The GRADE Working Group clarifies the construct of certainty of evidence. J. Clin. Epidemiol. 2017, 87, 4–13. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.; Akl, E.; E Brennan, S.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.L. Botulinum toxin A. An alternative treatment for migraine. Adv. Nurse Pract. 2009, 17, 40–41. [Google Scholar]
- Paul, A. Botulinum toxin also has an analgesic effect. Nerve poison against headache. MMW Fortschr. Med. 2001, 143, 12. [Google Scholar] [PubMed]
- Laskawi, R. Botulinum toxin in the head and neck region. HNO 2012, 60, 474. [Google Scholar] [CrossRef]
- Shah, S.; Calderon, M.-D.; Crain, N.; Pham, J.; Rinehart, J. Effectiveness of onabotulinumtoxinA (BOTOX) in pediatric patients experiencing migraines: A randomized, double-blinded, placebo-controlled crossover study in the pediatric pain population. Reg. Anesth. Pain Med. 2021, 46, 41–48. [Google Scholar] [CrossRef]
- Anand, K.; Prasad, A.; Singh, M.; Sharma, S.; Bala, K. Botulinum Toxin Type A in Prophylactic Treatment of Migraine. Am. J. Ther. 2006, 13, 183–187. [Google Scholar] [CrossRef]
- Belvis, R.; Mas, N. Treatment of chronic migraine with intramuscular pericranial injections of onabotulinumtoxin a. Recent Patents CNS Drug Discov. 2014, 9, 181–192. [Google Scholar] [CrossRef]
- Elkind, A.H.; O’carroll, P.; Blumenfeld, A.; DeGryse, R.; Dimitrova, R. A Series of Three Sequential, Randomized, Controlled Studies of Repeated Treatments With Botulinum Toxin Type A for Migraine Prophylaxis. J. Pain 2006, 7, 688–696. [Google Scholar] [CrossRef]
- Naprienko, M.V.; Smekalkina, L.V.; Safonov, M.I.; Filatova, E.G.; Latysheva, N.V.; Ekusheva, E.V.; Artemenko, A.R.; Osipova, V.V.; Baiushkina, L.I. Real-world migraine burden: Clinical and economic aspects. Zh. Nevrol. Psikhiatr. Im. S.S. Korsakova 2019, 119, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Varon, S.F.; Grosberg, B.; McAllister, P.J.; Freitag, F.; Aurora, S.K.; Dodick, D.W.; Silberstein, S.D.; Diener, H.C.; Degryse, R.E.; et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology 2011, 77, 1465–1472. [Google Scholar] [CrossRef]
- Vo, A.H.; Satori, R.; Jabbari, B.; Green, J.; Killgore, W.D.S.; Labutta, R.; Campbell, W.W. Botulinum toxin type-a in the prevention of migraine: A double-blind controlled trial. Aviat. Space Environ. Med. 2007, 78 (Suppl. S5), B113–B118. [Google Scholar]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Diener, H.-C.; Lipton, R.B.; Aurora, S.K.; Nolan, M.E.; Silberstein, S.D. Assessing Clinically Meaningful Treatment Effects in Controlled Trials: Chronic Migraine as an Example. J. Pain 2015, 16, 164–175. [Google Scholar] [CrossRef]
- Hou, M.; Xie, J.-F.; Kong, X.-P.; Zhang, Y.; Shao, Y.-F.; Wang, C.; Ren, W.-T.; Cui, G.-F.; Xin, L.; Hou, Y.-P. Acupoint Injection of Onabotulinumtoxin A for Migraines. Toxins 2015, 7, 4442–4454. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, J.A.; Kies, D.A.; Louter, M.A.; van Zwet, E.W.; Ferrari, M.D.; Terwindt, G.M. Acute withdrawal and botulinum toxin A in chronic migraine with medication overuse: A double-blind randomized controlled trial. Brain 2019, 142, 1203–1214. [Google Scholar] [CrossRef]
- Ondo, W.G.; Vuong, K.D.; Derman, H.S. Botulinum toxin A for chronic daily headache: A randomized, placebo-controlled, parallel design study. Cephalalgia Int. J. Headache 2004, 24, 60–65. [Google Scholar] [CrossRef]
