Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids
Abstract
1. Introduction
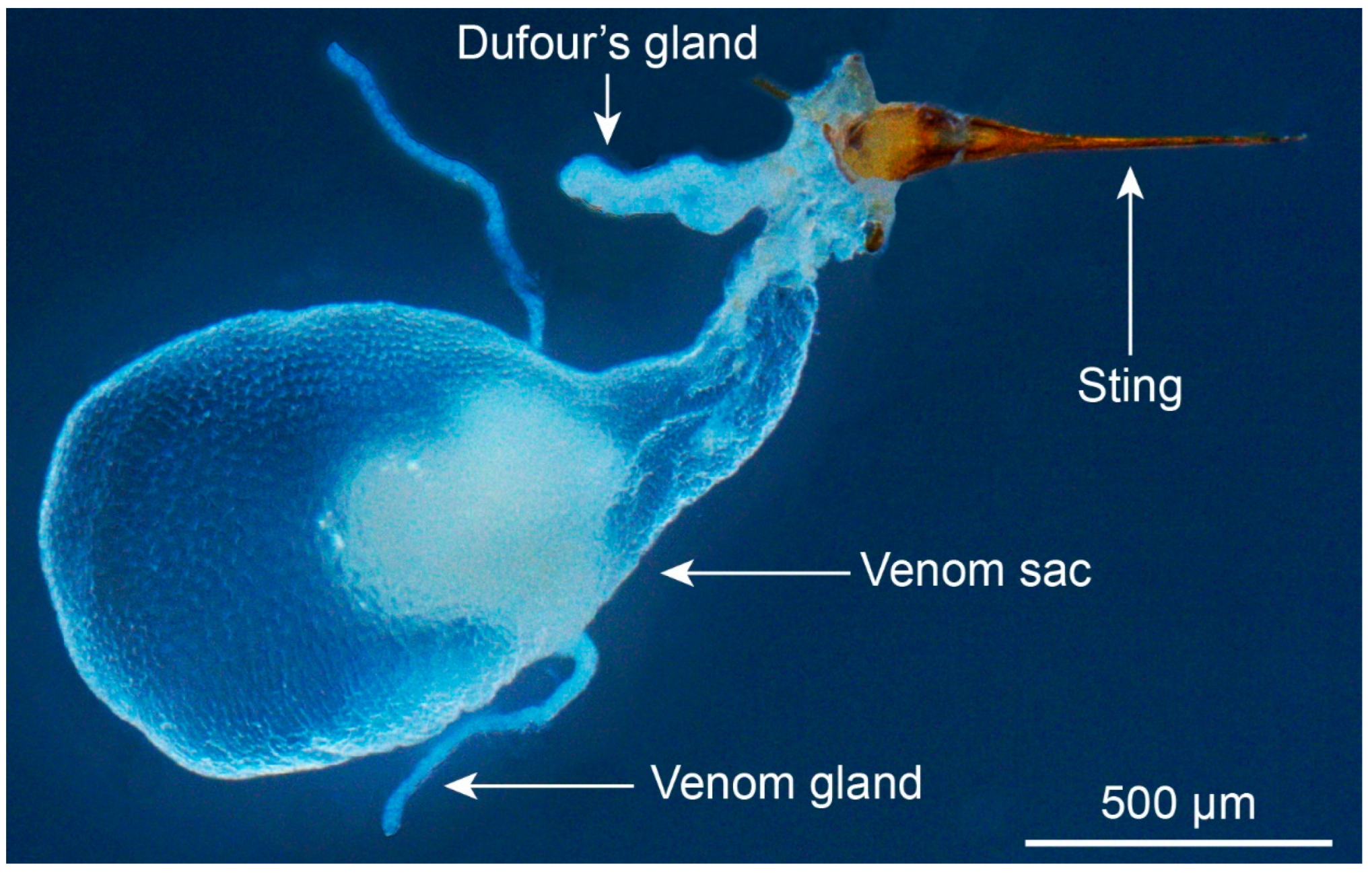
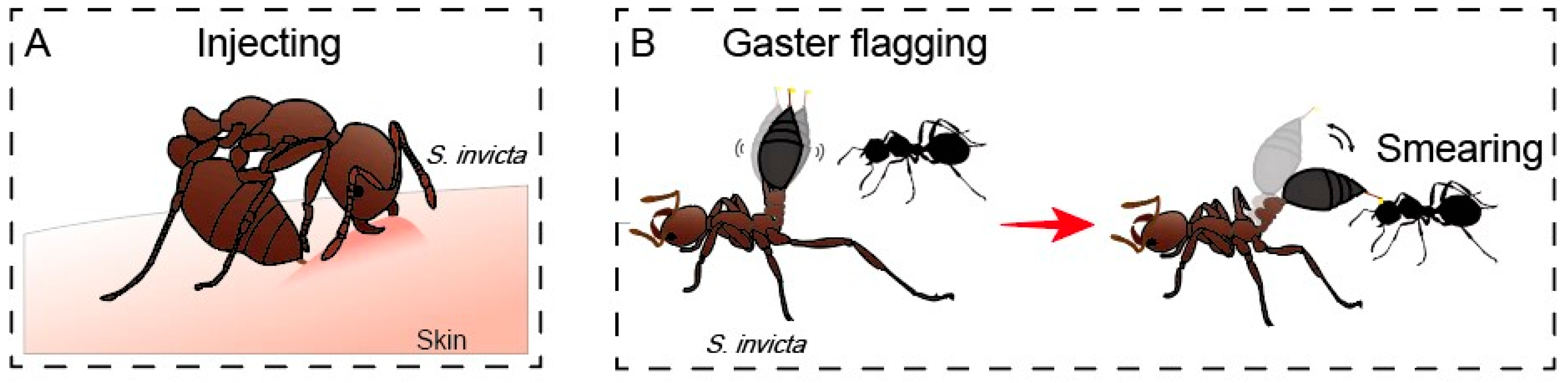
2. Patterns of Venom Use in Fire Ants
3. Alkaloids in Fire Ant Venom
4. Biological Activities of Piperidine Alkaloids from Fire Ant Venom
4.1. Insecticidal Activities
4.2. Bactericidal Activities
4.3. Fungicidal Activity
4.4. Anti-Protozoan Activity
5. Ecological Significance of Venom Alkaloids from Fire Ants
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fry, B.G.; Casewell, N.R.; Wuster, W.; Vidal, N.; Young, B.; Jackson, T.N.W. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.; Scheib, H.; Tyndall, J.; King, G.; Nevalainen, T.; Norman, J.; Lewis, R.; Norton, R.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo Vadis venomics? a roadmap to neglected venomous invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef] [PubMed]
- Wanchoo, A.; Zhang, W.; Ortiz-Urquiza, A.; Boswell, J.; Xia, Y.X.; Keyhani, N.O. Red imported fire ant (Solenopsis invicta) chemosensory proteins are expressed in tissue, developmental, and caste-specific patterns. Front. Physiol. 2020, 11, 585883. [Google Scholar] [CrossRef] [PubMed]
- Touchard, A.; Aili, S.; Fox, E.; Escoubas, P.; Orivel, J.; Nicholson, G.; Dejean, A. The biochemical toxin arsenal from ant venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef]
- Baer, H.; Liu, T.-Y.; Anderson, M.C.; Blum, M.; Schmid, W.H.; James, F.J. Protein components of fire ant venom (Solenopsis invicta). Toxicon 1979, 17, 397–405. [Google Scholar] [CrossRef]
- MacConnell, J.G.; Blum, M.S.; Fales, H.M. The chemistry of fire ant venom. Tetrahedron 1971, 27, 1129–1139. [Google Scholar] [CrossRef]
- Hoffman, D.R. Reactions to less common species of fire ants. J. Allergy Clin. Immunol. 1997, 100, 679–683. [Google Scholar] [CrossRef]
- Srisong, H.; Sukprasert, S.; Klaynongsruang, S.; Daduang, J.; Daduang, S. Identification, expression and characterization of the recombinant Sol g 4.1 protein from the venom of the tropical fire ant Solenopsis geminata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 23. [Google Scholar] [CrossRef]
- MacConnell, J.G.; Blum, M.S.; Fales, H.M. Alkaloid from fire ant venom: Identification and synthesis. Science 1970, 168, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.M. Fire ant venom alkaloids: Their contribution to chemosystematics and biochemical evolution. Biochem. Syst. Ecol. 1978, 6, 337–340. [Google Scholar] [CrossRef]
- Yu, Y.T.; Wei, H.Y.; Fadamiro, H.Y.; Chen, L. Quantitative analysis of alkaloidal constituents in imported fire ants by gas chromatography. J. Agric. Food Chem. 2014, 62, 5907–5915. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Blum, M.S.; Fales, H.M. Ant venom alkaloids from Solenopsis and Monomorium species: Recent developments. Tetrahedron 1982, 38, 1949–1958. [Google Scholar] [CrossRef]
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry in Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 2009, 53, 463–478. [Google Scholar] [CrossRef]
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry in Solenopsis fire ants. II. Identification of novel alkaloids in S. invicta. Toxicon 2009, 53, 479–486. [Google Scholar] [CrossRef]
