Effect of Monocerin, a Fungal Secondary Metabolite, on Endothelial Cells
Abstract
:1. Introduction
2. Results
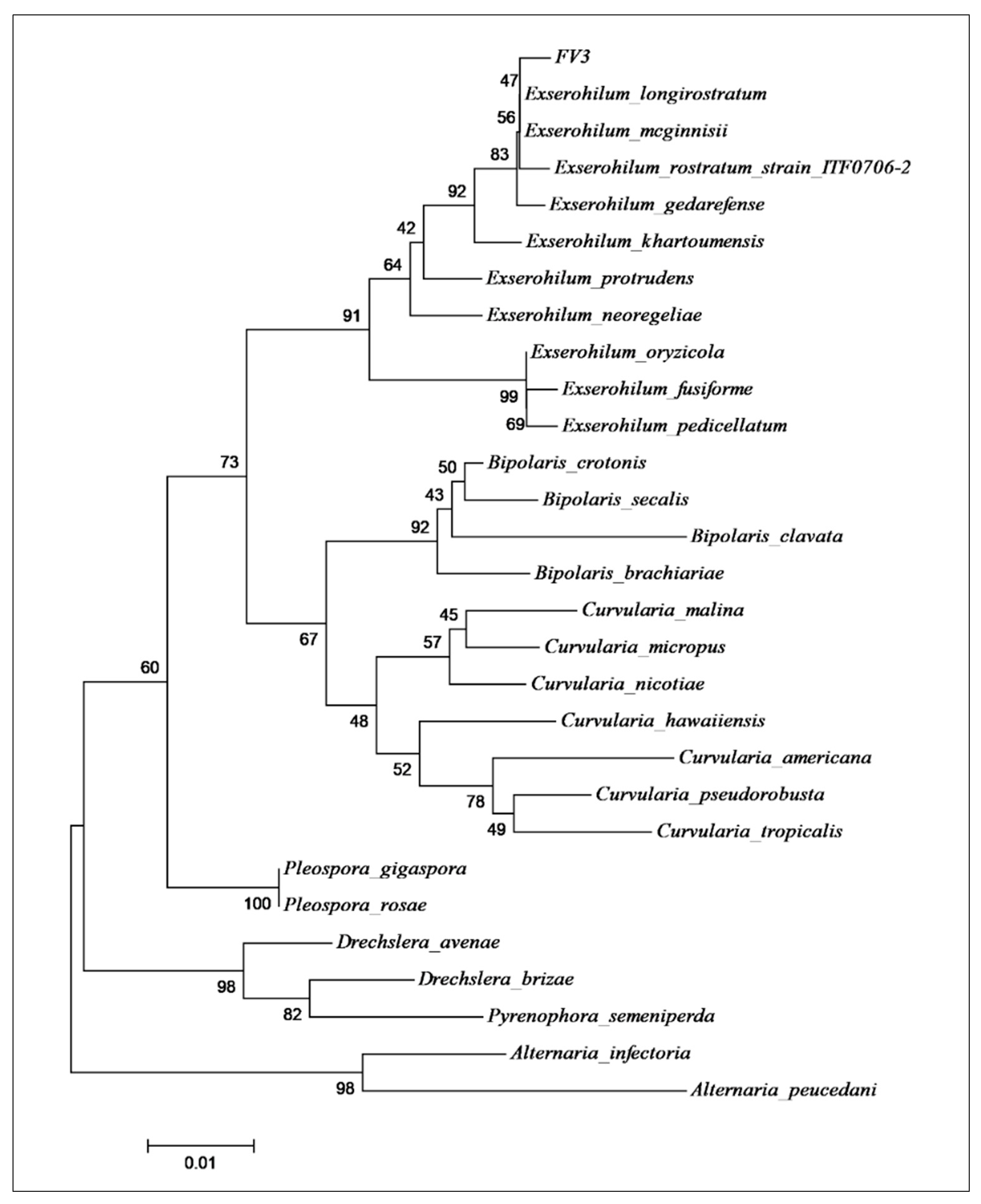
2.1. Identification and Morphological Characterization of the Fungal Isolate
2.2. Fungus Culture and Obtaining of Monocerin
2.3. Cell Viability
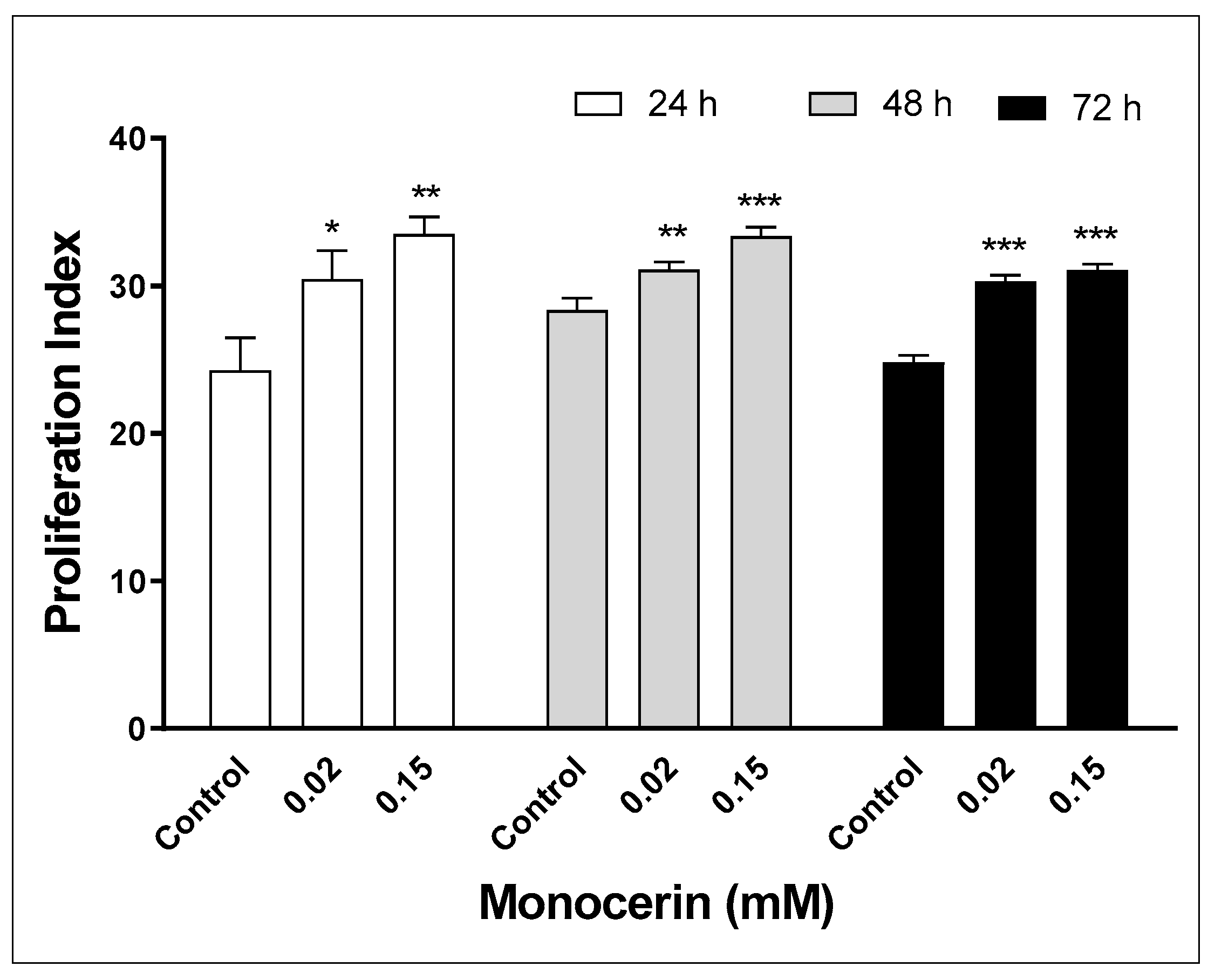
2.4. Proliferation Index by CFSE Assay
2.5. Annexin V/PI Labeling of Endothelial Cells Treated with Monocerin
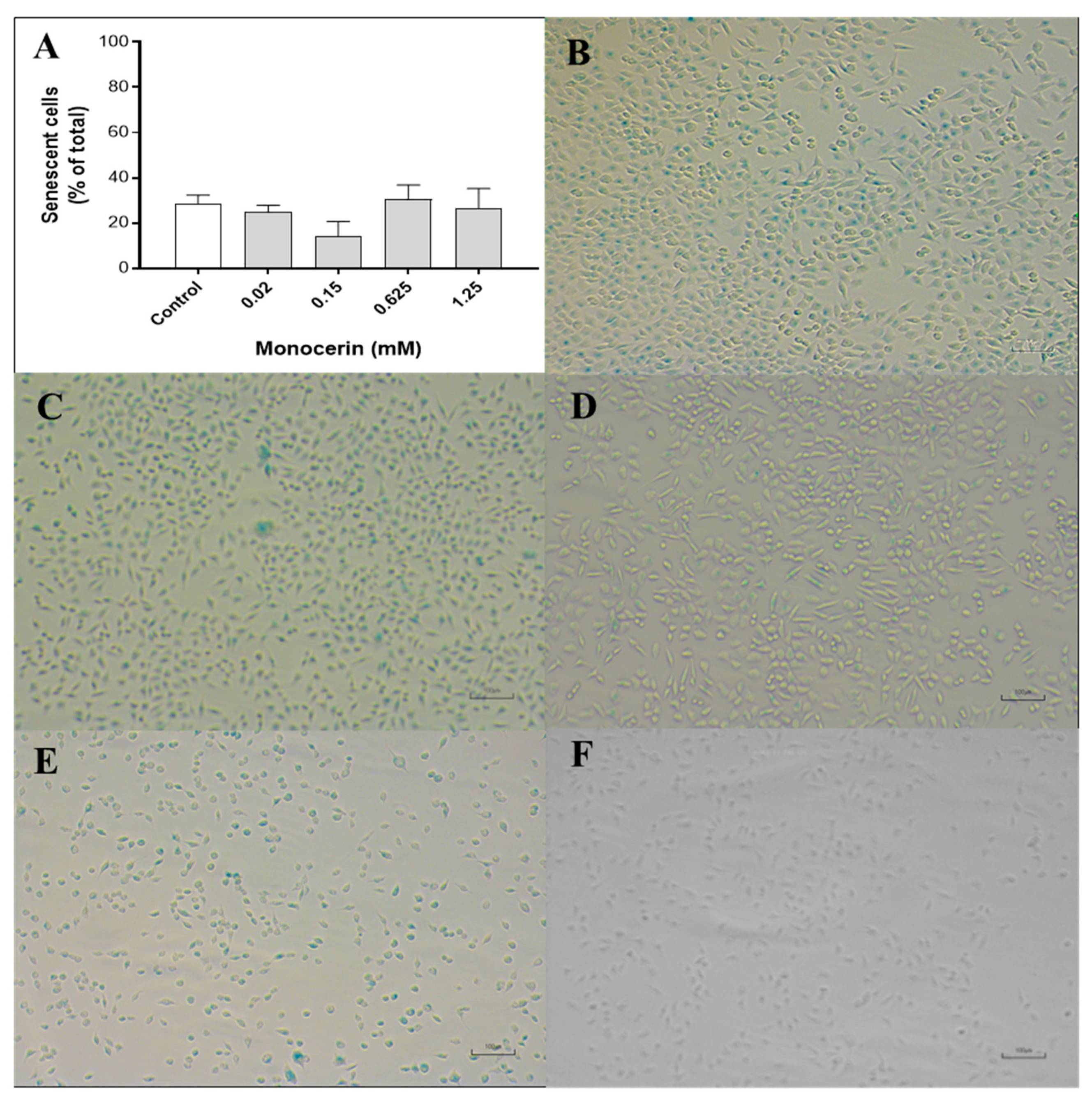
2.6. Endothelial Cell Senescence—β-Galactosidase Activity
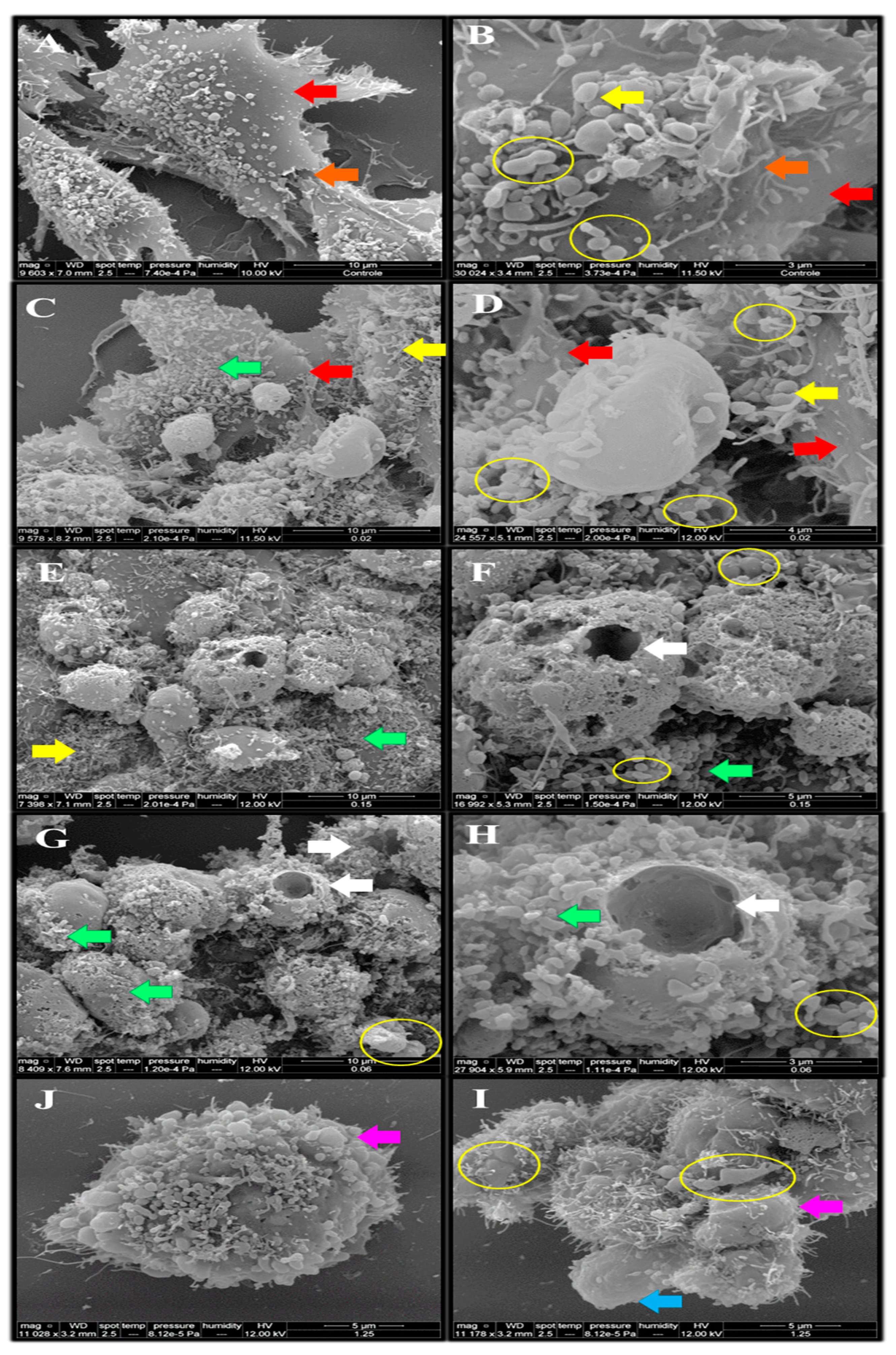
2.7. Morphological Analysis of the Endothelial Cells
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Materials, Chemicals and Cell Line
5.2. Isolation and Taxonomical Identification of the Fungal Strain
5.3. Morphological Analysis of the Fungus E. rostratum
5.3.1. Microcultive of the Fungus Strain and Analysis by Optical Microscopy
5.3.2. Morphological Analysis by Scanning Electron Microscopy (SEM)
5.3.3. Morphological Analysis by Transmission Electron Microscopy (TEM)
5.4. Fungus Culture and Purification of Monocerin
5.5. Biological Assays
5.5.1. Monocerin Preparation
5.5.2. Cell Culture
5.5.3. Cell Viability—MTT Assay
5.5.4. Senescence Associated β-Galactosidase (SA-β-gal) Activity
5.5.5. Annexin V/Propidium Iodide Apoptosis Assay
5.5.6. Analysis of Endothelial Cells Proliferation
5.5.7. Morphological Analysis of the Endothelial Cells by
Scanning Electron Microscopy (SEM)
Laser Scanning Confocal Microscopy
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conrado, R.; Gomes, T.C.; Roque, G.S.C.; De Souza, A.O. Overview of bioactive fungal secondary metabolites: Cytotoxic and antimicrobial compounds. Antibiotics 2022, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sappapan, R.; Sommit, D.; Ngamrojanavanich, N.; Pengpreecha, S.; Wiyakrutta, S.; Sriubolmas, N.; Pudhom, K. 11-Hydroxymonocerin from the plant endophytic fungus Exserohilum rostratum. J. Nat. Prod. 2008, 71, 1657–1659. [Google Scholar] [CrossRef]
