Handheld Fluorescence Spectrometer Enabling Sensitive Aflatoxin Detection in Maize
Abstract
1. Introduction
2. Optical Fluorescence Spectroscopy Results
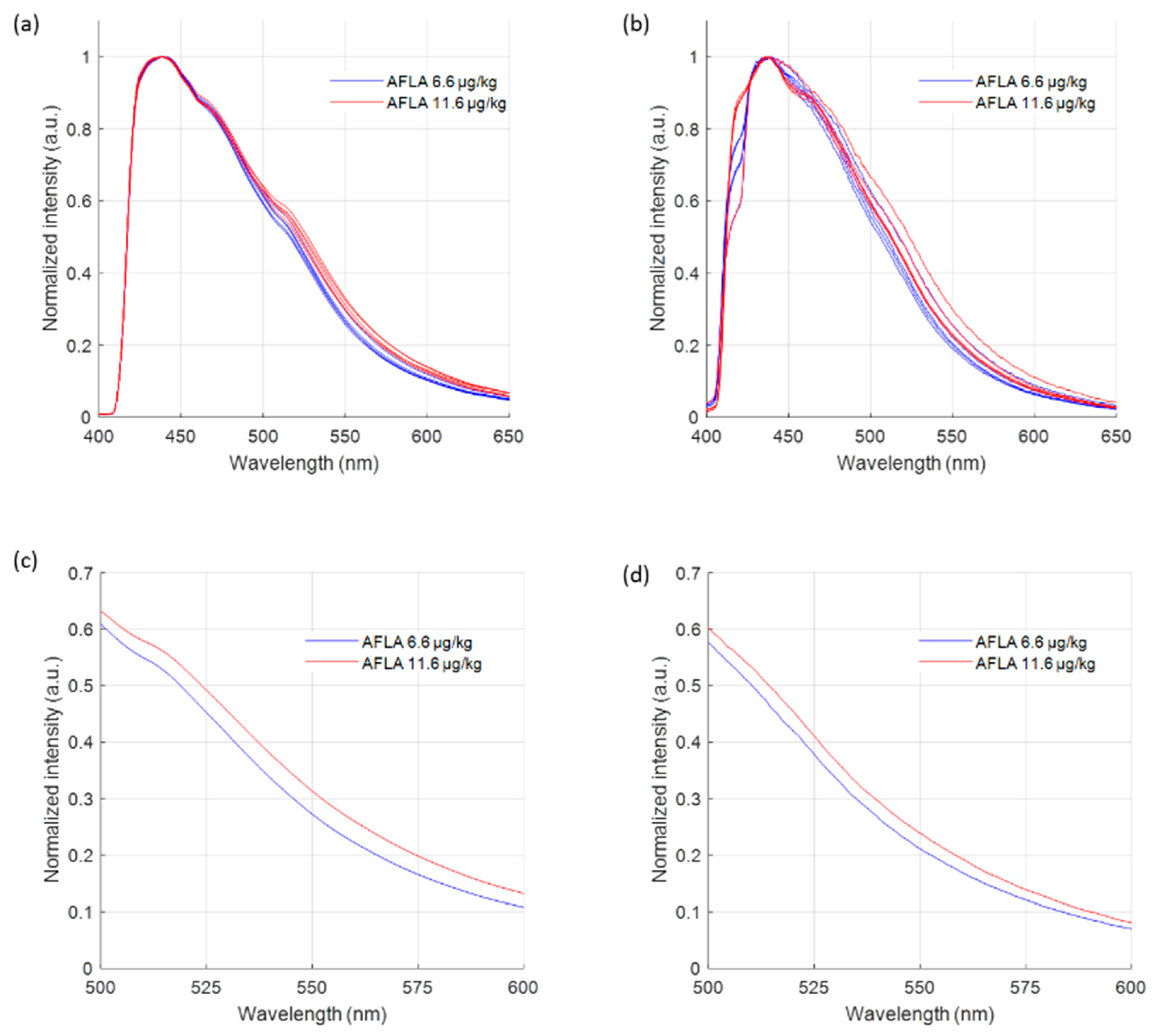
2.1. Fluorescence of Certified Aflatoxin-Contaminated Maize Powder
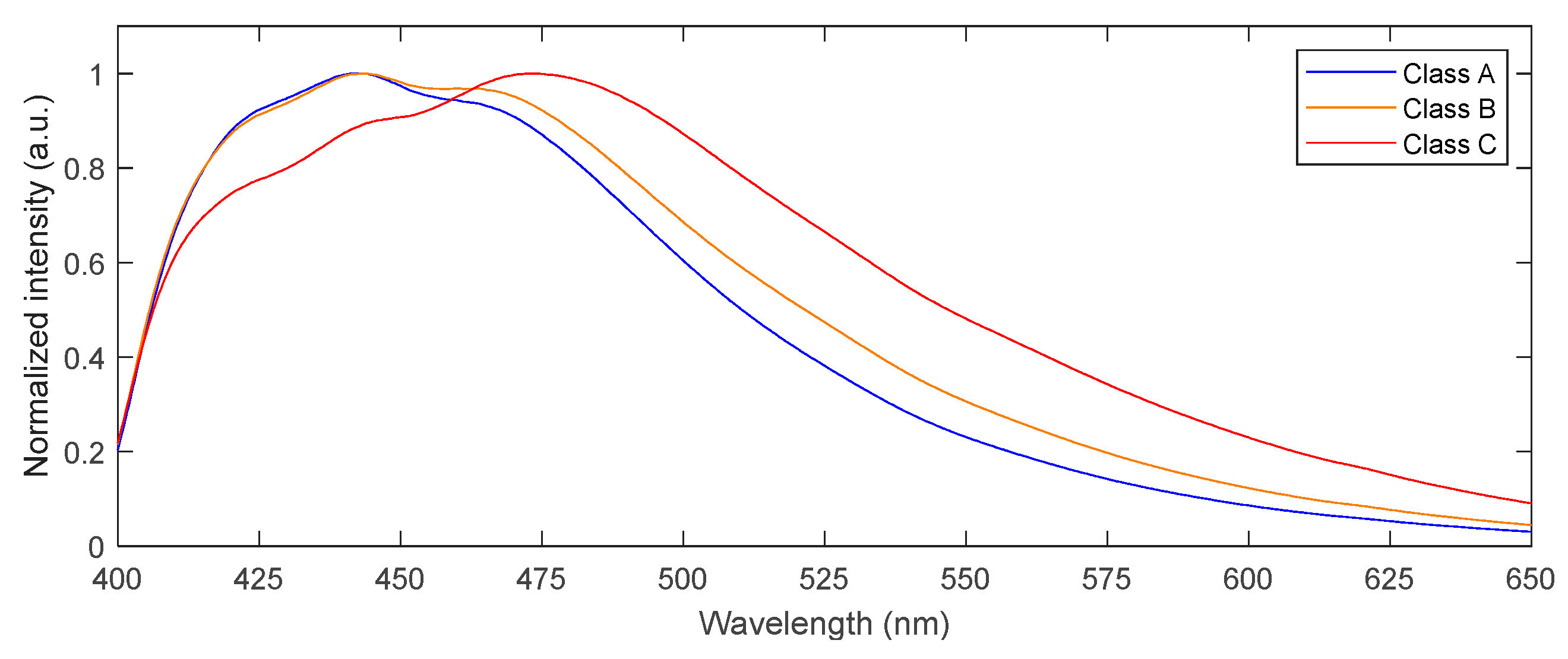
2.2. Fluorescence Emission of Naturally Contaminated Maize Kernels
3. Discussion
- (1)
- Miniaturization of the measurement unit, enabling the transition from a research-grade setup to a handheld unit.
- (2)
- Measurement of individual maize kernels, without the need for sample preparation, thus addressing the natural variation and inhomogeneity of the samples.
- (3)
- Minimization of the complexity of data processing, allowing fast processing and in-line integration.
- (4)
- Excellent sensitivity, enabling compliance with European legislation.
