Abstract
Consumption coagulopathy and hemorrhagic syndrome exacerbated by blood anticoagulability remain the most important causes of lethality associated with Bothrops snake envenomation. Bothrops venom also engages platelet aggregation on the injured endothelium via von Willebrand factor (vWF) interactions. Besides platelet aggregation, some Bothrops venom toxins may induce qualitative thrombopathy, which has been in part related to the inhibition of vWF activation. We tested whether B. lanceolatus venom impaired vWF to collagen(s) binding (vWF:CB) activity. Experiments were performed with B. lanceolatus crude venom, in the presence or absence of Bothrofav, a monospecific B. lanceolatus antivenom. Venom of B. lanceolatus fully inhibited vWF to collagen type I and III binding, suggesting venom interactions with the vWF A3 domain. In contrast, B. lanceolatus venom increased vWF to collagen type VI binding, suggesting the enhancement of vWF binding to collagen at the vWF A1 domain. Hence, B. lanceolatus venom exhibited contrasting in vitro effects in terms of the adhesive properties of vWF to collagen. On the other hand, the antivenom Bothrofav reversed the inhibitory effects of B. lanceolatus venom on vWF collagen binding activity. In light of the respective distribution of collagen type III and collagen type VI in perivascular connective tissue and the sub-endothelium, a putative association between an increase in vWF:CB activity for collagen type VI and the onset of thrombotic events in human B. lanceolatus envenomation might be considered.
Keywords:
Bothrops snake; B. lanceolatus; envenomation; von Willebrand factor; collagen binding activity Key Contribution:
vWF collagen binding (vWF:CB) activities are largely inhibited by B. lanceolatus venom incubation. Bothrofav reversed the inhibitory effects of B. lanceolatus venom on vWF:CB activity. An increase in vWF antigen (vWF:Ag) levels might be related to the proteolytic cleavage of vWF by B. lanceolatus venom, potentially reducing collagen binding activities.
1. Introduction
Features of human Bothrops spp. snake envenomation include marked local damage and, in some severe cases, abnormal hemostasis with systemic hemorrhage resulting from the synergistic action of several venom toxins [1,2]. The most toxic constituents of Bothrops spp. venom involved in impaired hemostasis and hemorrhage, include snake venom serine proteases (SVSPs), snake venom metalloproteinases (SVMPs), phospholipase A2 (PLA2), disintegrins (DIS), and C-type lectin-like proteins (CTL) [1]. Along with coagulation factor consumption and hemorrhagic activity, venom toxins can alter several platelet functions, including adhesion to the sub-endothelial extracellular matrix and aggregation, which may lead to initial clot or thrombus formation [3,4,5,6,7]. Of note, multistage processes that engage platelet aggregation have been described in Bothrops spp. envenomation [4].
Upon vascular injury, platelets initially adhere transiently to the sub-endothelial von Willebrand factor through interaction with the glycoprotein Ibα (GPIbα) platelet receptor. The von Willebrand factor (vWF) is a massive, shear-sensitive, homopolymeric protein that is critically involved in the initiation of platelet adhesion at high shear [8]. The shear-sensitive A1-A2-A3 region of vWF is important for platelet adhesion because this region contains the binding sites for glycoprotein Ibα and collagen structures [9,10]. In the extracellular matrix, collagen type I and collagen type III constitute the major part of the interstitial matrix [11,12,13]. Collagen type III is found extensively in connective tissues such as skin, lung, liver, intestine, and the perivascular connective tissue [12]. Collagen type III serves as a ligand for several proteins, such as the G-protein coupled receptor-56, von Willebrand factor, and α2β1 integrin [12]. Collagen type VI is a unique beaded filament collagen found in the interface between the basement membrane and interstitial matrix of many tissues, including the dermis, skeletal muscle, kidney, cornea, tendon, skin, cartilage, intervertebral discs, adipose tissue, and blood vessels [13]. Collagen type VI has a fundamental function anchoring endothelial basement membranes by interacting with collagen type IV, and also serves as a ligand for several proteins including biglycan, decorin, perlecan, neural/glial antigen 2 proteoglycan, fibronectin, tenascin, and α2β1 integrin [13].