- Aldridge, D.; Turner, W. Metabolites of Helminthosporium monoceras—Structures of monocerin and related benzopyrans. J. Chem. Soc. C-Org. 1970, 2598–2600. [Google Scholar] [CrossRef] [PubMed]
- Robeson, D.; Strobel, G. Monocerin, a phytotoxin from Exserohilum turcicum (= Drechslera turcica). Agric. Biol. Chem. 1982, 46, 2681–2683. [Google Scholar]
- Zhang, W.; Krohn, K.; Draeger, S.; Schulz, B. Bioactive isocoumarins isolated from the endophytic fungus Microdochium bolleyi. J. Nat. Prod. 2008, 71, 1078–1081. [Google Scholar] [CrossRef]
- Pinheiro, E.A.A.; Pina, J.R.S.; Feitosa, A.; Carvalho, J.; Borges, F.; Marinho, P.; Marinho, A. Bioprospecting of antimicrobial activity of extracts of endophytic fungi from Bauhinia guianensis. Rev. Argent. Microbiol. 2017, 49, 3–6. [Google Scholar] [CrossRef]
- Liu, S.; Su, M.; Song, S.J.; Jung, J.H. Marine-derived Penicillium species as producers of cytotoxic metabolites. Mar. Drugs 2017, 15, 2329. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. Exserohilum rostratum fungal meningitis associated with methylprednisolone injections. Future Microbiol. 2013, 8, 135–137. [Google Scholar] [CrossRef]
- Malani, A.N.; Kauffman, C.A.; Latham, R.; Peglow, S.; Ledtke, C.S.; Kerkering, T.M.; Kaufman, D.H.; Triplett, P.F.; Wright, P.W.; Bloch, K.C.; et al. Long-term outcomes of patients with fungal infections associated with contaminated methylprednisolone injections. Open Forum Infect. Dis. 2020, 7, ofaa164. [Google Scholar] [CrossRef]
- Kohashi, S.; Toyama, T.; Hashimoto, N.; Sakurai, M.; Kato, J.; Kikuchi, T.; Koda, Y.; Sugita, K.; Hasegawa, N.; Yarita, K.; et al. Sinusitis caused by Exserohilum rostratum after cord blood transplantation for myelodysplastic syndrome: A case report and literature review. Transpl. Infect. Dis. 2018, 20, e12805. [Google Scholar] [CrossRef] [PubMed]