4. Conclusions
5. Materials and Methods
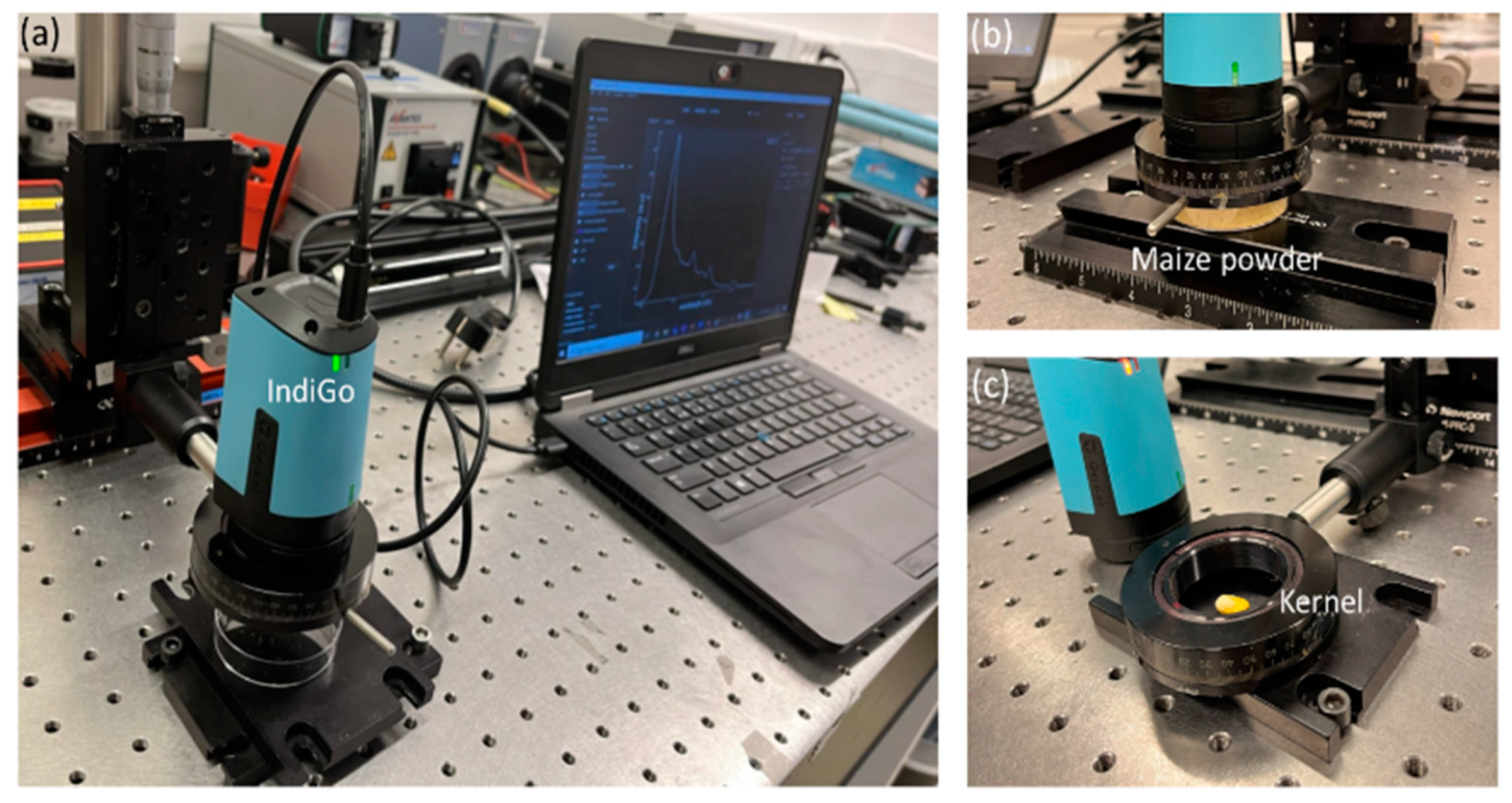
5.1. Maize Samples
5.2. Instrumentation
5.2.1. Research-Grade Fluorescence Measurement Setup
5.2.2. Handheld IndiGo Fluorescence Spectrometer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Commission Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- Unnevehr, L.; Grace, D. Aflatoxins: Finding Solutions for Improved Food Safety; International Food Policy Research Institute: Washington, DC, USA, 2013; pp. 1–18. [Google Scholar]
- Hruska, Z.; Yao, H.; Kincaid, R.; Darlington, D.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Fluorescence Imaging Spectroscopy (FIS) for Comparing Spectra from Corn Ears Naturally and Artificially Infected with Aflatoxin Producing Fungus. J. Food Sci. 2013, 78, T1313–T1320. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize Food Chain and Mycotoxins: A Review on Occurrence Studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops That Feed the World 6. Past Successes and Future Challenges to the Role Played by Maize in Global Food Security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- DSM (Dutch State Mines). World Mycotoxin Survey; DSM: Heerlen, The Netherlands, 2022. [Google Scholar]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk Assessment of Aflatoxins in Food. EFSA J. 2020, 18, 6040. [Google Scholar]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide Aflatoxin Contamination of Agricultural Products and Foods: From Occurrence to Control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef] [PubMed]
- Brankov, T.P.; Jovanovic, M.; Grujić, B. Aflatoxin Standards and Maize Trade. Econ. Agric. 2013, 3, 595–607. [Google Scholar]
- Miklós, G.; Angeli, C.; Ambrus, Á.; Nagy, A.; Kardos, V.; Zentai, A.; Kerekes, K.; Farkas, Z.; Jóźwiak, Á.; Bartók, T. Detection of Aflatoxins in Different Matrices and Food-Chain Positions. Front. Microbiol. 2020, 11, 1916. [Google Scholar]
- Romer Labs Worldwide Mycotoxin Regulations. Available online: https://www.romerlabs.com/en/knowledge-center/knowledge-library/articles/news/worldwide-mycotoxin-regulations/ (accessed on 13 May 2023).