Overall, vWF has a central role in primary hemostasis where it mediates platelet adhesion to damaged vascular sub-endothelium, and subsequently initiates platelet aggregation. Following a vascular injury, vWF binds specifically to fibrillar collagen. Binding sites for fibrillar collagen have been identified within vWF domains A1 and A3, although mutagenesis studies suggest that the major site in domain A3 and the minor site in domain A1 interact with different targets on collagen [9,10]. The vWF A3 domain is necessary and sufficient to support binding to fibrillar collagen types I and III, while the A1 domain is involved in binding to collagen type VI [9,10]. During platelet aggregation processes, vWF binds rapidly to exposed collagen vessel structures and enables platelet arrest from fast-flowing blood through the interaction of its A1 domain with the platelet GPIbα receptor. Rotational force imposed by flowing blood causes platelets to translocate over immobilized vWF until α2β integrin receptors engage their respective ligands and mediate permanent adhesion, spreading, and aggregation [8]. Collagen binding (vWF:CB) assays, usually performed using collagen(s) to capture vWF, determine quantitative binding [9,10]. These assays are initially diagnostic tests that quantify the binding capacity of vWF -A1 and -A3 domains to collagen (the main sub-endothelial matrix component), in order to improve the diagnosis and differentiation of the qualitative variant of von Willebrand disease, the most common inherited bleeding disorder [9,10].
Interactions between platelet function and snake venom constituents are multiple and complex. Thrombocytin, an SVSP from B. atrox venom, can induce platelet adhesion in vitro via calcium mobilization [14]. Bothrombin, an SVSP from B. jararaca, activates platelet aggregation in vitro by interacting with platelet GPIbα receptor in the presence of exogenous fibrinogen [15]. Bothrops PLA2 from B. jararacussu, such as bothropstoxin, is able to induce platelet aggregation through multiple signal transduction pathways, including thromboxane A2 formation and activation of protein kinase cascades [16]. Botrocetin, a CTL from B. jararaca venom, and aspercetin, a CTL from B. asper venom, can induce platelet aggregation in the presence of vWF, promoting its interaction with platelet GPIbα receptor [6,17,18,19]. Besides the activation of platelet aggregation, Bothrops spp. venom toxins may also induce qualitative thrombopathy [2]. For example, bothrasperin, a disintegrin from B. asper venom elicits platelet aggregation induced by collagen and ADP, thus altering the interaction of fibrinogen with the platelet integrin α2β3 receptor [20]. In human B. jararaca envenomation, platelets harvested from circulating blood display hypoaggregation to ristocetin and collagen [21]. Such antiaggregant properties have been attributed to the inhibitory effects of jararhagin, a P-III-type SVMP of B. jararaca, on vWF-to-collagen binding and its interaction with GPIb and the α2-subunit I domain of the platelet surface α2β integrin [22,23]. Atroxlysin-I and atroxlysin III (two SVMPs from B. atrox) and basparin A (an SVMP from B. asper) also inhibit collagen-dependent platelet aggregation independently of their proteolytic activities [24,25]. As a distinct mechanism, the proteolytic degradation of vWF by SVMPs [26,27,28], especially with the loss of high molecular weight bands and the increase in low molecular weight fragments, can further determine thrombopathy in Bothrops spp. envenomation [18,29,30].
In some cases of Bothrops spp. envenomation, platelet activation is associated with microthrombi formation, i.e., thrombotic microangiopathy [31,32,33,34,35,36]. The latter is usually observed in Bothrops spp. envenomation associated with consumption coagulopathy, which is typically marked by prolonged clotting times, clotting factor deficiencies (i.e., hypofibrinogenemia, low factor V, and low factor VIII), and elevated D-dimer [31,32,33,34,35,36]. Snakebite-associated thrombotic microangiopathy presents with red blood cell fragments (schistocytes) on the peripheral blood film and delayed phase thrombocytopenia [31,32,33,34,35,36]. Histologically, small vessel wall injury and micro-thrombosis are typically described and may lead to end-organ ischemia [31,32,33,34,35,36]. Multiple macro-thrombotic events, such as fatal pulmonary embolism, myocardial infarction, and cerebral ischemic stroke, have also been reported in human B. lanceolatus envenomation in the absence of thrombotic microangiopathy [37,38,39,40]. The pathogenesis of these thrombotic events still has to be fully elucidated. Proposed mechanisms include the switch of the endothelium to a prothrombotic phenotype with the overexpression of tissue factor, cytokines, and adhesion molecules, along with the activation of platelets, complement, and coagulation cascade [37,38,39,40].