- MacConnell, J.G.; Blum, M.S.; Buren, W.F.; Williams, R.N.; Fales, H.M. Fire ant venoms: Chemotaxonomic correlations with alkaloidal compositions. Toxicon 1976, 14, 69–78. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Adams, R.M.M. On the biological diversity of ant alkaloids. Annu. Rev. Entomol. 2022, 67, 367–385. [Google Scholar] [CrossRef]
- Wetterer, J.K. Exotic spread of Solenopsis invicta Buren (Hymenoptera: Formicidae) beyond North America. Sociobiology 2013, 60, 50–55. [Google Scholar] [CrossRef]
- Wittman, S.E. Impacts of invasive ants on native ant communities (Hymenoptera: Formicidae). Myrmecol. News 2014, 19, 111–123. [Google Scholar]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.J.; Zeng, L.; Lu, Y.Y. Impact of the red imported fire ant Solenopsis invicta Buren on biodiversity in South China: A review. J. Integr. Agric. 2019, 18, 788–796. [Google Scholar] [CrossRef]
- Obin, M.S.; Vander Meer, R.K. Gaster flagging by fire ants (Solenopsis spp.): Functional significance of venom dispersal behavior. J. Chem. Ecol. 1985, 11, 1757–1768. [Google Scholar] [CrossRef]
- Lai, L.C.; Chang, Y.Y.; Hua, K.H.; Wu, W.J.; Huang, R.N. Comparative toxicity of three fire ant (Hymenoptera: Formicidae) venoms to Spodoptera litura larvae. Sociobiology 2010, 56, 653–663. [Google Scholar] [CrossRef]
- Greenberg, L.; Kabashima, J.N.; Allison, C.J.; Rust, M.K.; Klotz, J.H.; Hurvois, J.P.; Paine, T.D. Lethality of red imported fire ant venom to Argentine ants and other ant species. Ann. Entomol. Soc. Am. 2008, 101, 1162–1168. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Flowers, H.; Rockhold, R.; Herath, H.M.T.B.; Nanayakkara, N.P.D. Antibacterial activity of synthetic fire ant venom: The solenopsins and isosolenopsins. Am. J. Med. Sci. 2009, 338, 287–291. [Google Scholar] [CrossRef]
- Li, S.Z.; Jin, X.X.; Chen, J.; Lu, S.E. Inhibitory activities of venom alkaloids of red imported fire ant against Clavibacter michiganensis subsp. michiganensis in vitro and the application of piperidine alkaloids to manage symptom development of bacterial canker on tomato in the greenhouse. Int. J. Pest Manag. 2013, 59, 150–156. [Google Scholar] [CrossRef]
- King, J.R.; Tschinkel, W.R. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proc. Natl. Acad. Sci. USA 2008, 105, 20339–20343. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Tillberg, C.V.; Suarez, A.V.; Folgarait, P.J.; Smith, C.R.; Holway, D.A. An experimental study of competition between fire ants and Argentine ants in their native range. Ecology 2007, 88, 63–75. [Google Scholar] [CrossRef]
- Morrison, L.W. Mechanisms of interspecific competition among an invasive and two native fire ants. Oikos 2000, 90, 238–252. [Google Scholar] [CrossRef]
- Porter, S.D.; van Eimeren, B.; Gilbert, L.E. Invasion of red imported fire ants (Hymenoptera: Formicidae): Microgeography of competitive replacement. Ann. Entomol. Soc. Am. 1988, 81, 913–918. [Google Scholar] [CrossRef]
- Callahan, P.S.; Blum, M.S.; Walker, J.R. Morphology and histology of the poison glands and sting of the imported fire ant (Solenopsis saevissima v. richteri Forel). Ann. Entomol. Soc. Am. 1959, 52, 573–590. [Google Scholar] [CrossRef]