- Chaidaroon, W.; Phaocharoen, N.; Srisomboon, T.; Vanittanakom, N. Keratitis in a Patient with Human Immunodeficiency Virus. Case Rep. Ophthalmol. 2019, 10, 127–133. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Katragkou, A.; Maung, B.B.W.; Moradi, P.W.; Sussman-Straus, G.E.; Naing, E.; Kovanda, L.L.; Finkelman, M.A.; Walsh, T.J. Antifungal efficacy of isavuconazole and liposomal amphotericin B in a rabbit model of Exserohilum rostratum meningoencephalitis: A preclinical paradigm for management of CNS phaeohyphomycosis. Med. Mycol. 2021, 59, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Jensen, P.R.; Williams, P.G.; Fenical, W. Isolation and structure assignments of rostratins A-D, cytotoxic disulfides produced by the marine-derived fungus Exserohilum rostratum. J. Nat. Prod. 2004, 67, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.A.A.; Borges, F.C.; Pina, J.R.S.; Ferreira, L.R.S.; Cordeiro, J.S.; Carvalho, J.M.; Feitosa, A.O.; Campos, F.R.; Barison, A.; Souza, A.D.L.; et al. Annularins I and J: New metabolites isolated from endophytic fungus Exserohilum rostratum. J. Braz. Chem. Soc. 2016, 27, 1432–1436. [Google Scholar]
- Li, C.; Nitka, M.V.; Gloer, J.B.; Campbell, J.; Shearer, C.A. Annularins A-H: New polyketide metabolites from the freshwater aquatic fungus Annulatascus triseptatus. J. Nat. Prod. 2003, 66, 1302–1306. [Google Scholar] [CrossRef]
- Erkel, G.; Belahmer, H.; Serwe, A.; Anke, T.; Kunz, H.; Kolshorn, H.; Liermann, J.; Opatz, T. Oxacyclododecindione, a novel inhibitor of IL-4 signaling from Exserohilum rostratum. J. Antibiot. (Tokyo) 2008, 61, 285–290. [Google Scholar] [CrossRef]