The potential role of B. lanceolatus venom (frequent trigger of pro-thrombotic events) in inducing changes in vWF collagen binding (vWF:CB) activity has not been previously explored. The main objective of the present study was to test whether crude venom of B. lanceolatus, captured in the wild on the French Caribbean island of Martinique, induces in vitro changes in the adhesive properties of vWF on collagen(s), and whether the monospecific antivenom Bothrofav prevents venom-induced alterations of vWF:CB activities.
2. Results
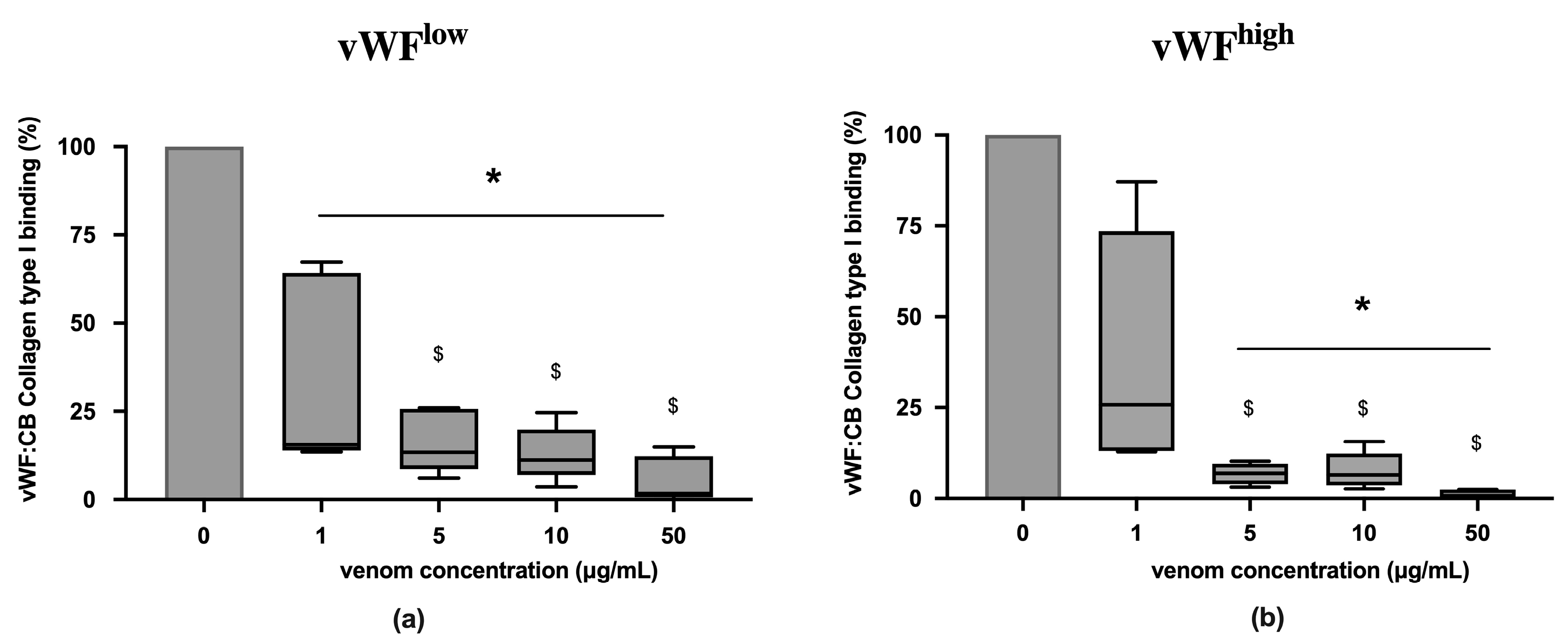
In conditions of low vWF concentrations (vWFlow) and high vWF concentrations (vWFhigh), vWF collagen type I binding (vWF:CB) activities were partially inhibited in the presence of B. lanceolatus venom at the concentration 1 µg/mL (Figure 1a,b). Venom concentrations above 1 µg/mL fully inhibited vWF collagen type I binding (vWF:CB) activities in vWFlow and vWFhigh conditions (Figure 1a,b). Experiments with concentrations below 1 µg/mL were not performed.