- Evers, S.; Vollmer-Haase, J.; Schwaag, S.; Rahmann, A.; Husstedt, I.W.; Frese, A. Botulinum toxin A in the prophylactic treatment of migraine--a randomized, double-blind, placebo-controlled study. Cephalalgia Int. J. Headache 2004, 24, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Petri, S.; Tölle, T.; Straube, A.; Pfaffenrath, V.; Stefenelli, U.; Ceballos-Baumann, A. Botulinum Toxin as Preventive Treatment for Migraine: A Randomized Double-Blind Study. Eur. Neurol. 2009, 62, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.M.; Schim, J.D.; Chippendale, T.J. Botulinum Toxin Type A and Divalproex Sodium for Prophylactic Treatment of Episodic or Chronic Migraine. Headache 2008, 48, 210–220. [Google Scholar] [CrossRef]
- Silberstein, S.; Mathew, N.; Saper, J.; Jenkins, S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache 2000, 40, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.; Schreiber, C. Botulinum Toxin Type A as Migraine Preventive Treatment in Patients Previously Failing Oral Prophylactic Treatment Due To Compliance Issues. Headache 2008, 48, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Hollanda, L.; Monteiro, L.; Melo, A. Botulinum Toxin Type A for Cephalic Cutaneous Allodynia in Chronic Migraine: A Randomized, Double-Blinded, Placebo-Controlled Trial. Neurol. Int. 2014, 6, 70–73. [Google Scholar] [CrossRef]
- Dodick, D.W.; Mauskop, A.; Elkind, A.H.; DeGryse, R.; Brin, M.F.; Silberstein, S.D. Botulinum toxin type A for the prophylaxis of chronic daily headache: Subgroup analysis of patients not receiving other prophylactic medications: A randomized double-blind, placebo-controlled study. Headache 2005, 45, 315–324. [Google Scholar] [CrossRef]
- Grazzi, L.; Usai, S. Onabotulinum toxin A (Botox) for chronic migraine treatment: An Italian experience. Neurol. Sci. 2015, 36 (Suppl. S1), 33–35. [Google Scholar] [CrossRef]
- Grazzi, L. Onabotulinum toxin A for treatment of chronic migraine with medication overuse. Neurol. Sci. 2013, 34 (Suppl. S1), S27–S28. [Google Scholar] [CrossRef]
- Millán-Guerrero, R.O.; Isais-Millán, S.; Barreto-Vizcaíno, S.; Rivera-Castaño, L.; Rios-Madariaga, C. Subcutaneous histamine versus botulinum toxin type A in migraine prophylaxis: A randomized, double-blind study. Eur. J. Neurol. 2009, 16, 88–94. [Google Scholar] [CrossRef]
- Chankrachang, S.; Arayawichanont, A.; Poungvarin, N.; Nidhinandana, S.; Boonkongchuen, P.; Towanabut, S.; Sithinamsuwan, P.; Kongsaengdao, S. Prophylactic Botulinum Type A Toxin Complex (Dysport®) for Migraine Without Aura. Headache 2011, 51, 52–63. [Google Scholar] [CrossRef]
- Loeb, L.M.; Amorim, R.P.; Mazzacoratti, M.D.G.N.; Scorza, F.A.; Peres, M.F.P. Botulinum toxin A (BT-A) versus low-level laser therapy (LLLT) in chronic migraine treatment: A comparison. Arq. Neuro-Psiquiatr. 2018, 76, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Grazzi, L.; Usai, S. Botulinum toxin A: A new option for treatment of chronic migraine with medication overuse. Neurol. Sci. 2014, 35 (Suppl. S1), 37–39. [Google Scholar] [CrossRef] [PubMed]
- Freitag, F.G.; Diamond, S.; Diamond, M.; Urban, G. Botulinum Toxin Type A in the Treatment of Chronic Migraine Without Medication Overuse. Headache 2008, 48, 201–209. [Google Scholar] [CrossRef]
- Magalhães, E.; Menezes, C.; Cardeal, M.; Melo, A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin. Neurol. Neurosurg. 2010, 112, 463–466. [Google Scholar] [CrossRef]