- Morgan, E.D.; Parry, K.; Tyler, R.C. The chemical composition of the Dufour gland secretion of the ant Myrmica scrabrinodis. Insect Biochem. 1979, 9, 117–121. [Google Scholar] [CrossRef]
- Yang, T.C.; Jianchu, M.; Fu, G.; Zhong, S.; Ding, G.; Ren, Z.; Wei, L.; Lu, L. Ultrastructure of the poison gland in the red imported fire ant, Solenopsis invicta Buren (Hymenoptera: Formicidae). Sociobiology 2007, 50, 343–353. [Google Scholar]
- Wilson, E. Source and possible nature of the odor trail of fire ants. Science 1959, 129, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.G.P.; Bueno, O.C.; Yabuki, A.T.; de Jesus, C.M.; Solis, D.R.; Rossi, M.L.; Nogueira, N.D. General morphology and ultrastructure of the venom apparatus and convoluted gland of the fire ant, Solenopsis saevissima. J. Insect Sci. 2010, 10, 24. [Google Scholar] [CrossRef]
- Haight, K.L.; Tschinkel, W.R. Patterns of venom synthesis and use in the fire ant, Solenopsis invicta. Toxicon 2003, 42, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Haight, K.L. Ontogeny of the defensive stinging behavior of the fire ant, Solenopsis invicta. J. Insect Behav. 2008, 21, 147–152. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Jones, N.T.; Gilbert, L.E. Chemical warfare among invaders: A detoxification interaction facilitates an ant invasion. Science 2014, 343, 1014–1017. [Google Scholar] [CrossRef]
- LeBrun, E.G.; Abbott, J.; Gilbert, L.E. Imported crazy ant displaces imported fire ant, reduces and homogenizes grassland ant and arthropod assemblages. Biol. Invasions 2013, 15, 2429–2442. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.L.; Oi, D.; Grodowitz, M.J. Update on the defensive chemicals of the little black ant, Monomorium minimum (Hymenoptera: Formicidae). Toxicon 2016, 122, 127–132. [Google Scholar] [CrossRef]
- Adams, E.S.; Traniello, J.F.A. Chemical interference competition by Monomorium minimum (Hymenoptera: Formicidae). Oecologia 1981, 51, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.M.M.; Jones, T.H.; Longino, J.T.; Weatherford, R.G.; Mueller, U.G. Alkaloid venom weaponry of three Megalomyrmex thief ants and the behavioral response of Cyphomyrmex costatus host ants. J. Chem. Ecol. 2015, 41, 373–385. [Google Scholar] [CrossRef]
- Fox, E.G.P. Venom toxins of fire ants. In Venom Genomics and Proteomics; Gopalakrishnakone, P., Calvete, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–16. [Google Scholar]
- Chen, J.; Shang, H.W. Advances in research on the venom chemistry of imported fire ants. In Recent Advances in Entomological Research: From Molecular Biology to Pest Management; Liu, T., Kang, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 417–433. [Google Scholar]
- Pianaro, A.; Fox, E.G.P.; Bueno, O.C.; Marsaioli, A.J. Rapid configuration analysis of the solenopsins. Tetrahedron Asymmetry 2012, 23, 635–642. [Google Scholar] [CrossRef]
- Leclercq, S.; Thirionet, I.; Broeders, F.; Daloze, D.; Vander Meer, R.K.; Braekman, J.C. Absolute configuration of the solenopsins, venom alkaloids of the fire ants. Tetrahedron 1994, 50, 8465–8478. [Google Scholar] [CrossRef]
- Araujo, F.D.S.; Botelho, M.A.; Trigo, J.R.; Marsaioli, A.J. Absolute configuration of Solenopsis piperidines is a tool to classify fire ants (Formicidae: Myrmicinae). J. Braz. Chem. Soc. 2018, 29, 398–403. [Google Scholar] [CrossRef]
