Behavioral and Physiological Alterations in Angus Steers Grazing Endophyte-Infected Toxic Fescue during Late Fall
Abstract
1. Introduction
2. Results
2.1. Environmental Temperature
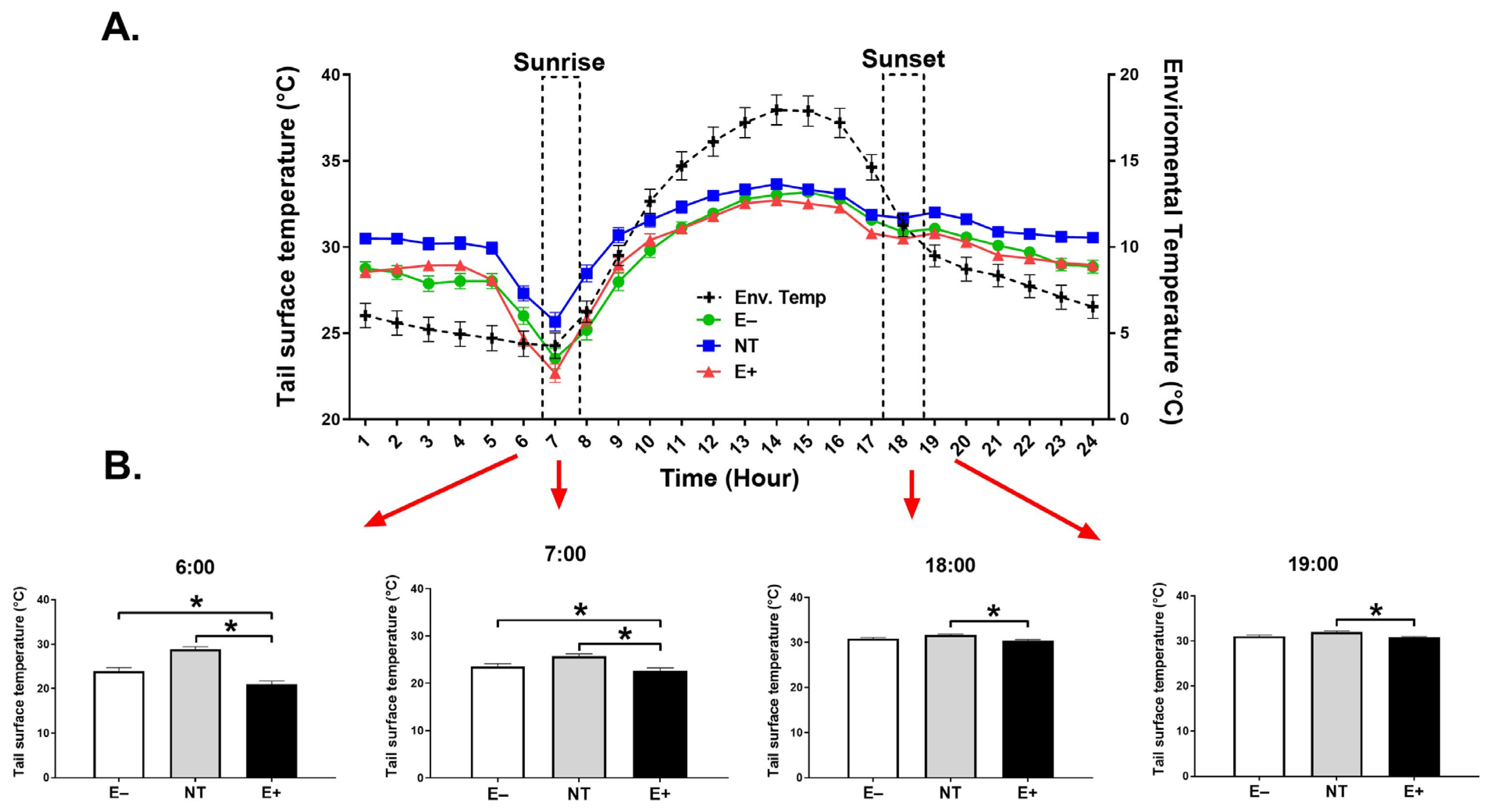
2.2. Skin Surface Temperature (SST)
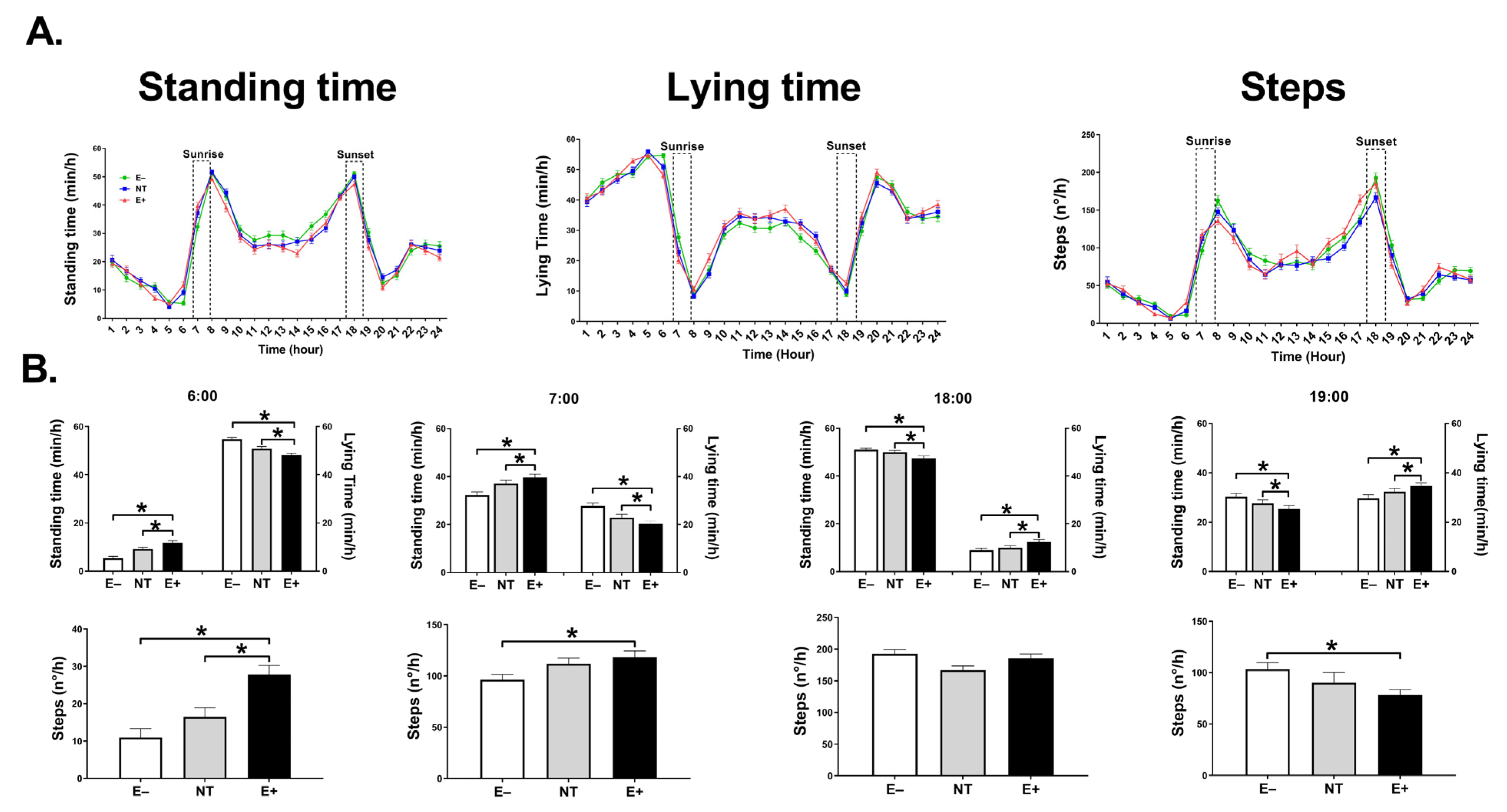
2.3. Behavioral Activity
2.4. Endophyte and Total Plant Ergot Alkaloids
2.5. Nutritional Analyses and Forage Availability of Fescue Pastures
2.6. Animal Weight Gains and Physiological Parameters
2.7. Ruminal pH, and Rumen and Fecal Volatile Fatty Acid (VFA) Concentrations
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals, Treatments, and Experimental Design
5.2. Sample Collection and Processing
5.2.1. Detection of Endophyte and Total Plant Ergot Alkaloids Analysis
5.2.2. Nutritional Analyses and Forage Availability of Fescue Pastures
5.3. Data Loggers
5.3.1. IceTag® Leg Sensor
5.3.2. iButton Temperature Logger
5.3.3. Environmental Temperature Data Loggers
5.4. Volatile Fatty Acid Analysis
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hannaway, D.B.; Daly, C.; Halbleib, M.D.; James, D.; West, C.P.; Volenec, J.J.; Chapman, D.; Li, X.; Cao, W.; Shen, J.; et al. Development of Suitability Maps with Examples for the United States and China. In Tall Fescue for the Twenty-First Century; Fribourg., H.A., Hannaway, D.B., West, C.P., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2009; Volume 53, pp. 31–47. [Google Scholar]
- Ball, D.; Pederson, J.; Lacefield, G. The tall-fescue endophyte. Am. Sci. 1993, 81, 370–379. [Google Scholar]
- Thompson, F.; Stuedemann, J.; Hill, N. Anti-quality factors associated with alkaloids in eastern temperate pasture. Rangel. Ecol. Manag. /J. Range Manag. Arch. 2001, 54, 474–489. [Google Scholar] [CrossRef]
- Hoveland, C.S. Importance and economic significance of the Acremonium endophytes to performance of animals and grass plant. Agric. Ecosyst. Environ. 1993, 44, 3–12. [Google Scholar] [CrossRef]
- Hoveland, C.S. Origin and history. In Tall Fescue for the Twenty-First Century; Fribourg., H.A., Hannaway, D.B., West, C.P., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2009; Volume 53, pp. 1–10. [Google Scholar]