- Dillon, M.; Simpson, T.; Sweeney, J. Enantioselective synthesis of monocerin and fusarentin ethers—antifungal and insecticidal fungal metabolites. Tetrahedron Lett. 1992, 33, 7569–7572. [Google Scholar] [CrossRef]
- Cuq, F.; Herrmanngorline, S.; Klaebe, A.; Rossignol, M.; Petitprez, M. Monocerin in Exserohilum turcicum isolates from maize and a study of its phytotoxicity. Phytochemistry 1993, 34, 1265–1270. [Google Scholar] [CrossRef]
- Claydon, N.; Grove, J.; Pople, M. Insecticidal secondary metabolic products from the entomogenous fungus Fusarium larvarum. J. Inver. Pathol. 1979, 33, 364–367. [Google Scholar] [CrossRef]
- Lim, C.-H. Monocerin and ziganein: Phytotoxins from pathogenic fungus Exserohilum monoceras Inu-1. J. Applied Biol. Chem. 1999, 42, 45–47. [Google Scholar]
- Johnson, K.E.; Wilgus, T.A. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Wang, X.F.; Li, M.L.; Fang, Q.Q.; Zhao, W.Y.; Lou, D.; Hu, Y.Y.; Chen, J.; Wang, X.Z.; Tan, W.Q. Flexible electrical stimulation device with Chitosan-Vaseline® dressing accelerates wound healing in diabetes. Bioact. Mater. 2021, 6, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, L.; Zhang, X.; Chang, H.; Ma, S.; Xie, Z.; Tang, S.; Ju, X.; Zhu, H.; Shen, B.; et al. MiR-221-3p targets HIPK2 to promote diabetic wound healing. Microvasc. Res. 2022, 140, 104306. [Google Scholar] [CrossRef] [PubMed]
- Jian, K.; Yang, C.; Li, T.; Wu, X.; Shen, J.; Wei, J.; Yang, Z.; Yuan, D.; Zhao, M.; Shi, J. PDGF-BB-derived supramolecular hydrogel for promoting skin wound healing. J. Nanobiotechnol. 2022, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Pang, L.; Li, J.J.; Zhang, C.; Li, J.X.; Zhang, X.; Mao, T.; Wu, D.T.; Ma, X.Y.; Geng, F.N.; et al. Characterization and diabetic wound healing benefits of protein-polysaccharide complexes isolated from an animal ethno-medicine Periplaneta americana L. Int. J. Biol. Macromol. 2022, 195, 466–474. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Madrid, H.; Tan, Y.P.; da Cunha, K.C.; Gené, J.; Guarro, J.; Crous, P.W. Multi-locus phylogeny and taxonomy of Exserohilum. Persoonia 2018, 41, 71–108. [Google Scholar] [CrossRef]