- Brand, J.M.; Blum, M.S.; Fales, H.M.; MacConnell, J.G. Fire ant venoms: Comparative analyses of alkaloidal components. Toxicon 1972, 10, 259–271. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.L.; Shang, H.W.; Rojas, M.G. Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: Formicidae). J. Agric. Food Chem. 2009, 57, 3128–3133. [Google Scholar] [CrossRef]
- Chen, J.; Shang, H.W.; Jin, X.X. Interspecific variation of Δ1,6-piperideines in imported fire ants. Toxicon 2010, 55, 1181–1187. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.Y.; Hu, Q.B.; Fadamiro, H.Y. Similarity in venom alkaloid chemistry of alate queens of imported fire ants: Implication for hybridization between Solenopsis richteri and S. invicta in the Southern United States. Chem. Biodivers. 2012, 9, 702–713. [Google Scholar] [CrossRef]
- Hill, R.K.; Yuri, T. An approach to natural 2-alkyl-6-methylpiperidines via N-acyllactam rearrangement. Tetrahedron 1977, 33, 1569–1571. [Google Scholar] [CrossRef]
- Jefford, C.W.; Wang, J.B. An enantiospecific synthesis of solenopsin A. Tetrahedron Lett. 1993, 34, 2911–2914. [Google Scholar] [CrossRef]
- Blum, M.S.; Brand, J.M.; Duffield, R.M.; Snelling, R.R. Chemistry of the venom of Solenopsis aurea (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 1973, 66, 702. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Q.B.; Fadamiro, H.Y. Reduction of venom alkaloids in Solenopsis richteri × Solenopsis invicta hybrid: An attempt to identify new alkaloidal components. J. Agric. Food Chem. 2010, 58, 11534–11542. [Google Scholar] [CrossRef]
- Leclercq, S.; Braekman, J.C.; Daloze, D.; Pasteels, J.M.; Vander Meer, R.K. Biosynthesis of the solenopsins, venom alkaloids of the fire ants. Naturwissenschaften 1996, 83, 222–225. [Google Scholar] [CrossRef]
- Shi, Q.H.; Hu, L.; Wang, W.K.; Vander Meer, R.K.; Porter, S.D.; Chen, L. Workers and alate queens of Solenopsis geminata share qualitatively similar but quantitatively different venom alkaloid chemistry. Front. Ecol. Evol. 2015, 3, 76. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Li, X.C.; Zhao, J.H. Pyridine alkaloids in the venom of imported fire ants. J. Agric. Food Chem. 2019, 67, 11388–11395. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Chinta, S.P.; Jones, T.H. Novel alkaloids from the fire ant, Solenopsis geminata. Sci. Nat. 2022, 109, 15. [Google Scholar] [CrossRef]
- Tankersley, M.S. The stinging impact of the imported fire ant. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 354–359. [Google Scholar] [CrossRef]
- Xu, Y.J.; Huang, J.; Zhou, A.M.; Zeng, L. Prevalence of Solenopsis invicta (Hymenoptera: Formicidae) venom allergic reactions in mainland China. Fla. Entomol. 2012, 95, 961–965. [Google Scholar] [CrossRef]
- Blum, M.S.; Walder, J.R.; Callahan, P.S. Chemical, insecticidal, and antibiotic properties of fire ant venom. Science 1958, 128, 307–308. [Google Scholar] [CrossRef]
- Sannasi, A.; Blum, M. Pathological effects of fire ant venom on the integument and blood of house fly larvae. J. Ga. Entomol. Soc. 1969, 4, 103–110. [Google Scholar]