- Bacon, C.; Porter, J.; Robbins, J.; Luttrell, E. Epichloe typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 1977, 34, 576–581. [Google Scholar] [CrossRef]
- Kallenbach, R.L. BILL E. KUNKLE INTERDISCIPLINARY BEEF SYMPOSIUM: Coping with tall fescue toxicosis: Solutions and realities. J. Anim. Sci. 2015, 93, 5487–5495. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.R.; Aiken, G.E.; Spiers, D.E.; Fletcher, L.R.; Oliver, J.W. Physiological basis of fescue toxicosis. In Tall Fescue for the Twenty-First Century; Fribourg., H.A., Hannaway, D.B., West, C.P., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2009; Volume 53, pp. 203–227. [Google Scholar]
- Roberts, C.; Andrae, J. Tall fescue toxicosis and management. Crop Manag. 2004, 3, 1–18. [Google Scholar] [CrossRef]
- Johnson, J.; Aiken, G.; Phillips, T.; Barrett, M.; Klotz, J.; Schrick, F. Steer and pasture responses for a novel endophyte tall fescue developed for the upper transition zone. J. Anim. Sci. 2012, 90, 2402–2409. [Google Scholar] [CrossRef]
- Bouton, J.H.; Latch, G.C.; Hill, N.S.; Hoveland, C.S.; McCann, M.A.; Watson, R.H.; Parish, J.A.; Hawkins, L.L.; Thompson, F.N. Reinfection of tall fescue cultivars with non-ergot alkaloid–producing endophytes. Agron. J. 2002, 94, 567–574. [Google Scholar] [CrossRef]
- Oliver, J.W. Pathophysiologic response to endophyte toxins. In Neotyphodium in Cool-Season Grasses; Craig, A.R., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; Volume 1, pp. 291–304. [Google Scholar]
- Berde, B. Ergot compounds: A synopsis. Adv. Biochem. Psychopharmacol. 1980, 23, 3–23. [Google Scholar]
- Strickland, J.R.; Looper, M.L.; Matthews, J.; Rosenkrans, C.F., Jr.; Flythe, M.; Brown, K. Board-invited review: St. Anthony’s Fire in livestock: Causes, mechanisms, and potential solutions. J. Anim. Sci. 2011, 89, 1603–1626. [Google Scholar] [CrossRef]
- Nicol, A.M.; Klotz, J.L. Ergovaline, an endophytic alkaloid. 2. Intake and impact on animal production, with reference to New Zealand. Anim. Prod. Sci. 2016, 56, 1775–1786. [Google Scholar] [CrossRef]