- Pak, A.T.; Üstün, I.; Sengul, Y. Botulinum toxin type A wear-off phenomenon in chronic migraine patients: How long does the maximum efficiency last? Arq. Neuro-Psiquiatr. 2021, 79, 886–890. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Martínez-Camblor, P.; Ramón, C.; Larrosa, D.; Serrano-Pertierra, E.; Pascual, J. CGRP and VIP levels as predictors of efficacy of Onabotulinumtoxin type A in chronic migraine. Headache 2014, 54, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; E DeGryse, R.; Adams, A.M.; Diener, H.-C. Early onset of effect of onabotulinumtoxinA for chronic migraine treatment: Analysis of PREEMPT data. Cephalalgia 2019, 39, 945–956. [Google Scholar] [CrossRef]
- Demiryurek, B.E.; Ertem, D.H.; Tekin, A.; Ceylan, M.; Aras, Y.G.; Gungen, B.D. Effects of onabotulinumtoxinA treatment on efficacy, depression, anxiety, and disability in Turkish patients with chronic migraine. Neurol. Sci. 2016, 37, 1779–1784. [Google Scholar] [CrossRef]
- Zidan, A.; Hussaini, S.; Gibson, S.; Brooks, G.; Mejico, L. Onabotulinumtoxin Type A reconstitution with preserved versus preservative-free saline in chronic migraine (B-RECON). A randomised, double-blind trial. Int. J. Clin. Pract. 2020, 74, e13522. [Google Scholar] [CrossRef]
- Lipton, R.B.; Rosen, N.L.; Ailani, J.; E DeGryse, R.; Gillard, P.J.; Varon, S.F. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: Pooled results from the PREEMPT randomized clinical trial program. Cephalalgia 2016, 36, 899–908. [Google Scholar] [CrossRef]
- Blumenfeld, A.M.; Patel, A.T.; Turner, I.M.; Mullin, K.B.; Adams, A.M.; Rothrock, J.F. Patient-Reported Outcomes from a 1-Year, Real-World, Head-to-Head Comparison of OnabotulinumtoxinA and Topiramate for Headache Prevention in Adults With Chronic Migraine. J. Prim. Care Community Health 2020, 11, 2150132720959936. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D.; Dodick, D.W.; Aurora, S.K.; Diener, H.-C.; E DeGryse, R.; Lipton, R.B.; Turkel, C.C. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J. Neurol. Neurosurg. Psychiatry 2015, 86, 996–1001. [Google Scholar] [CrossRef]
- Davies, B.; Gaul, C.; Martelletti, P.; García-Moncó, J.C.; Brown, S. Real-life use of onabotulinumtoxinA for symptom relief in patients with chronic migraine: REPOSE study methodology and baseline data. J. Headache Pain 2017, 18, 93. [Google Scholar] [CrossRef]
- Kollewe, K.; Gaul, C.; Gendolla, A.; Sommer, K. Real-life use of onabotulinumtoxinA reduces healthcare resource utilization in individuals with chronic migraine: The REPOSE study. J. Headache Pain 2021, 22, 50. [Google Scholar] [CrossRef]
- García-Azorín, D.; Martínez, B.; Gutiérrez, M.; Ruiz-Piñero, M.; Echavarría, A.; Sierra, Á.; Guerrero, Á.L. Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study. Toxins 2022, 14, 850. [Google Scholar] [CrossRef] [PubMed]
- Butera, C.; Colombo, B.; Bianchi, F.; Cursi, M.; Messina, R.; Amadio, S.; Guerriero, R.; Comi, G.; Del Carro, U. Refractory chronic migraine: Is drug withdrawal necessary before starting a therapy with onabotulinum toxin type A? Neurol. Sci. 2016, 37, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- di Cola, F.S.; Caratozzolo, S.; Liberini, P.; Rao, R.; Padovani, A. Response Predictors in Chronic Migraine: Medication Overuse and Depressive Symptoms Negatively Impact Onabotulinumtoxin-A Treatment. Front. Neurol. 2019, 10, 678. [Google Scholar] [CrossRef]
- Wenzel, R.; Jones, D.; Borrego, J.A. Comparing two botulinum toxin type A formulations using manufacturers? product summaries. J. Clin. Pharm. Ther. 2007, 32, 387–402. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; Degryse, R.E.; VanDenburgh, A.M.; Nolan, M.E.; Turkel, C.C. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Analyses of the 56-Week PREEMPT Clinical Program. Headache 2011, 51, 1358–1373. [Google Scholar] [CrossRef]