- McGinnis, M.R.; Rinaldi, M.G.; Winn, R.E. Emerging agents of phaeohyphomycosis: Pathogenic species of Bipolaris and Exserohilum. J. Clin. Microbiol. 1986, 24, 250–259. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Dong, C.; Yao, Y. Isolation, characterization of melanin derived from Ophiocordyceps sinensis, an entomogenous fungus endemic to the Tibetan Plateau. J. Biosci. Bioeng. 2012, 113, 474–479. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell. Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Krizhanovsky, V.; Yon, M.; Dickins, R.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Espin, D.; Canamero, M.; Maraver, A.; Gomez-Lopez, G.; Contreras, J.; Murillo-Cuesta, S.; Rodriguez-Baeza, A.; Varela-Nieto, I.; Ruberte, J.; Collado, M.; et al. Programmed cell senescence during mammalian embryonic development. Cell 2013, 155, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.; Rodier, F.; Toussaint, W.; Mitchell, J.; Laberge, R.; Vijg, J.; Van Steeg, H.; Dolle, M.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Munoz-Espin, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.; Morton, J.; Sansom, O.; Zender, L.; Keyes, W. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef]
- Kurz, D.J.; Decary, S.; Hong, Y.; Erusalimsky, J.D. Senescence-associated beta-galactosidase reflects an increase in lysosomal mass with replicative age in human endothelial cells. J. Cell Sci. 2002, 113, 3613–3622. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four faces of cellular senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Bruno, S.; Del Bino, G.; Gorczyca, W.; Hotz, M.A.; Lassota, P.; Traganos, F. Features of apoptotic cells measured by flow cytometry. Cytometry 1992, 13, 795–808. [Google Scholar] [CrossRef]