- Rhodes, M.; Paterson, J.; Kerley, M.; Garner, H.; Laughlin, M. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 1991, 69, 2033–2043. [Google Scholar] [CrossRef]
- Aiken, G.; Kirch, B.; Strickland, J.; Bush, L.; Looper, M.; Schrick, F. Hemodynamic responses of the caudal artery to toxic tall fescue in beef heifers. J. Anim. Sci. 2007, 85, 2337–2345. [Google Scholar] [CrossRef]
- Hoveland, C.S. The fescue toxicosis story: An update. In Proceedings of the Beef Improvement Federation 35th An Rnueasel arch Symposium Annual Meeting, Lexington, KY, USA, 28–31 May 2003; pp. 20–24. [Google Scholar]
- Stuedemann, J.; Seman, D.H. Integrating genetics, environment and management to minimize animal toxicosis. In Neotyphodium in Cool-Season Grasses; Craig, A.R., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; Volume 1, pp. 305–317. [Google Scholar]
- Thompson, F.; Stuedemann, J. Pathophysiology of fescue toxicosis. Agric. Ecosyst. Environ. 1993, 44, 263–281. [Google Scholar] [CrossRef]
- Terrien, J.; Perret, M.; Aujard, F. Behavioral thermoregulation in mammals: A review. Front. Biosci.-Landmark 2011, 16, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martinez-Burnes, J.; Gomez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.J. In pursuit of ‘normal’: A review of the behaviour of cattle at pasture. Appl. Anim. Behav. Sci. 2012, 138, 1–11. [Google Scholar] [CrossRef]
- Scaglia, G.; Boland, H.; Wyatt, W. Effects of time of supplementation on beef stocker calves grazing ryegrass. II. Grazing behavior and dry matter intake. Prof. Anim. Sci. 2009, 25, 749–756. [Google Scholar] [CrossRef]
- Authority, E.F.S. Scientific report on the effects of farming systems on dairy cow welfare and disease. EFSA J. 2009, 7, 1143r. [Google Scholar] [CrossRef]
- Seman, D.H.; Stuedemann, J.A.; Anderson, J.E. Spectral analysis of bovine grazing behavior on Neotyphodium coenophialum infested tall fescue. Appl. Anim. Behav. Sci. 1997, 54, 73–87. [Google Scholar] [CrossRef]
- Senft, R.; Rittenhouse, L.; Woodmansee, R. Factors influencing patterns of cattle grazing behavior on shortgrass steepe. Rangel. Ecol. Manag./J. Range Manag. Arch. 1985, 38, 82–87. [Google Scholar] [CrossRef]