- Aurora, S.K.; Dodick, D.W.; Diener, H.; DeGryse, R.E.; Turkel, C.C.; Lipton, R.B.; Silberstein, S.D. OnabotulinumtoxinA for chronic migraine: Efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol. Scand. 2014, 129, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; Schreiber, C.P.; Porter, J.A.; Blumenfeld, A.M.; Farmer, K.U. A Multi-Center Double-Blind Pilot Comparison of OnabotulinumtoxinA and Topiramate for the Prophylactic Treatment of Chronic Migraine. Headache 2011, 51, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Mathew, N.T.; Jaffri, S.F.A. A Double-Blind Comparison of OnabotulinumtoxinA (BOTOX (R)) and Topiramate (TOPAMAX (R)) for the Prophylactic Treatment of Chronic Migraine: A Pilot Study. Headache 2009, 49, 1466–1478. [Google Scholar] [CrossRef]
- Naderinabi, B.; Saberi, A.; Hashemi, M.; Haghighi, M.; Biazar, G.; Sedighinejad, A.; Chavoshi, T. Acupuncture and botulinum toxin A injection in the treatment of chronic migraine: A randomized controlled study. Casp. J. Intern. Med. 2017, 8, 196–204. [Google Scholar] [CrossRef]
- Rothrock, J.F.; Adams, A.M.; Lipton, R.B.; Silberstein, S.D.; Jo, E.; Zhao, X.; Blumenfeld, A.M.; on behalf of the FORWARD Study investigative group. FORWARD Study: Evaluating the Comparative Effectiveness of OnabotulinumtoxinA and Topiramate for Headache Prevention in Adults With Chronic Migraine. Headache 2019, 59, 1700–1713. [Google Scholar] [CrossRef]
- Shehata, H.S.; Esmail, E.H.; Abdelalim, A.; El-Jaafary, S.; Elmazny, A.; Sabbah, A.; Shalaby, N.M. Repetitive transcranial magnetic stimulation versus botulinum toxin injection in chronic migraine prophylaxis: A pilot randomized trial. J. Pain Res. 2016, 9, 771–777. [Google Scholar] [CrossRef]
- Winner, P.K.; Kabbouche, M.; Yonker, M.; Wangsadipura, V.; Lum, A.; Brin, M.F. A Randomized Trial to Evaluate OnabotulinumtoxinA for Prevention of Headaches in Adolescents With Chronic Migraine. Headache 2020, 60, 564–575. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Harbord, R. Funnel plots in meta-analysis. Stata J. 2004, 4, 127–141. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Fischer, F.; et al. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Jones, J.; Hunter, D. Qualitative Research: Consensus methods for medical and health services research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef]
- Scuteri, D.; Tonin, P.; Nicotera, P.; Vulnera, M.; Altieri, G.C.; Tarsitano, A.; Bagetta, G.; Corasaniti, M.T. Pooled Analysis of Real-World Evidence Supports Anti-CGRP mAbs and OnabotulinumtoxinA Combined Trial in Chronic Migraine. Toxins 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; Blumenfeld, A.M. Combination CGRP monoclonal antibody and onabotulinumtoxinA treatment for preventive treatment in chronic migraine. Headache 2022, 62, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Adornetto, A.; Rombolà, L.; Naturale, M.D.; Morrone, L.A.; Bagetta, G.; Tonin, P.; Corasaniti, M.T. New Trends in Migraine Pharmacology: Targeting Calcitonin Gene–Related Peptide (CGRP) With Monoclonal Antibodies. Front. Pharmacol. 2019, 10, 363. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Hegron, A.; Landini, L.; Jensen, D.D.; Latorre, R.; Ding, J.; Marini, M.; de Araujo, D.S.M.; Ramírez-Garcia, P.; et al. Schwann cell endosome CGRP signals elicit periorbital mechanical allodynia in mice. Nat. Commun. 2022, 13, 646. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Do, T.P.; Ashina, H.; Ashina, M.; Burstein, R. Dual Therapy With Anti-CGRP Monoclonal Antibodies and Botulinum Toxin for Migraine Prevention: Is There a Rationale? Headache 2020, 60, 1056–1065. [Google Scholar] [CrossRef]