- Hussain, H.; L, S.R.; Ahmad, S.; Razak, M.; Mohamud, W.; Bakar, J.; Ghazali, H.; Yildiz, F. Determination of cell viability using acridine orange/propidium iodide dual-spectrofluorometry assay. Cogent Food Agric. 2019, 5, 1582398. [Google Scholar] [CrossRef]
- Mcmaster, G.; Carmichael, G. Analysis of single-stranded and double-stranded nucleic-acids on polyacrylamide and agarose gels by using glyoxal and acridine-orange. Proc. Nat. Acad. Sci. USA 1977, 74, 4835–4838. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Young, M.R. Uptake of dyes and drugs by living cells in culture. Life Sci. 1964, 3, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.; Cahill, D. A rapid cell counting method utilising acridine orange as a novel discriminating marker for both cultured astrocytes and microglia. J. Neurosci. Methods 2007, 165, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.M.; White, K.; Fessler, L.I.; Steller, H. Programmed cell death during Drosophila embryogenesis. Development 1993, 117, 29–43. [Google Scholar] [CrossRef]
- Gambier, R.; Mulcahy, D. Confocal laser-scanning microscopy of mitochondria within microspore tetrads of plants using rhodamine-123 as a fluorescent vital stain. Biotech. Histochem. 1994, 69, 311–316. [Google Scholar] [CrossRef]
- Wu, F. Localization of mitochondria in plant-cells by vital staining with rhodamine-123. Planta 1987, 171, 346–357. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Maria, D.A.; Goncalves, P.; de Araujo, W.L.; de Souza, A.O. Biogenic Aspergillus tubingensis silver nanoparticles’ in vitro effects on human umbilical vein endothelial cells, normal human fibroblasts, HEPG2, and Galleria mellonella. Toxicol. Res. 2019, 8, 789–801. [Google Scholar] [CrossRef]