- Lai, L.-C.; Kuo, T.-C.; Huang, R.-N.; Wu, W.-J. The insecticidal activities of fire ant (Hymenoptera: Formicidae) venoms against Plutella xylostella (Lepidoptera: Plutellidae) larvae. J. Econ. Entomol. 2012, 105, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.C.; Hua, K.H.; Yang, C.C.; Huang, R.N.; Wu, W.J. Secretion profiles of venom alkaloids in Solenopsis geminata (Hymenoptera: Formicidae) in Taiwan. Environ. Entomol. 2009, 38, 879–884. [Google Scholar] [CrossRef]
- Lai, L.-C.; Huang, R.-N.; Wu, W.-J. Venom alkaloids of monogyne and polygyne forms of the red imported fire ant, Solenopsis invicta, in Taiwan. Insectes Sociaux 2008, 55, 443–449. [Google Scholar] [CrossRef]
- Brand, J.M.; Blum, M.S.; Ross, H.H. Biochemical evolution in fire ant venoms. Insect Biochem. 1973, 3, 45–51. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Wu, X.; Wang, L.; Chen, L.; Lu, Y.-Y.; Xu, Y.J. Queen venom isosolenopsin a delivers rapid incapacitation of fire ant competitors. Toxicon 2019, 158, 77–83. [Google Scholar] [CrossRef]
- Blum, M.S. Biocidal and deterrent activities of nitrogen heterocycles produced by venomous myrmicine ants. In Biologically Active Products; Cutler, H.G., Ed.; ACS Publications: Washington, DC, USA, 1988; pp. 438–449. [Google Scholar]
- Rashid, T.; Chen, J.; McLeod, P. Toxicity of newly isolated piperideine alkaloids from the red imported fire ant, Solenopsis invicta Buren, against the green peach aphid, Myzus persicae (Sulzer). Adv. Entomol. 2013, 1, 20–23. [Google Scholar] [CrossRef]
- Wu, X.; Wang, G.; Xu, G.; Chen, L. Synthesis and insecticidal activity of fire ant venom alkaloid-based 2-methyl-6-alkyl-Δ1,6-piperideines. Molecules 2022, 27, 1107. [Google Scholar] [CrossRef]
- Blum, M.S.; Everett, D.M.; Jones, T.H.; Fales, H.M. Arthropod natural products as insect repellents. In Naturally Occurring Pest Bioregulators; Hedin, P.A., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1991; Volume 449, pp. 14–26. [Google Scholar]
- Jouvenaz, D.P.; Blum, M.S.; MacConnell, J.G. Antibacterial activity of venom alkaloids from the imported fire ant, Solenopsis invicta Buren. Antimicrob. Agents Chemother. 1972, 2, 291–293. [Google Scholar] [CrossRef]
- Park, J.; Kaufmann, G.F.; Bowen, J.P.; Arbiser, J.L.; Janda, K.D. Solenopsin A, a venom alkaloid from the fire ant Solenopsis invicta, inhibits quorum-sensing signaling in Pseudomonas aeruginosa. J. Infect. Dis. 2008, 198, 1198–1201. [Google Scholar] [CrossRef]
- Xu, D.; Jia, R.; Li, Y.; Gu, T. Advances in the treatment of problematic industrial biofilms. World J. Microbiol. Biotechnol. 2017, 33, 97. [Google Scholar] [CrossRef] [PubMed]
- Hinsa, S.M.; Espinosa-Urgel, M.; Ramos, J.; O’Toole, G. Transition from reversible to irreversible attachment during biofilm formation by Pseudomonas fluorescens WCS365 requires an ABC transporter and a large secreted protein. Mol. Microbiol. 2003, 49, 905–918. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, D.B.; Fox, E.G.P.; dos Santos, D.G.; de Sousa, J.S.; Freire, D.M.G.; Nogueira, F.C.S.; Domont, G.B.; de Castilho, L.V.A.; Machado, E.D. Fire ant venom alkaloids inhibit biofilm formation. Toxins 2019, 11, 420. [Google Scholar] [CrossRef]
- Kumar, S.; Paliya, B.S.; Singh, B.N. Superior inhibition of virulence and biofilm formation of Pseudomonas aeruginosa PAO1 by phyto-synthesized silver nanoparticles through anti-quorum sensing activity. Microb. Pathog. 2022, 170, 105678. [Google Scholar] [CrossRef]