- Houseal, G.; Olson, B. Cattle use of microclimates on a northern latitude winter range. Can. J. Anim. Sci. 1995, 75, 501–507. [Google Scholar] [CrossRef]
- Gonyou, H.; Christopherson, R.; Young, B. Effects of cold temperature and winter conditions on some aspects of behaviour of feedlot cattle. Appl. Anim. Ethol. 1979, 5, 113–124. [Google Scholar] [CrossRef]
- Whittow, G.C. UNGULATES. In Comparative Physiology of Thermoregulation; Whittow, G.C., Ed.; Academic Press: New York, NY, USA, 1971; Volume 1, pp. 191–281. [Google Scholar]
- Taylor, N.A.; Tipton, M.J.; Kenny, G.P. Considerations for the measurement of core, skin and mean body temperatures. J. Therm. Biol. 2014, 46, 72–101. [Google Scholar] [CrossRef]
- Tor-Agbidye, J. Correlation of Endophyte Toxins (Ergovaline and Lolitrem B) with Clinical Disease: Fescue Foot and Perennial Ryegrass Staggers. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1993. [Google Scholar]
- Robichaud, M.V.; Rushen, J.; de Passille, A.M.; Vasseur, E.; Orsel, K.; Pellerin, D. Associations between on-farm animal welfare indicators and productivity and profitability on Canadian dairies: I. On freestall farms. J. Dairy Sci. 2019, 102, 4341–4351. [Google Scholar] [CrossRef]
- Hendriks, S.J.; Phyn, C.V.C.; Huzzey, J.M.; Mueller, K.R.; Turner, S.A.; Donaghy, D.J.; Roche, J.R. Graduate Student Literature Review: Evaluating the appropriate use of wearable accelerometers in research to monitor lying behaviors of dairy cows. J. Dairy Sci. 2020, 103, 12140–12157. [Google Scholar] [CrossRef]
- Richeson, J.T.; Lawrence, T.E.; White, B.J. Using advanced technologies to quantify beef cattle behavior. Transl. Anim. Sci. 2018, 2, 223–229. [Google Scholar] [CrossRef]
- Kou, H.; Zhao, Y.; Ren, K.; Chen, X.; Lu, Y.; Wang, D. Automated measurement of cattle surface temperature and its correlation with rectal temperature. PLoS ONE 2017, 12, e0175377. [Google Scholar] [CrossRef]
- Saha, U.; Hancock, D.; Kissel, D. How Do We Calculate Relative Forage Quality in Georgia. Available online: https://aesl.ces.uga.edu/publications/Feeds/RFQ_Calc_Circ.pdf (accessed on 10 March 2023).