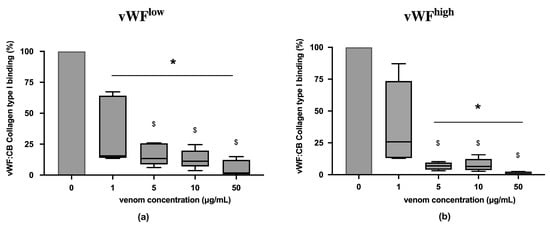
Figure 1.
(a) Effects of B. lanceolatus venom on vWF collagen type I binding activity (vWF:CB collagen type I binding), expressed as percent of control, in conditions of low vWF concentrations (vWFlow); (b) Effects of B. lanceolatus venom on vWF collagen type I binding activity (vWF:CB collagen type I binding) in conditions of high vWF concentrations (vWFhigh). Data are displayed as box plots (median, whiskers min–max) of 5–8 independent experiments. * indicates statistical difference (p < 0.05) with control (phosphate saline buffer, PBS). $ indicates statistical difference (p < 0.05) with venom concentration of 1 µg/mL.
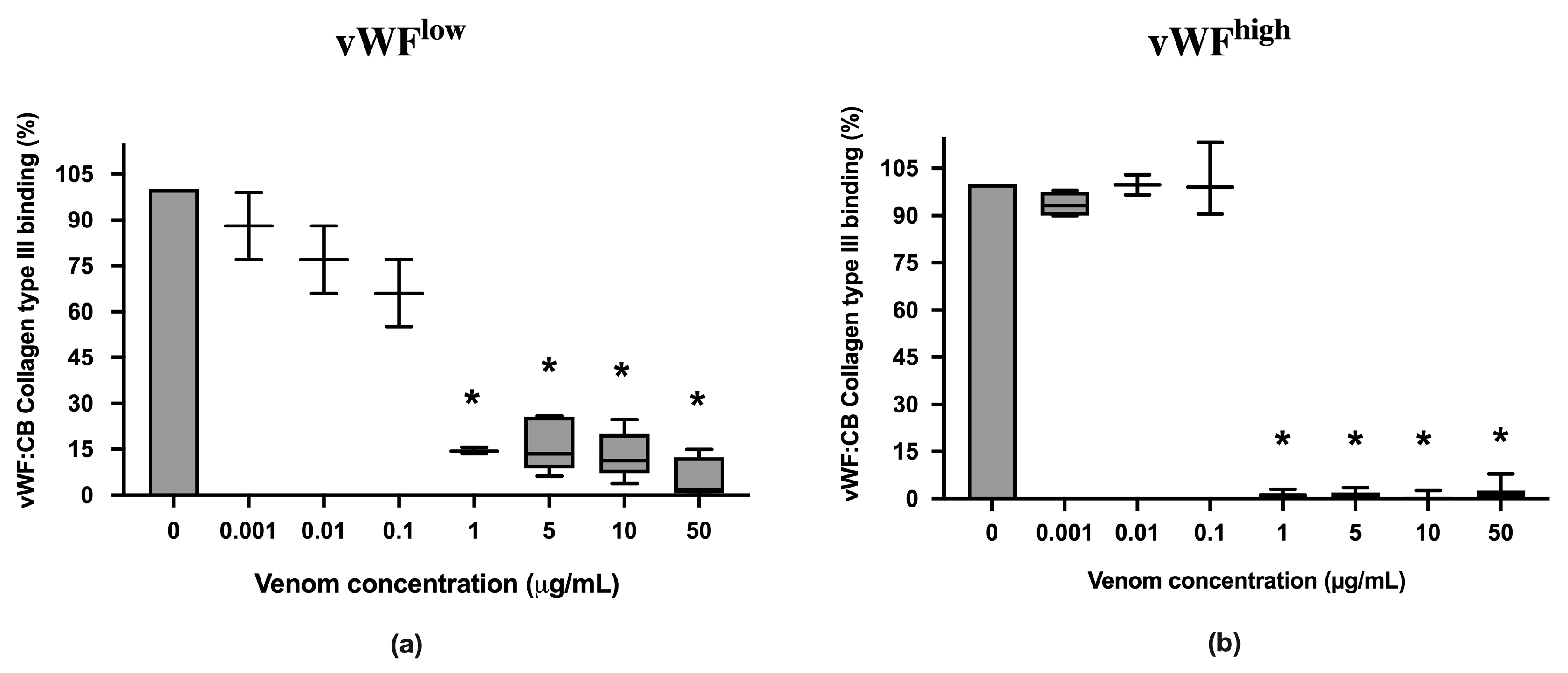
vWF collagen type III binding (vWF:CB) activities in conditions of low vWF concentrations (vWFlow) and high vWF concentrations (vWFhigh) were not inhibited in the presence of B. lanceolatus venom concentrations under 1 µg/mL (Figure 2a,b). Conversely, venom concentrations at 1 µg/mL and above fully inhibited vWF collagen type III binding (vWF:CB) activities in vWFlow and vWFhigh conditions (Figure 2a,b).