- Yan, Y.; An, Y.; Wang, X.; Chen, Y.; Jacob, M.R.; Tekwani, B.L.; Dai, L.; Li, X.-C. Synthesis and antimicrobial evaluation of fire ant venom alkaloid based 2-methyl-6-alkyl-Δ1,6-piperideines. J. Nat. Prod. 2017, 80, 2795–2798. [Google Scholar] [CrossRef]
- Dawadi, S.; Baysal-Gurel, F.; Addesso, K.M.; Liyanapathiranage, P.; Simmons, T. Fire ant venom alkaloids: Possible control measure for soilborne and foliar plant pathogens. Pathogens 2021, 10, 659. [Google Scholar] [CrossRef]
- Storey, G.K.; Vander Meer, R.K.; Boucias, D.G.; McCoy, C.W. Effect of fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J. Invertebr. Pathol. 1991, 58, 88–95. [Google Scholar] [CrossRef]
- Blum, M.S. Alkaloidal ant venoms: Chemistry and biological activities. In Bioregulators for Pest Control; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 276, pp. 393–408. [Google Scholar]
- Li, S.Z.; Jin, X.X.; Chen, J. Effects of piperidine and piperideine alkaloids from the venom of red imported fire ants, Solenopsis invicta Buren, on Pythium ultimum Trow growth in vitro and the application of piperideine alkaloids to control cucumber damping-off in the greenhouse. Pest Manag. Sci. 2012, 68, 1546–1552. [Google Scholar] [CrossRef]
- Dai, L.; Jacob, M.; Khan, S.; Khan, I.; Clark, A.; Li, X.C. Synthesis and antifungal activity of natural product-based 6-alkyl-2,3,4,5-tetrahydropyridines. J. Nat. Prod. 2011, 74, 2023–2026. [Google Scholar] [CrossRef]
- Silva, R.C.M.C.; Fox, E.G.P.; Gomes, F.M.; Feijo, D.F.; Ramos, I.; Koeller, C.M.; Costa, T.F.R.; Rodrigues, N.S.; Lima, A.P.; Atella, G.C.; et al. Venom alkaloids against Chagas disease parasite: Search for effective therapies. Sci. Rep. 2020, 10, 16. [Google Scholar] [CrossRef]
- Fox, E.G.P.; Pianaro, A.; Solis, D.R.; Delabie, J.H.C.; Vairo, B.C.; Machado, E.A.M.; Bueno, O.C. Intraspecific and intracolonial variation in the profile of venom alkaloids and cuticular hydrocarbons of the fire ant Solenopsis saevissima Smith (Hymenoptera: Formicidae). Psyche 2012, 2012, 398061. [Google Scholar] [CrossRef]
- Chen, L.; Sharma, K.R.; Fadamiro, H.Y. Fire ant venom alkaloids act as key attractants for the parasitic phorid fly, Pseudacteon tricuspis (Diptera: Phoridae). Naturwissenschaften 2009, 96, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Vander Meer, R.K.; Morel, L. Ant queens deposit pheromones and antimicrobial agents on eggs. Naturwissenschaften 1995, 82, 93–95. [Google Scholar] [CrossRef]
- Yang, C.C.; Yu, Y.C.; Valles, S.M.; Oi, D.H.; Chen, Y.C.; Shoemaker, D.; Wu, W.J.; Shih, C.J. Loss of microbial (pathogen) infections associated with recent invasions of the red imported fire ant Solenopsis invicta. Biol. Invasions 2010, 12, 3307–3318. [Google Scholar] [CrossRef]
- Zettler, J.A.; McInnis, T.M.; Allen, C.R.; Spira, T.P. Biodiversity of fungi in red imported fire ant (Hymenoptera: Formicidae) mounds. Ann. Entomol. Soc. Am. 2002, 95, 487–491. [Google Scholar] [CrossRef]
- Gunawan, S.; Tufts, D.M.; Bextine, B.R. Molecular identification of hemolymph-associated symbiotic bacteria in red imported fire ant larvae. Curr. Microbiol. 2008, 57, 575–579. [Google Scholar] [CrossRef]