- Hynd, P. Beef cattle nutrition. In Animal Nutrition: From Theory to Practice; Storer, P., Ed.; Csiro Publishing: Clayton South, Australia, 2019; pp. 122–159. [Google Scholar]
- Spiers, D.E.; Evans, T.J.; Rottinghaus, G.E. Interaction between thermal stress and fescue toxicosis: Animal models and new perspectives. In Neotyphodium in Cool-Season Grasses; Craig, A.R., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing: Ames, IA, USA, 2005; Volume 1, pp. 243–270. [Google Scholar]
- Peters, C.; Grigsby, K.; Aldrich, C.; Paterson, J.; Lipsey, R.; Kerley, M.; Garner, G. Performance, forage utilization, and ergovaline consumption by beef cows grazing endophyte fungus-infected tall fescue, endophyte fungus-free tall fescue, or orchardgrass pastures. J. Anim. Sci. 1992, 70, 1550–1561. [Google Scholar] [CrossRef]
- Hoveland, C.; Haaland, R.; King, C., Jr.; Anthony, W.; Clark, E.; McGuire, J.; Smith, L.; Grimes, H.; Holliman, J. Association of Epichloe typhina fungus and steer performance on tall fescue pasture. Agron. J. 1980, 72, 1064–1065. [Google Scholar] [CrossRef]
- Aldrich, C.; Paterson, J.; Tate, J.; Kerley, M. The effects of endophyte-infected tall fescue consumption on diet utilization and thermal regulation in cattle. J. Anim. Sci. 1993, 71, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Al-Haidary, A.; Spiers, D.; Rottinghaus, G.; Garner, G.; Ellersieck, M. Thermoregulatory ability of beef heifers following intake of endophyte-infected tall fescue during controlled heat challenge. J. Anim. Sci. 2001, 79, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Forcherio, C.; Larson, B.; Samford, M.; Kerley, M. The effects of fescue toxicosis on beef cattle productivity. J. Anim. Sci. 1995, 73, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Parish, J.; McCann, M.; Watson, R.; Paiva, N.; Hoveland, C.; Parks, A.; Upchurch, B.; Hill, N.; Bouton, J. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in stocker cattle. J. Anim. Sci. 2003, 81, 2856–2868. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.P.; Hoveland, C.S.; Clark, E.M.; Davis, N.D.; Smith, L.A.; Grimes, H.W.; Holliman, J.L. Association of an endophytic fungus with fescue toxicity in steers fed Kentucky 31 tall fescue seed or hay. J. Anim. Sci. 1982, 55, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Latch, G.C. Physiological interactions of endophytic fungi and their hosts. Biotic stress tolerance imparted to grasses by endophytes. Agric. Ecosyst. Environ. 1993, 44, 143–156. [Google Scholar] [CrossRef]
- Matthews, A.; Poore, M.; Huntington, G.; Green, J. Intake, digestion, and N metabolism in steers fed endophyte-free, ergot alkaloid-producing endophyte-infected, or nonergot alkaloid-producing endophyte-infected fescue hay. J. Anim. Sci. 2005, 83, 1179–1185. [Google Scholar] [CrossRef]
- Stuedemann, J.; Wilkinson, S.; Belesky, D.; Devine, O.; Breedlove, D.; Thompson, F.; Hoveland, C.; Ciordia, H.; Townsend, W. Utilization and management of endophyte-infested tall fescue: Affects on steer performance and behavior. In Proceedings of the Southern Pasture and Forage Crop Improvement Conference, Raleigh, NC, USA, 20–22 May 1985; pp. 17–20. [Google Scholar]
- Foote, A.P.; Kristensen, N.B.; Klotz, J.L.; Kim, D.H.; Koontz, A.F.; McLeod, K.R.; Bush, L.P.; Schrick, F.N.; Harmon, D.L. Ergot alkaloids from endophyte-infected tall fescue decrease reticuloruminal epithelial blood flow and volatile fatty acid absorption from the washed reticulorumen. J. Anim. Sci. 2013, 91, 5366–5378. [Google Scholar] [CrossRef]