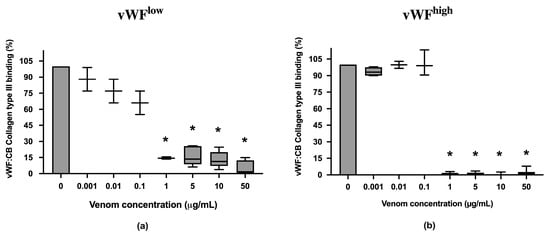
Figure 2.
(a) Effects of B. lanceolatus venom on vWF collagen type III binding activity (vWF:CB collagen type III binding), expressed as percent of control, in conditions of low vWF concentrations (vWFlow); (b) Effects of B. lanceolatus venom on vWF collagen type III binding activity (vWF:CB collagen type III binding) expressed as percent of control in conditions of high vWF concentrations (vWFhigh). Data are displayed as box plots (median, whiskers min–max) of 5–8 independent experiments. * indicates statistical difference (p < 0.05) with control (phosphate saline buffer, PBS).
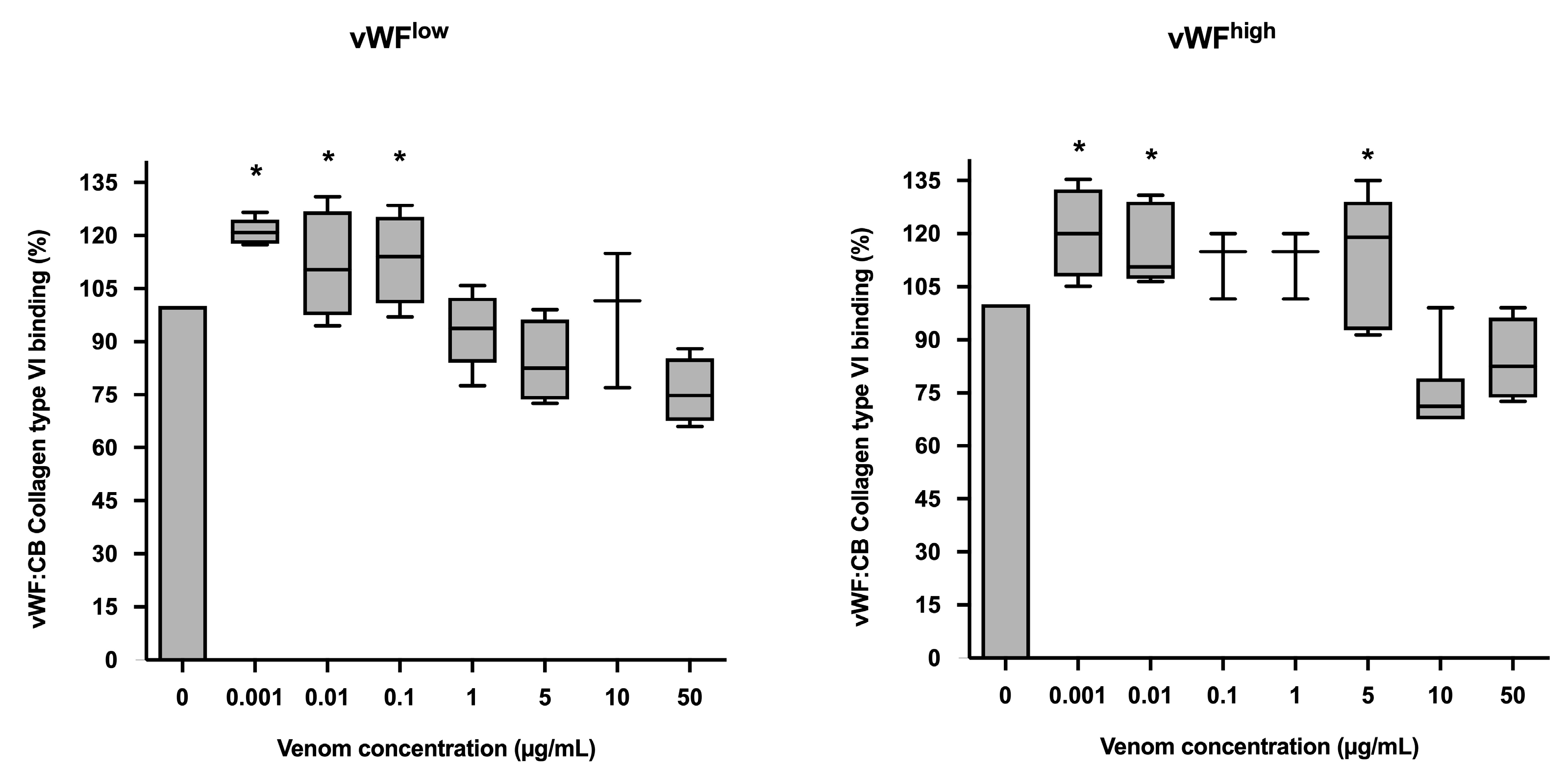
In conditions of low vWF concentrations (vWFlow), vWF collagen type VI binding (vWF:CB) activities were increased in the presence of B. lanceolatus venom at concentrations of 0.001, 0.01, and 0.1 µg/mL, while venom concentrations of 1 µg/mL and above had no effect. In conditions of high vWF concentrations (vWFhigh), venom concentrations of 0.001, 0.01, and 5 µg/mL were associated with increases in vWF collagen type VI binding (vWF:CB) activity (Figure 3a,b).