- Rojewska, E.; Piotrowska, A.; Popiolek-Barczyk, K.; Mika, J. Botulinum Toxin Type A—A Modulator of Spinal Neuron–Glia Interactions under Neuropathic Pain Conditions. Toxins 2018, 10, 145. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Makuch, W.; Luvisetto, S.; Pavone, F.; Marinelli, S.; Przewlocka, B.; Mika, J. Participation of pro- and anti-nociceptive interleukins in botulinum toxin A-induced analgesia in a rat model of neuropathic pain. Eur. J. Pharmacol. 2016, 791, 377–388. [Google Scholar] [CrossRef]
- Piotrowska, A.; Popiolek-Barczyk, K.; Pavone, F.; Mika, J. Comparison of the Expression Changes after Botulinum Toxin Type A and Minocycline Administration in Lipopolysaccharide-Stimulated Rat Microglial and Astroglial Cultures. Front. Cell. Infect. Microbiol. 2017, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, J.; Sun, L.; Wang, Y.; Meng, H. Combination of Botulinum Toxin and minocycline Ameliorates Neuropathic Pain Through Antioxidant Stress and Anti-Inflammation via Promoting SIRT1 Pathway. Front. Pharmacol. 2021, 11, 602417. [Google Scholar] [CrossRef]
- Nørgaard, A.; Jensen-Dahm, C.; Gasse, C.; Hansen, E.S.; Waldemar, G. Psychotropic Polypharmacy in Patients with Dementia: Prevalence and Predictors. J. Alzheimer’s Dis. 2017, 56, 707–716. [Google Scholar] [CrossRef]
- Riedl, L.; Kiesel, E.; Hartmann, J.; Fischer, J.; Roßmeier, C.; Haller, B.; Kehl, V.; Priller, J.; Trojan, M.; Diehl-Schmid, J. A bitter pill to swallow—Polypharmacy and psychotropic treatment in people with advanced dementia. BMC Geriatr. 2022, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Letinier, L.; Pujade, I.; Duthoit, P.; Evrard, G.; Salvo, F.; Gil-Jardine, C.; Pariente, A. Emergency department admissions induced by drug–drug interactions in the elderly: A cross-sectional study. Clin. Transl. Sci. 2022, 15, 1472–1481. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Rombolà, L.; Watanabe, C.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Tonin, P.; Russo, R.; Nucci, C.; Morrone, L.A. Impact of nutraceuticals on glaucoma: A systematic review. Prog. Brain Res. 2020, 257, 141–154. [Google Scholar] [CrossRef]
- Scuteri, D.; Crudo, M.; Rombolà, L.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Sakurada, T.; Greco, R.; Corasaniti, M.T.; Morrone, L.A.; et al. Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 2018, 129, 20–24. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolá, L.; Tridico, L.; Mizoguchi, H.; Watanabe, C.; Sakurada, T.; Sakurada, S.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Neuropharmacological Properties of the Essential Oil of Bergamot for the Clinical Management of Pain-Related BPSDs. Curr. Med. Chem. 2019, 26, 3764–3774. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Watanabe, C.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Role of 5-HT1A Receptor in the Anxiolytic-Relaxant Effects of Bergamot Essential Oil in Rodent. Int. J. Mol. Sci. 2020, 21, 2597. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Sandrini, G.; Tamburin, S.; Corasaniti, M.T.; Nicotera, P.; Tonin, P.; Bagetta, G. Bergamot rehabilitation AgaINst agitation in dementia (BRAINAID): Study protocol for a randomized, double-blind, placebo-controlled trial to assess the efficacy of furocoumarin-free bergamot loaded in a nanotechnology-based delivery system of the essential oil in the treatment of agitation in elderly affected by severe dementia. Phytother. Res. PTR 2021, 35, 5333–5338. [Google Scholar]