- Welch, C.B.; Lourenco, J.M.; Krause, T.R.; Seidel, D.S.; Fluharty, F.L.; Pringle, T.D.; Callaway, T.R. Evaluation of the Fecal Bacterial Communities of Angus Steers With Divergent Feed Efficiencies Across the Lifespan From Weaning to Slaughter. Front. Vet. Sci. 2021, 8, 597405. [Google Scholar] [CrossRef]
- Reece, W.O.; Erickson, H.; Goff, J.; Uemura, E. Body temperature and its regulation. In Dukes’ Physiology of Domestic Animals; Reece, W.O., Erickson, H.H., Goff, J.P., Goff, J.P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; Volume 13, pp. 149–154. [Google Scholar]
- Murray, D.; Yeates, N. Walking trials with cattle II. A comparison of bulls, steers and heifers. J. Agric. Sci. 1967, 69, 71–78. [Google Scholar] [CrossRef]
- Lim, D.-H.; Kim, T.-I.; Kim, H.-J.; Kim, S.-B.; Park, S.-M.; Park, J.-H.; Ha, S.-M.; Lee, J.-H.; Lim, H.-J.; Jeong, H.-Y. Effect of short-distance walking activity on milk production and metabolic status of lactating dairy cows. J. Korean Soc. Grassl. Forage Sci. 2018, 38, 343–348. [Google Scholar] [CrossRef]
- Li, G.; Chen, S.; Chen, J.; Peng, D.; Gu, X. Predicting rectal temperature and respiration rate responses in lactating dairy cows exposed to heat stress. J. Dairy Sci. 2020, 103, 5466–5484. [Google Scholar] [CrossRef]
- Godyn, D.; Herbut, P.; Angrecka, S. Measurements of peripheral and deep body temperature in cattle—A review. J. Therm. Biol. 2019, 79, 42–49. [Google Scholar] [CrossRef]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Loew, D.; Van Deusen, E.B.; Meier-Ruge, W. Effects on the central nervous system. In Ergot Alkaloids and Related Compounds; Berde., B., Schild, H.O., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 1, pp. 421–531. [Google Scholar]
- Wagner, D.G. Effects of cold stress on cattle performance and management factors to reduce cold stress and improve performance. In Proceedings of the The Bovine Practitioner, Calgary, AB, Canada, 28 October 1988; pp. 88–93. [Google Scholar]
- Young, B. Cold stress as it affects animal production. J. Anim. Sci. 1981, 52, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ames, D.R. The Concept of Adjusting Energy Level in Maintenance Rations for Cold Weather; Kansas Agricultural Experiment Station Research Reports; Kansas State University: Manhattan, KS, USA, 1978; pp. 94–97. [Google Scholar]
- Hughes, G.P.; Reid, D. Studies on the behaviour of cattle and sheep in relation to the utilization of grass. J. Agric. Sci. 1951, 41, 350–366. [Google Scholar] [CrossRef]
- Tucker, C.B.; Jensen, M.B.; de Passillé, A.M.; Hänninen, L.; Rushen, J. Invited review: Lying time and the welfare of dairy cows. J. Dairy Sci. 2021, 104, 20–46. [Google Scholar] [CrossRef]
- Thorup, V.M.; Munksgaard, L.; Robert, P.-E.; Erhard, H.; Thomsen, P.; Friggens, N. Lameness detection via leg-mounted accelerometers on dairy cows on four commercial farms. Animal 2015, 9, 1704–1712. [Google Scholar] [CrossRef]
- Westin, R.; Vaughan, A.; de Passillé, A.M.; Devries, T.J.; Pajor, E.A.; Pellerin, D.; Siegford, J.M.; Vasseur, E.; Rushen, J. Lying times of lactating cows on dairy farms with automatic milking systems and the relation to lameness, leg lesions, and body condition score. J. Dairy Sci. 2016, 99, 551–561. [Google Scholar] [CrossRef]
- Rashamol, V.P.; Sejian, V.; Bagath, M.; Krishnan, G.; Archana, P.R.; Bhatta, R. Physiological adaptability of livestock to heat stress: An updated review. J. Anim. Behav. Biometeorol. 2020, 6, 62–71. [Google Scholar] [CrossRef]