- Araujo, W.L.; Maccheroni, W.; Aguilar-Vildoso, C.I.; Barroso, P.A.V.; Saridakis, H.O.; Azevedo, J.L. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef]
- Martin, K.J.; Rygiewicz, P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005, 5, 28. [Google Scholar] [CrossRef]
- Samson, R.A.; Stalpers, J.A.; Verkerke, W. A simplified technique to prepare fungal specimens for scanning electron microscopy. Cytobios 1979, 24, 7–11. [Google Scholar]
- Karnovsky, M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Heymann, J.A.; Hayles, M.; Gestmann, I.; Giannuzzi, L.A.; Lich, B.; Subramaniam, S. Site-specific 3D imaging of cells and tissues with a dual beam microscope. J. Struct. Biol. 2006, 155, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Jamalzadeh, L.; Ghafoori, H.; Sariri, R.; Rabuti, H.; Nasirzade, J.; Hasani, H.; Aghamaali, M.R. Cytotoxic effects of some common organic solvents on MCF-7, Raw-264.7 and human umbilical vein endothelial cells. Avicenna J. Med. Biochem. 2016, 4, e33453. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival—application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.H.; Basile, G.; Acosta, M.; Scott, C.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereirasmith, O.; et al. A biomarker that identifies senescent human-cells in culture and in aging skin in vivo. Proc. Nat. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Milovanova, T.; Manevich, Y.; Haddad, A.; Chatterjee, S.; Moore, J.S.; Fisher, A.B. Endothelial cell proliferation associated with abrupt reduction in shear stress is dependent on reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 245–258. [Google Scholar] [CrossRef]
- Geisow, M.; Beaven, G.; Darcyhart, P.; Young, M. Site of action of a polyanion inhibitor of phagosome-lysome fusion in cultured macrophages. Exp. Cell Res. 1980, 126, 159–165. [Google Scholar] [CrossRef]