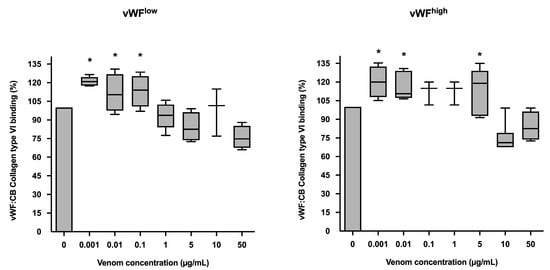
Figure 3.
(a) Effects of B. lanceolatus venom on vWF collagen type VI binding activity (vWF:CB collagen type VI binding), expressed as percent of control, in conditions of low vWF concentrations (vWFlow); (b) Effects of B. lanceolatus venom on vWF collagen type VI binding activity (vWF:CB collagen type VI binding) expressed as percent of control in conditions of high vWF concentrations (vWFhigh). Data are displayed as box plots (median, whiskers min–max) of 5–8 independent experiments. * indicates statistical difference (p < 0.05) with control (phosphate saline buffer, PBS).
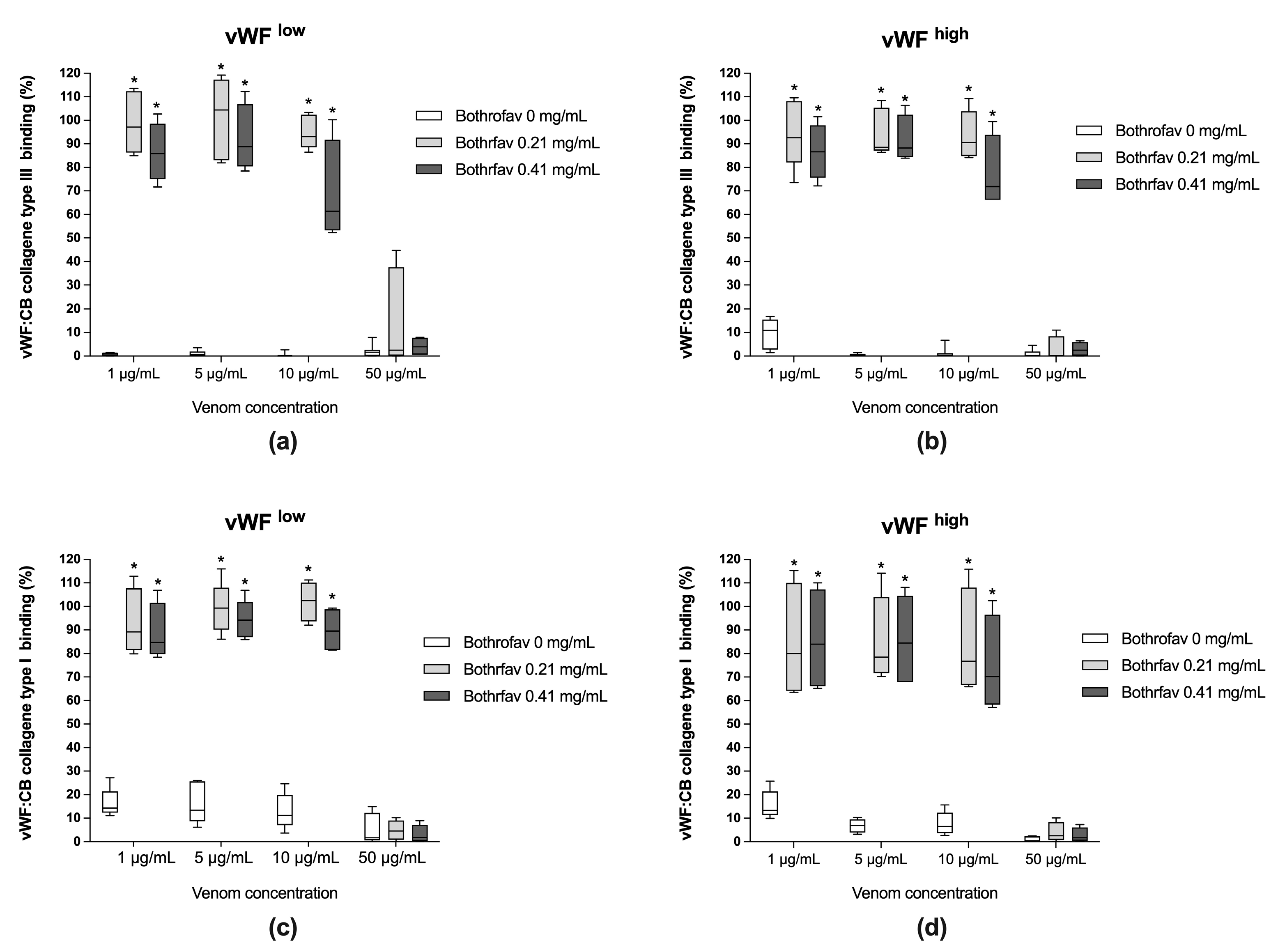
The monospecific antivenom Bothrofav was able to fully reverse vWF collagen binding activities in conditions of B. lanceolatus venom concentrations of 1, 5, and 10 µg/mL, which inhibited vWF collagen type III and type I binding activities (vWF:CB) in both low vWF concentrations (vWFlow) (Figure 4a,c) and high vWF concentrations (vWFhigh) (Figure 4b,d). At a 50 µg/mL B. lanceolatus venom concentration, the protective effects of Bothrofav were significantly suppressed (Figure 4).