- Scuteri, D.; Cassano, R.; Trombino, S.; Russo, R.; Mizoguchi, H.; Watanabe, C.; Hamamura, K.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; et al. Development and Translation of NanoBEO, a Nanotechnology-Based Delivery System of Bergamot Essential Oil Deprived of Furocumarins, in the Control of Agitation in Severe Dementia. Pharmaceutics 2021, 13, 379. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolà, L.; Crudo, M.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Tonin, P.; Corasaniti, M.T.; et al. Preclinical Characterization of Antinociceptive Effect of Bergamot Essential Oil and of Its Fractions for Rational Translation in Complementary Therapy. Pharmaceutics 2022, 14, 312. [Google Scholar] [CrossRef]
- Scuteri, D.; Rombolà, L.; Crudo, M.; Watanabe, C.; Mizoguchi, H.; Sakurada, S.; Hamamura, K.; Sakurada, T.; Morrone, L.A.; Tonin, P.; et al. Translational Value of the Transdermal Administration of Bergamot Essential Oil and of Its Fractions. Pharmaceutics 2022, 14, 1006. [Google Scholar] [CrossRef]
- Hamamura, K.; Katsuyama, S.; Komatsu, T.; Scuteri, D.; Bagetta, G.; Aritake, K.; Sakurada, T. Behavioral Effects of Continuously Administered Bergamot Essential Oil on Mice With Partial Sciatic Nerve Ligation. Front. Pharmacol. 2020, 11, 1310. [Google Scholar] [CrossRef]
- Sakurada, T.; Kuwahata, H.; Katsuyama, S.; Komatsu, T.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Sakurada, S. Intraplantar injection of bergamot essential oil into the mouse hindpaw: Effects on capsaicin-induced nociceptive behaviors. Int. Rev. Neurobiol. 2009, 85, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Ernst, E. Complementary and Alternative Approaches to the Treatment of Persistent Musculoskeletal Pain. Clin. J. Pain 2004, 20, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; Gauthier, S.; Cummings, J.L.; Brodaty, H.; Grossberg, G.T.; Robert, P.; Lyketsos, C.G. Management of agitation and aggression associated with Alzheimer disease. Nat. Rev. Neurol. 2009, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Contrada, M.; Loria, T.; Sturino, D.; Cerasa, A.; Tonin, P.; Sandrini, G.; Tamburin, S.; Bruni, A.C.; Nicotera, P.; et al. Pain and agitation treatment in severe dementia patients: The need for Italian Mobilization-Observation-Behavior-Intensity-Dementia (I-MOBID2) pain scale translation, adaptation and validation with psychometric testing. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 113013. [Google Scholar] [CrossRef]
- Scuteri, D.; Contrada, M.; Loria, T.; Tonin, P.; Sandrini, G.; Tamburin, S.; Nicotera, P.; Bagetta, G.; Corasaniti, M.T. Pharmacological Treatment of Pain and Agitation in Severe Dementia and Responsiveness to Change of the Italian Mobilization–Observation–Behavior–Intensity–Dementia (I-MOBID2) Pain Scale: Study Protocol. Brain Sci. 2022, 12, 573. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, R.; James, L.; Liu, C.; Orejudos, A.; Yushmanova, I.; Brin, M.F. Safety of OnabotulinumtoxinA with Concomitant Antithrombotic Therapy in Patients with Muscle Spasticity: A Retrospective Pooled Analysis of Randomized Double-Blind Studies. CNS Drugs 2020, 34, 433–445. [Google Scholar] [CrossRef]