- Olson, B.; T Wallander, R. Influence of winter weather and shelter on activity patterns of beef cows. Can. J. Anim. Sci. 2002, 82, 491–501. [Google Scholar] [CrossRef]
- Brown, D.; Livesey, G.; Dauncey, M. Influence of mild cold on the components of 24 hour thermogenesis in rats. J. Physiol. 1991, 441, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Yates, S.G.; Powell, R.G. Analysis of ergopeptine alkaloids in endophyte-infected tall fescue. J. Agric. Food Chem. 1988, 36, 337–340. [Google Scholar] [CrossRef]
- Lyons, P.C.; Plattner, R.D.; Bacon, C.W. Occurrence of peptide and clavine ergot alkaloids in tall fescue grass. Science 1986, 232, 487–489. [Google Scholar] [CrossRef]
- Klotz, J.L.; Aiken, G.E.; Bussard, J.R.; Foote, A.P.; Harmon, D.L.; Goff, B.M.; Schrick, F.N.; Strickland, J.R. Vasoactivity and Vasoconstriction Changes in Cattle Related to Time off Toxic Endophyte-Infected Tall Fescue. Toxins 2016, 8, 271. [Google Scholar] [CrossRef]
- Rottinghaus, G.E.; Garner, G.B.; Cornell, C.N.; Ellis, J.L. HPLC method for quantitating ergovaline in endophyte-infested tall fescue: Seasonal variation of ergovaline levels in stems with leaf sheaths, leaf blades, and seed heads. J. Agric. Food Chem. 1991, 39, 112–115. [Google Scholar] [CrossRef]
- Hovermale, J.T.; Craig, A.M. Correlation of ergovaline and lolitrem B levels in endophyte-infected perennial ryegrass (Lolium perenne). J. Vet. Diagn. Investig. 2001, 13, 323–327. [Google Scholar] [CrossRef]
- Rogers, W.M.; Roberts, C.A.; Andrae, J.G.; Davis, D.K.; Rottinghaus, G.E.; Hill, N.S.; Kallenbach, R.L.; Spiers, D.E. Seasonal fluctuation of ergovaline and total ergot alkaloid concentrations in tall fescue regrowth. Crop Sci. 2011, 51, 1291–1296. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Uppal, K.; Tran, V.T.; Jones, D.P.; Filipov, N.M. Metabolomics of fescue toxicosis in grazing beef steers. Food Chem. Toxicol. 2017, 105, 285–299. [Google Scholar] [CrossRef]
- Browning, R., Jr.; Leite-Browning, M.L. Effect of ergotamine and ergonovine on thermal regulation and cardiovascular function in cattle. J. Anim. Sci. 1997, 75, 176–181. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Kieran, T.J.; Seidel, D.S.; Glenn, T.C.; Silveira, M.F.D.; Callaway, T.R.; Stewart, R.L., Jr. Comparison of the ruminal and fecal microbiotas in beef calves supplemented or not with concentrate. PLoS ONE 2020, 15, e0231533. [Google Scholar] [CrossRef] [PubMed]
- Rushing, J.B.; Saha, U.K.; Lemus, R.; Sonon, L.; Baldwin, B.S. Analysis of some important forage quality attributes of Southeastern Wildrye (Elymus glabriflorus) using near-infrared reflectance spectroscopy. Am. J. Anal. Chem. 2016, 7, 642. [Google Scholar] [CrossRef]
- Saha, U.; Endale, D.; Tillman, P.G.; Johnson, W.C.; Gaskin, J.; Sonon, L.; Schomberg, H.; Yang, Y. Analysis of various quality attributes of sunflower and soybean plants by near infrared reflectance spectroscopy: Development and validation calibration models. Am. J. Anal. Chem. 2017, 8, 462–492. [Google Scholar] [CrossRef]
- Ungar, E.D.; Nevo, Y.; Baram, H.; Arieli, A. Evaluation of the IceTag leg sensor and its derivative models to predict behaviour, using beef cattle on rangeland. J. Neurosci. Methods 2018, 300, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Kokin, E.; Praks, J.; Veermäe, I.; Poikalainen, V.; Vallas, M. IceTag3D™ accelerometric device in cattle lameness detection. Agron. Res. 2014, 12, 223–230. [Google Scholar]
- Peacock Technology. Available online: https://www.peacocktechnology.com/en/careers (accessed on 16 February 2023).