- Yoshimori, T.; Yamamoto, A.; Moriyama, Y.; Futai, M.; Tashiro, Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 1991, 266, 17707–17712. [Google Scholar] [CrossRef]


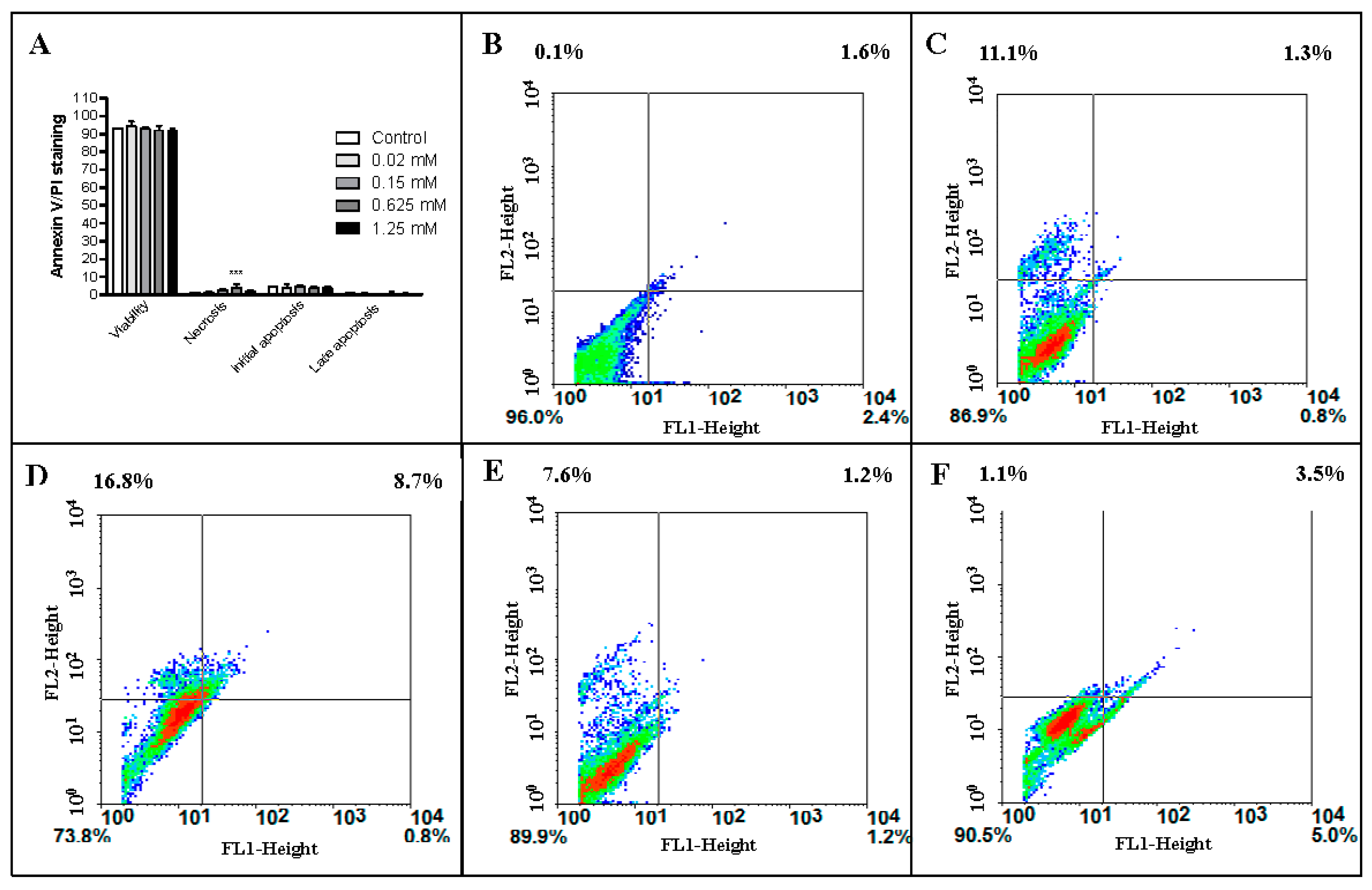

| Monocerin (mM) | Viability | Necrosis | Initial Apoptosis | Late Apoptosis |
|---|---|---|---|---|
| Control | 93.1 ± 0.0 | 1.4 ± 0.0 | 4.6 ± 0.0 | 0.9 ± 0.0 |
| 0.02 | 94.3 ± 0.8 | 1.2 ± 0.2 | 4.0 ± 0.6 | 0.6 ± 0.2 |
| 0.15 | 92.7 ± 0.3 | 2.3 ± 0.2 | 4.5 ± 0.3 | 0.5 ± 0.1 |
| 0.625 | 91.9 ± 0.8 | 3.9 ± 0.5 | 3.6 ± 0.3 | 0.6 ± 0.3 |
| 1.25 | 91.5 ± 0.5 | 2.2 ± 0.1 | 3.6 ± 0.3 | 0.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, T.C.; Conrado, R.; Oliveira, R.C.d.; Selari, P.J.R.G.; Melo, I.S.d.; Araújo, W.L.; Maria, D.A.; De Souza, A.O. Effect of Monocerin, a Fungal Secondary Metabolite, on Endothelial Cells. Toxins 2023, 15, 344. https://doi.org/10.3390/toxins15050344
Gomes TC, Conrado R, Oliveira RCd, Selari PJRG, Melo ISd, Araújo WL, Maria DA, De Souza AO. Effect of Monocerin, a Fungal Secondary Metabolite, on Endothelial Cells. Toxins. 2023; 15(5):344. https://doi.org/10.3390/toxins15050344
Chicago/Turabian StyleGomes, Tainah Colombo, Rafael Conrado, Rodrigo Cardoso de Oliveira, Priscila Jane Romano Gonçalves Selari, Itamar Soares de Melo, Welington Luiz Araújo, Durvanei Augusto Maria, and Ana Olívia De Souza. 2023. "Effect of Monocerin, a Fungal Secondary Metabolite, on Endothelial Cells" Toxins 15, no. 5: 344. https://doi.org/10.3390/toxins15050344
APA StyleGomes, T. C., Conrado, R., Oliveira, R. C. d., Selari, P. J. R. G., Melo, I. S. d., Araújo, W. L., Maria, D. A., & De Souza, A. O. (2023). Effect of Monocerin, a Fungal Secondary Metabolite, on Endothelial Cells. Toxins, 15(5), 344. https://doi.org/10.3390/toxins15050344