- Vinson, S.B. Invasion of the red imported fire ant (Hymenoptera: Formicidae): Spread, biology, and impact. Am. Entomol. 1997, 43, 23–39. [Google Scholar] [CrossRef]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guenard, B.; Lewis, O.T. The cryptic impacts of invasion: Functional homogenization of tropical ant communities by invasive fire ants. Oikos 2020, 129, 585–597. [Google Scholar] [CrossRef]
- Barbieri, R.F.; Grangier, J.; Lester, P.J. Arrival sequence and diet mediate interspecific competition in an ant community. Insectes Sociaux 2013, 60, 463–473. [Google Scholar] [CrossRef]
- Westermann, F.L.; McPherson, L.S.; Jones, T.H.; Milicich, L.; Lester, P.J. Toxicity and utilization of chemical weapons: Does toxicity and venom utilization contribute to the formation of species communities? Ecol. Evol. 2015, 5, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Wilder, S.M.; Barnum, T.R.; Holway, D.A.; Suarez, A.V.; Eubanks, M.D. Introduced fire ants can exclude native ants from critical mutualist-provided resources. Oecologia 2013, 172, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Tschinkel, W.R. The Fire Ants; Harvard University Press: Cambridge, MA, USA, 2006; p. 723. [Google Scholar]
- Caldwell, J. The evolution of myrmecophagy and its correlates in poison frogs (Family Dendrobatidae). J. Zool. 2009, 240, 75–101. [Google Scholar] [CrossRef]
- Coleman, J.L.; Cannatella, D.C. How phylogenetics can elucidate the chemical ecology of poison frogs and their arthropod prey. J. Chem. Ecol. 2022, 48, 384–400. [Google Scholar] [CrossRef]
- Daly, J.W.; Spande, T.F.; Garraffo, H.M. Alkaloids from amphibian skin: A tabulation of over eight-hundred compounds. J. Nat. Prod. 2005, 68, 1556–1575. [Google Scholar] [CrossRef]
- Davison, I.; Saporito, R.A.; Schulte, L.M.; Summers, K. Piperidine alkaloids from fire ants are not sequestered by the green and black poison frog (Dendrobates auratus). Chemoecology 2021, 31, 391–396. [Google Scholar] [CrossRef]
- Ajayi, O.S.; Chen, L.; Fadamiro, H.Y. Host preference in parasitic phorid flies: Response of Pseudacteon curvatus and P. obtusus to venom alkaloids of native and imported Solenopsis fire ants. Chemoecology 2020, 30, 197–204. [Google Scholar] [CrossRef]
- Chen, L.; Fadamiro, H.Y. Pseudacteon phorid flies: Host specificity and impacts on Solenopsis fire ants. Annu. Rev. Entomol. 2018, 63, 47–67. [Google Scholar] [CrossRef]
- Chen, L.; Porter, S.D. Biology of Pseudacteon decapitating flies (Diptera: Phoridae) that parasitize ants of the Solenopsis saevissima complex (Hymenoptera: Formicidae) in South America. Insects 2020, 11, 107. [Google Scholar] [CrossRef]
- Chen, L.; Morrison, L.W. Importation biological control of invasive fire ants with parasitoid phorid flies-progress and prospects. Biol. Control 2021, 154, 104509. [Google Scholar] [CrossRef]
- Arbiser, J.L.; Kau, T.; Konar, M.; Narra, K.; Ramchandran, R.; Summers, S.A.; Vlahos, C.J.; Ye, K.Q.; Perry, B.N.; Matter, W.; et al. Solenopsin, the alkaloidal component of the fire ant (Solenopsis invicta), is a naturally occurring inhibitor of phosphatidylinositol-3-kinase signaling and angiogenesis. Blood 2007, 109, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Arbiser, J.L.; Nowak, R.; Michaels, K.; Skabytska, Y.; Biedermann, T.; Lewis, M.J.; Bonner, M.Y.; Rao, S.; Gilbert, L.C.; Yusuf, N.; et al. Evidence for biochemical barrier restoration: Topical solenopsin analogs improve inflammation and acanthosis in the KC-Tie2 mouse model of psoriasis. Sci. Rep. 2017, 7, 11198. [Google Scholar] [CrossRef] [PubMed]