- Scuteri, D.; Tarsitano, A.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Focus on zavegepant: The first intranasal third-generation gepant. Pain Manag. 2022, 12, 879–885. [Google Scholar] [CrossRef]
- Scuteri, D.; Tonin, P.; Nicotera, P.; Bagetta, G.; Corasaniti, M.T. Real world considerations for newly approved CGRP receptor antagonists in migraine care. Expert Rev. Neurother. 2022, 22, 221–230. [Google Scholar] [CrossRef]
- Guerzoni, S.; Baraldi, C.; Pani, L. The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: A reliable option for the optimal treatment of chronic migraine. Neurol. Sci. 2022, 43, 5687–5695. [Google Scholar] [CrossRef] [PubMed]


| Study | Aurora et al., 2011 [91] | Aurora et al., 2014 [92] | Cady et al., 2011 [93] | Diener et al., 2010 [25] | Mathew et al., 2009 [94] | Naderinabi et al., 2017 [95] | Rothrock et al., 2019 [96] | Shehata et al., 2016 [97] | Winner et al., 2020 [98] |
|---|---|---|---|---|---|---|---|---|---|
| Study design | Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program—Pooled analysis | Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) clinical program—Subgroup analysis | Multi-center double-blind pilot trial | Phase III REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT) 2 clinical trial | Single-center, double-blind trial | Iranian Registry of Clinical Trial IRCT 201404146186N3 randomized clinical trial | Multicenter, randomized, parallel-group, post-authorization, open-label prospective study (FORWARD; Clinicaltrials.gov NCT02191579) | Pilot, randomized trial on a small-scale | Multicenter, double-blind, parallel-group, randomized trial |
| Intervention | Onabotulinumtoxin A (155–195 U)-pooled analysis of two phase III, 24-week, double-blind, parallel-group, placebo-controlled studies, followed by a 32-week, open-label, single-treatment, onabotulinumtoxin A phase. n = 687 | Onabotulinumtoxin A (155–195 U)-secondary analysis assessed patients who received all five treatment cycles. n = 515 | Onabotulinumtoxin A injections plus placebo tablets. Up to 200 units of onabotulinumtoxin A or placebo injected with 100 units into fixed locations and up to additional 100 units in a follow-the-pain scheme determined by the investigator. Average dosage of 109 units for the first injection cycle. n = 29 | Onabotulinumtoxin A (155–195 U)-Phase 3 study, with a 24-week, double-blind, placebo-controlled phase, followed by a 32-week, open-label phase. n = 347 | Onabotulinumtoxin A, maximum 200 units (U) and 100 U fixed-site and 100 U follow-the-pain at month 3. n = 26 | Onabotulinumtoxin A 155U following the protocol of the Phase ΙΙΙ Research Evaluating Migraine Prophylaxis Therapy I (PREEMPT1). n = 50 | Onabotulinumtoxin A 155 U every 12 weeks for 3 cycles. n = 140 | Onabotulinumtoxin A following the Phase III Research Evaluating Migraine Prophylaxis Therapy injection paradigm. n = 15 | Single treatment of onabotulinumtoxin A (155 U or 74 U) fixed-dose and fixed-site paradigm. n = 43 |
| Comparator | Placebo n = 692 | Placebo n = 490 | Topiramate plus placebo injections. Initiated at 25 mg daily and escalated to 100 mg in weekly incremental changes of 25 mg. The dosage can be further escalated after one month to a maximum dosage of 200 mg per day. Average daily dosage of 136 mg by week 12. n = 30 | Placebo n = 358 | Topiramate (4-week titration to 100 mg/day with option for additional 4-week titration to 200 mg/day, plus placebo injections. n = 29 | Acupuncture group (n = 50) and control group (sodium valproate 500 mg/day; n = 50) | Topiramate “immediate release” 50–100 mg/day to week 36 (n = 142). After 12 weeks, patients initially randomized to topiramate can cross over to onabotulinumtoxin A treatment (n = 80) | Repetitive transcranial magnetic stimulation (rTMS). n = 14 | Placebo. n = 37 |