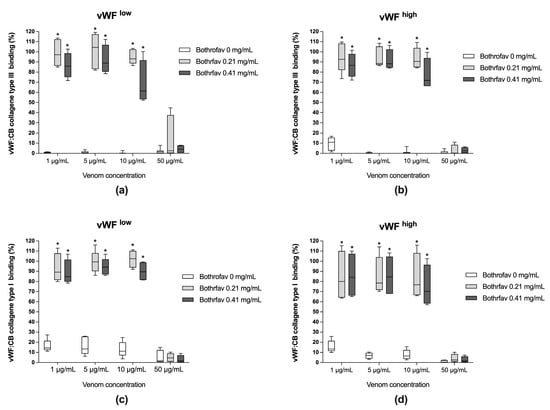
Figure 4.
(a,b) Effects of Bothrofav antivenom on the inhibitory action of different B. lanceolatus venom concentrations (1, 5, 10, and 50 µg/mL) on vWF collagen type III binding activity (vWF:CB collagen type III binding), expressed as percent of control in conditions of low vWF concentrations (vWFlow) and high vWF concentrations (vWFhigh); (c,d) Effects of Bothrofav antivenom on the inhibitory action of different B. lanceolatus venom concentrations (1, 5, 10, and 50 µg/mL) on vWF collagen type I binding activity (vWF:CB collagen type I binding), expressed as percent of control, in conditions of low vWF concentrations (vWFlow) and high vWF concentrations (vWFhigh). Data are displayed as box plots (median, whiskers min–max) of 5–8 independent experiments. * indicates statistical difference (p < 0.05) with control (Bothrofav antivenom phosphate saline buffer vehicle).