| Name | Structure | Name | Structure |
|---|---|---|---|
| cis Piperidines | trans Piperidines | ||
| cis-C7 | 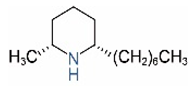 | ||
| cis-C9:1 | 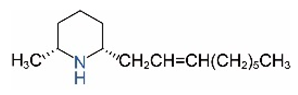 | ||
| cis-C9 | 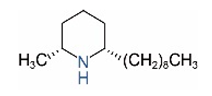 | ||
| cis-C11:1 a | 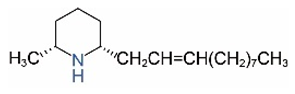 | trans-C11:1 | 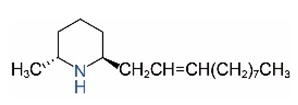 |
| cis-C11 |  | trans-C11 | 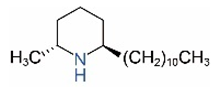 |
| cis-C13:1 | 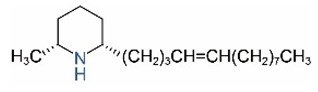 | trans-C13:1 |  |
| cis-C13 | 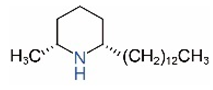 | trans-C13 | 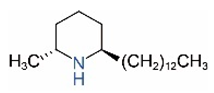 |
| cis-C15:1 | 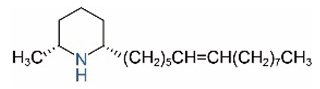 | trans-C15:1 |  |
| cis-C15 | 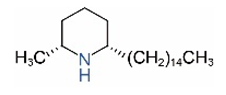 | trans-C15 |  |
| cis-C17:1 |  | trans-C17:1 | 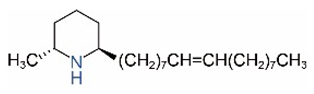 |
| cis-C17 | 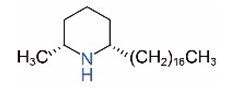 | trans-C17 | 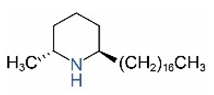 |
| Piperideines | |||
| Δ1,2-C11 |  | Δ1,6-C11 |  |
| Δ1,2-C13:1 | 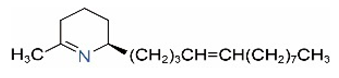 | Δ1,6-C13:1 | 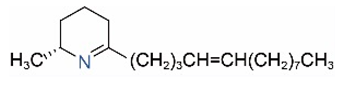 |
| Δ1,2-C13 | 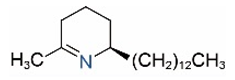 | Δ1,6-C13 | 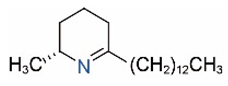 |
| Δ1,2-C15:1 |  | Δ1,6-C15:1 | 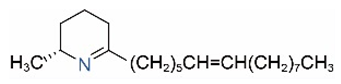 |
| Δ1,2-C15 | 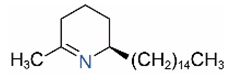 | Δ1,6-C15 | 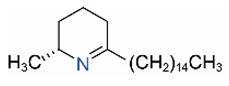 |
| Δ1,2-C17:1 | 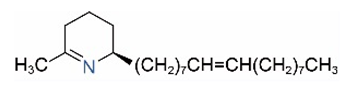 | Δ1,6-C17:1 | 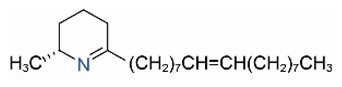 |
| Δ1,2-C17 | 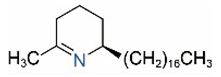 | Δ1,6-C17 | 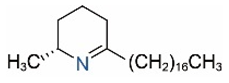 |
| Pyridines | |||
| 6UP11 |  | ||
| 2M6UP11 |  | 2M6UP11:1 | 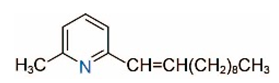 |
| 2M6TP13 | 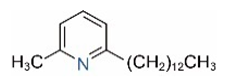 | 2M6TP13:1 | 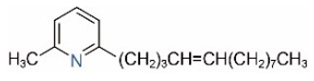 |
| 2M6PP15 | 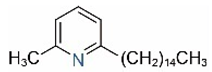 | 2M6PP15:1 |  |
| Structure | Termite | Ant * | Caterpillar | Bacteria | Fungus |
|---|---|---|---|---|---|
| Saturated alkaloids, side carbon chain length ↑ | ↓ | ↑ | ↓ | ||
| Unsaturated alkaloids, side carbon chain length ↑ | ↓ | ||||
| cis-Isomers → trans-isomers | ↑ | ↑ | |||
| Addition of a double bond to the side carbon chain | ↑ | ||||
| Piperideine alkaloids, side carbon chain length ↑ | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Chen, L. Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids. Toxins 2023, 15, 439. https://doi.org/10.3390/toxins15070439
Xu G, Chen L. Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids. Toxins. 2023; 15(7):439. https://doi.org/10.3390/toxins15070439
Chicago/Turabian StyleXu, Guangxin, and Li Chen. 2023. "Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids" Toxins 15, no. 7: 439. https://doi.org/10.3390/toxins15070439
APA StyleXu, G., & Chen, L. (2023). Biological Activities and Ecological Significance of Fire Ant Venom Alkaloids. Toxins, 15(7), 439. https://doi.org/10.3390/toxins15070439