| Summary of safety findings | The proportion of patients experiencing a serious adverse event during the double-blind phase was 4.8% for onabotuli-numtoxin A group and 2.3% for the placebo group. The incidence of individual treatment-related adverse events (TRAEs) was 6.7% in the onabotulinumtoxin A group. Total TRAEs occur in n = 202 (29.4%) patients in the onabotulinumtoxin A group and in n = 88 (12.7%) patients in the placebo group | TRAEs were experienced by n = 147 (28.5%) of onabotulinumtoxin A group and by n = 61 (12.4%) of placebo group in the double-blind phase | Nausea occurred in 59.1% patients treated with onabotulinumtoxin A in comparison with 27.3% patients receiving topiramate | TRAEs in n = 116 (33.4%) of onabotulinumtoxin A-receiving patients and in n = 49 (13.7%) from the placebo-receiving patients | TRAEs occurred in 18 (69.2%) patients from the onabotulinumtoxin A group and in 25 (86.2%) patients from the topiramate group | Significant lower rate of AEs was recorded for the acupuncture group rather than the group receiving botulinum toxin (6% vs. 22%; p = 0.021) | TRAEs occurred in 17% of patients treated with onabotulinumtoxin A and in 70% of patients treated with topiramate | No systemic reactions or serious AEs were recorded | TRAEs occurred in 10 (23%) patients in the onabotulinumtoxin A 155 U group, in 7 (16%) patients in the onabotulinumtoxin A 74 U group and in 4 (11%) patients in the placebo group |
| Onabotulinumtoxin A vs. | Placebo | Topiramate | Other Comparators |
|---|---|---|---|
| Frequency of adverse events (% of patients experiencing the adverse event in the onabotulinumtoxin A group) | Neck pain (4.3–7.5%), muscular weakness (1.6–5.2%), musculoskeletal pain (5%), injection site pain (2.1%) and eyelid ptosis (1.9%). | Nausea (59.1%), neck pain (4%), musculoskeletal pain (2%), migraine (1%) and blurred vision (1%). | Ptosis, facial masking or asymmetry (22%; respect to acupuncture and sodium valproate); No serious adverse event with respect to repetitive transcranial magnetic stimulation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corasaniti, M.T.; Bagetta, G.; Nicotera, P.; Tarsitano, A.; Tonin, P.; Sandrini, G.; Lawrence, G.W.; Scuteri, D. Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins 2023, 15, 332. https://doi.org/10.3390/toxins15050332
Corasaniti MT, Bagetta G, Nicotera P, Tarsitano A, Tonin P, Sandrini G, Lawrence GW, Scuteri D. Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins. 2023; 15(5):332. https://doi.org/10.3390/toxins15050332
Chicago/Turabian StyleCorasaniti, Maria Tiziana, Giacinto Bagetta, Pierluigi Nicotera, Assunta Tarsitano, Paolo Tonin, Giorgio Sandrini, Gary W. Lawrence, and Damiana Scuteri. 2023. "Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials" Toxins 15, no. 5: 332. https://doi.org/10.3390/toxins15050332
APA StyleCorasaniti, M. T., Bagetta, G., Nicotera, P., Tarsitano, A., Tonin, P., Sandrini, G., Lawrence, G. W., & Scuteri, D. (2023). Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins, 15(5), 332. https://doi.org/10.3390/toxins15050332