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for Detection of Aflatoxins in Agricultural Food Crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Bhatnagar, D.; Cleveland, T. Classification of Corn Kernels Contaminated with Aflatoxins Using Fluorescence and Reflectance Hyperspectral Images Analysis. In Proceedings of the Sensing for Agriculture and Food Quality and Safety VII; Kim, M.S., Chao, K., Chin, B.A., Eds.; SPIE: Baltimore, MD, USA, 2015; Volume 9488, p. 94880M. [Google Scholar] [CrossRef]
- Mishra, G.; Panda, B.K.; Ramirez, W.A.; Jung, H.; Singh, C.B.; Lee, S.H.; Lee, I. Research Advancements in Optical Imaging and Spectroscopic Techniques for Nondestructive Detection of Mold Infection and Mycotoxins in Cereal Grains and Nuts. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4612–4651. [Google Scholar] [CrossRef]
- Ghilardelli, F.; Barbato, M.; Gallo, A. A Preliminary Study to Classify Corn Silage for High or Low Mycotoxin Contamination by Using near Infrared Spectroscopy. Toxins 2022, 14, 323. [Google Scholar] [CrossRef]
- Rathna Priya, T.S.; Manickavasagan, A. Characterising Corn Grain Using Infrared Imaging and Spectroscopic Techniques: A Review. J. Food Meas. Charact. 2021, 15, 3234–3249. [Google Scholar] [CrossRef]
- Smeesters, L.; Meulebroeck, W.; Raeymaekers, S.; Thienpont, H. Optical Detection of Aflatoxins in Maize Using One-and Two-Photon Induced Fluorescence Spectroscopy. Food Control 2015, 51, 408–416. [Google Scholar] [CrossRef]
- Smeesters, L.; Meulebroeck, W.; Raeymaekers, S.; Thienpont, H. One- and Two-Photon Induced Fluorescence Spectroscopy Enabling the Detection of Localized Aflatoxin Contamination in Individual Maize Kernels. In Proceedings of the Optical Sensing and Detection IV; Berghmans, F., Mignani, A.G., Eds.; Proc. of SPIE: Brussels, Belgium, 2016; Volume 9899, pp. 98990X-1–98990X-15. [Google Scholar]
- Liu, T.; He, J.; Yao, W.; Jiang, H.; Chen, Q. Determination of Aflatoxin B1 Value in Corn Based on Fourier Transform Near-Infrared Spectroscopy: Comparison of Optimization Effect of Characteristic Wavelengths. LWT 2022, 164, 113657. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, H.; Chen, Q. Characteristic Wavelengths Optimization Improved the Predictive Performance of Near-Infrared Spectroscopy Models for Determination of Aflatoxin B 1 in Maize. J. Cereal Sci. 2022, 105, 103474. [Google Scholar] [CrossRef]
- Cheng, X.; Vella, A.; Stasiewicz, M.J. Classification of Aflatoxin Contaminated Single Corn Kernels by Ultraviolet to near Infrared Spectroscopy. Food Control 2019, 98, 253–261. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, H.; Chen, Q. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Determination of Aflatoxin B 1 (AFB 1) in Maize Based on a Portable Raman Spectroscopy System and Multivariate Analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 275, 121148. [Google Scholar] [CrossRef]