- Mattachini, G.; Riva, E.; Bisaglia, C.; Pompe, J.C.; Provolo, G. Methodology for quantifying the behavioral activity of dairy cows in freestall barns. J Anim Sci 2013, 91, 4899–4907. [Google Scholar] [CrossRef]
- Endres, M.I.; Barberg, A.E. Behavior of dairy cows in an alternative bedded-pack housing system. J. Dairy Sci. 2007, 90, 4192–4200. [Google Scholar] [CrossRef]
- Mote, R.S.; Hill, N.S.; Skarlupka, J.H.; Tran, V.T.; Walker, D.I.; Turner, Z.B.; Sanders, Z.P.; Jones, D.P.; Suen, G.; Filipov, N.M. Toxic tall fescue grazing increases susceptibility of the Angus steer fecal microbiota and plasma/urine metabolome to environmental effects. Sci. Rep. 2020, 10, 2497. [Google Scholar] [CrossRef]

| Treatment | ||||
|---|---|---|---|---|
| E+ | E− | NT | p-Value | |
| Physiological parameters | ||||
| Rectal temperature (°C) | 39.5 b ± 0.07 | 39.3 a ± 0.06 | 39.3 a + 0.09 | 0.05 |
| Skin surface temperature (°C) | 29.5 b ± 0.07 | 29.6 b ± 0.07 | 30.9 a ± 0.07 | 0.001 |
| Ear surface temperature (°C) | 27.9 ± 1.23 | 25.2 ± 0.61 | 26.8 ± 0.63 | 0.2 |
| Ankle surface temperature (°C) | 20.03 ± 0.62 | 20.3 ± 0.59 | 20.5 ± 039 | 0.4 |
| Respiration rate (BPM; breath per minute) | 40.5 ± 1.70 | 38.01 ± 2.69 | 38.4 ± 2.20 | 0.7 |
| Behavioral activity | ||||
| Standing time (h/day/animal) | 10.1 b ± 0.36 | 10.6 a ± 0.34 | 10.5 a ± 0.26 | 0.0001 |
| Laying time (h/day/animal) | 13.9 b ± 0.36 | 13.4 a ± 0.34 | 13.5 a ± 0.26 | 0.0001 |
| Steps (n/day) | 1865.8 b ± 8.79 | 1864.4 ab ± 8.18 | 1771.1 a ± 8.38 | 0.01 |
| Lying bouts (n/day) | 10.8 ± 0.42 | 11.7 ± 0.41 | 10.5 ± 0.42 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llada, I.M.; Lourenco, J.M.; Dycus, M.M.; Carpenter, J.M.; Suen, G.; Hill, N.S.; Filipov, N.M. Behavioral and Physiological Alterations in Angus Steers Grazing Endophyte-Infected Toxic Fescue during Late Fall. Toxins 2023, 15, 343. https://doi.org/10.3390/toxins15050343
Llada IM, Lourenco JM, Dycus MM, Carpenter JM, Suen G, Hill NS, Filipov NM. Behavioral and Physiological Alterations in Angus Steers Grazing Endophyte-Infected Toxic Fescue during Late Fall. Toxins. 2023; 15(5):343. https://doi.org/10.3390/toxins15050343
Chicago/Turabian StyleLlada, Ignacio M., Jeferson M. Lourenco, Mikayla M. Dycus, Jessica M. Carpenter, Garret Suen, Nicholas S. Hill, and Nikolay M. Filipov. 2023. "Behavioral and Physiological Alterations in Angus Steers Grazing Endophyte-Infected Toxic Fescue during Late Fall" Toxins 15, no. 5: 343. https://doi.org/10.3390/toxins15050343
APA StyleLlada, I. M., Lourenco, J. M., Dycus, M. M., Carpenter, J. M., Suen, G., Hill, N. S., & Filipov, N. M. (2023). Behavioral and Physiological Alterations in Angus Steers Grazing Endophyte-Infected Toxic Fescue during Late Fall. Toxins, 15(5), 343. https://doi.org/10.3390/toxins15050343