- Wang, B.; Deng, J.; Jiang, H. Markov Transition Field Combined with Convolutional Neural Network Improved the Predictive Performance of Near-Infrared Spectroscopy Models for Determination of Aflatoxin B1 in Maize. Foods 2022, 11, 2210. [Google Scholar] [CrossRef]
- Bartolić, D.; Mutavdžić, D.; Carstensen, J.M.; Stanković, S. Fluorescence Spectroscopy and Multispectral Imaging for Fingerprinting of Aflatoxin-B 1 Contaminated (Zea mays L.) Seeds: A Preliminary Study. Sci. Rep. 2022, 12, 4849. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, W.; Liang, D.; Tian, X. Classification of Aflatoxin B1 Concentration of Single Maize Kernel Based on Near-Infrared Hyperspectral Imaging and Feature Selection. Sensors 2021, 21, 4257. [Google Scholar] [CrossRef]
- Kim, Y.; Baek, I.; Lee, K.; Qin, J.; Kim, G.; Kon, B.; Chan, D.E.; Herrman, T.J.; Cho, S.; Kim, M.S. Investigation of Reflectance, Fluorescence, and Raman Hyperspectral Imaging Techniques for Rapid Detection of Aflatoxins in Ground Maize. Food Control 2022, 132, 108479. [Google Scholar] [CrossRef]
- Aoun, M.; Siegel, C.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Application of Reflectance Spectroscopy to Identify Maize Genotypes and Aflatoxin Levels in Single Kernels Abstract. World Mycotoxin J. 2022, 15, 327–342. [Google Scholar] [CrossRef]
- Rasch, C.; Kumke, M.; Löhmannsröben, H.G.G. Sensing of Mycotoxin Producing Fungi in the Processing of Grains. Food Bioprocess Technol. 2010, 3, 908–916. [Google Scholar] [CrossRef]
- Ghalkhani, K.; Chaichi, M.J.; Hashemi, M.; Fatemi, M.H. Effects of Solvent, PH and Ionic Strength on the Fluorescence Features of Aflatoxin B1, B2, G1 and G2. Iran. Chem. Soc. Anal. Bioanal. Chem. Res. 2019, 6, 449–456. [Google Scholar]
- Held, P. Quantitation of Peptides and Amino Acids with a SynergyTM HT Using UV Fluorescence; Agilent Technologies, BioTek Instruments: Winooski, VT, USA, 2006. [Google Scholar]
- Smeesters, L.; Meulebroeck, W.; Raeymaekers, S.; Thienpont, H. The Use of One- and Two- Photon Induced Fluorescence Spectroscopy for the Optical Characterization of Carcinogenic Aflatoxins. In Proceedings of the SPIE Ultrafast Nonlinear Imaging and Spectroscopy II; Liu, Z., Khoo, I.C., Psaltis, D., Eds.; SPIE: San Diego, CA, USA, 2014; Volume 9198, pp. 1–11. [Google Scholar]
- Whitaker, T.B.; Johansson, A.S. Sampling Uncertainties for the Detection of Chemical Agents in Complex Food Matrices. J. Food Prot. 2005, 68, 1306–1313. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef] [PubMed]
- ISO ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 9 May 2023).




| Number of Tested Samples | Percentage of Contaminated Samples | Average Contamination (µg/kg) | Median Contamination (µg/kg) | Maximum Contamination (µg/kg) | |
|---|---|---|---|---|---|
| Europe | 783 | 11 | 30 | 6 | 370 |
| North America | 428 | 7 | 47 | 18 | 602 |
| South and Central America | 3928 | 18 | 6 | 2 | 565 |
| Asia | 983 | 27 | 44 | 20 | 478 |
| Africa | 467 | 6 | 45 | 22 | 247 |
| Middle East and North Africa | 11 | 45 | 1 | 1 | 3 |
| Spectroscopic Sensing Technique | Sample Type | Measurement Device | Sensing Performance | References |
|---|---|---|---|---|
| One- and two-photon induced fluorescence spectroscopy | Individual maize kernels | Research-grade laser setup | Classification between 0 µg/kg and 72 µg/kg AFLA | [18,19] |
| SWIR hyperspectral imaging | Bulk kernels | / | Classification accuracies of 70–96% | [17] |
| Fluorescence hyperspectral imaging | Bulk kernels | / | Classification accuracy of 87% for 20 µg/kg AFLA | [17] |
| Fourier-transform NIR + neural network models | Maize powder | Antaris FT-NIR laboratory spectrometer | Root mean square error of prediction = 1.5606 µg/kg for AFLA between 2.5 µg/kg and 41.5 µg/kg | [20] |
| NIR spectroscopy + support vector machines | Ground samples | Research NIR setup 901.78–1661.24 nm | Root mean square error of prediction = 3.5967 µg/kg for AFLA between 2.6 µg/kg and 61 µg/kg. | [21] |
| UV-VIS-NIR reflectance + UV-excited fluorescence + random forest model | Single kernel | Custom research LED setup | Accuracy of 95% for 20 µg/kg AFLA | [22] |
| Raman spectroscopy + support vector machines | Crushed maize | Handheld Raman spectrometer with 785 nm laser | Root mean square error of prediction = 3.5377 µg/kg for AFLA between 2.6 µg/kg and 61 µg/kg. | [23] |
| NIR spectroscopy + deep learning | Ground samples | Custom NIR spectrometer (901.78–1661.24 nm) | Root mean square error of prediction = 1.3691 µg/kg for AFLA between 2.7 µg/kg and 61 µg/kg | [24] |
| Fluorescence spectroscopy + multispectral imaging + linear discriminant analysis | Whole kernels | Spectrofluorimeter with xenon lamp + photomultiplier | Classification between 0 µg/kg and 1475 µg/kg AFLA | [25] |
| NIR hyperspectral imaging + linear discriminant analysis | Individual kernels | In-line laboratory setup using halogen light | Accuracy 88.67–95.56% for AFLA between 0 µg/kg and 100 µg/kg. | [26] |
| VIS-NIR-SWIR reflectance + machine learning | Ground maize | Camera-based lab setup with imaging spectrograph | 82.6–95.7% (cut-off 10 µg/kg) | [27] |
| Fluorescence spectroscopy + support vector machines | Ground maize | UV LED lab setup with imaging spectrograph | 95.7% (cut-off 10 µg/kg) | [27] |
| Raman spectroscopy + support vector machines | Ground maize | Research lab setup using 785 nm laser + ImSpector spectrograph | 87% (cut-off 10 µg/kg) | [27] |
| UV-VIS-NIR spectrometer + partial least squares | Single kernels | Reflectance research grade setup (304 to 1085 nm) | 71, 82 and 92% for 20, 100 and 1000 μg/kg AFLA | [28] |
| Maize Sample | Aflatoxin B1 (µg/kg) | Aflatoxin B2 (µg/kg) | Aflatoxin G1 (µg/kg) | Aflatoxin G2 (µg/kg) |
|---|---|---|---|---|
| Class A | 0 | 0 | 0 | 0 |
| Class B | 0.6 | <Limit of quantification | 0 | 0 |
| Class C | 1527.1 | 120.7 | 0 | 0 |
| Maize Sample | Aflatoxin B1 (µg/kg) | Aflatoxin B2 (µg/kg) | Aflatoxin G1 (µg/kg) | Aflatoxin G2 (µg/kg) |
|---|---|---|---|---|
| Low level | 5.3 ± 2.1 | 1.3 ± 0.5 | <1 | <1 |
| Medium level | 9.5 ± 3.5 | 2.1 ± 0.7 | <1 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smeesters, L.; Kuntzel, T.; Thienpont, H.; Guilbert, L. Handheld Fluorescence Spectrometer Enabling Sensitive Aflatoxin Detection in Maize. Toxins 2023, 15, 361. https://doi.org/10.3390/toxins15060361
Smeesters L, Kuntzel T, Thienpont H, Guilbert L. Handheld Fluorescence Spectrometer Enabling Sensitive Aflatoxin Detection in Maize. Toxins. 2023; 15(6):361. https://doi.org/10.3390/toxins15060361
Chicago/Turabian StyleSmeesters, Lien, Thomas Kuntzel, Hugo Thienpont, and Ludovic Guilbert. 2023. "Handheld Fluorescence Spectrometer Enabling Sensitive Aflatoxin Detection in Maize" Toxins 15, no. 6: 361. https://doi.org/10.3390/toxins15060361
APA StyleSmeesters, L., Kuntzel, T., Thienpont, H., & Guilbert, L. (2023). Handheld Fluorescence Spectrometer Enabling Sensitive Aflatoxin Detection in Maize. Toxins, 15(6), 361. https://doi.org/10.3390/toxins15060361