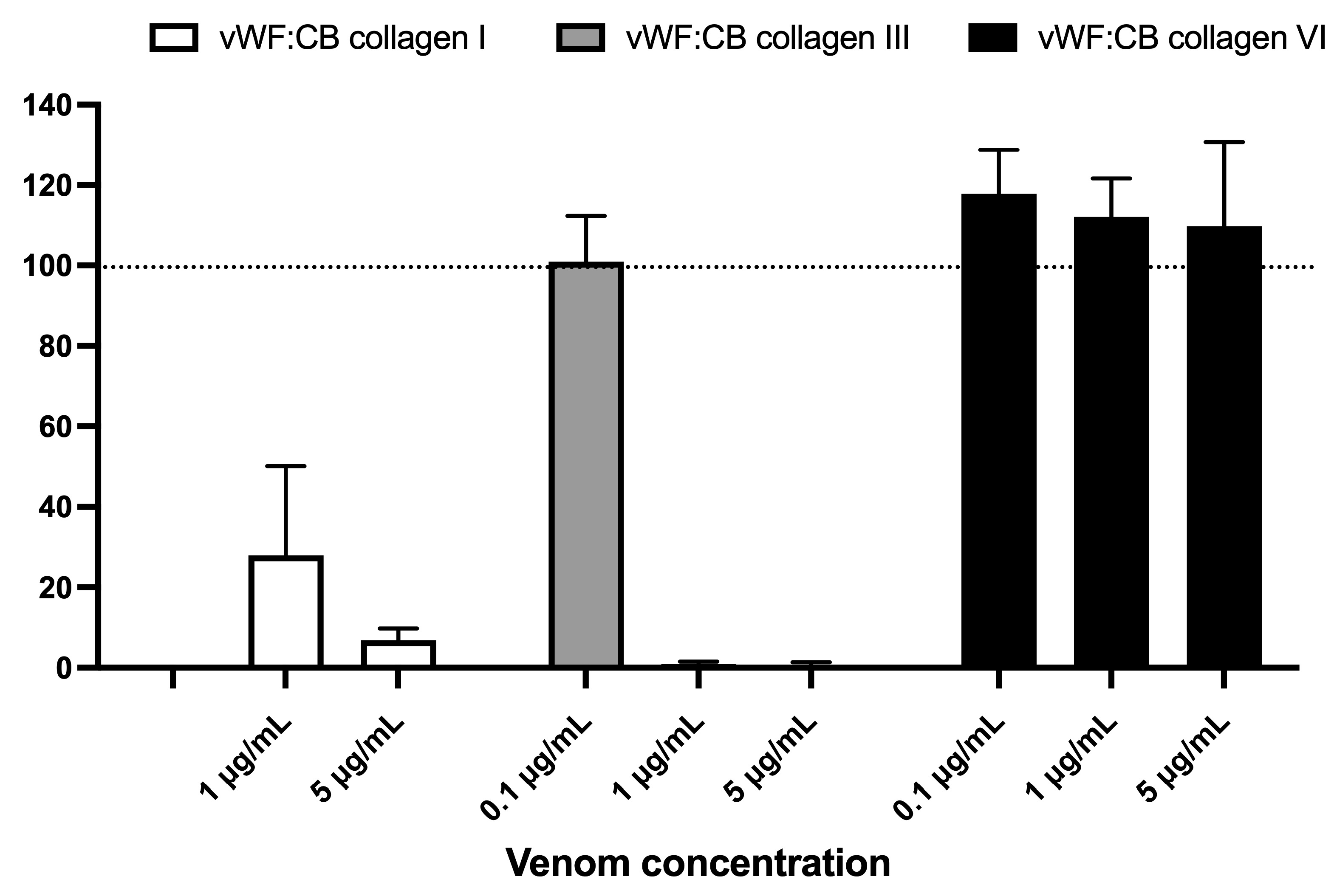
Figure 5 displays the effects of varying concentrations of B. lanceolatus venom (0.1, 1, and 5 µg/mL) on vWF collagen type I, III, and VI binding activities (vWF:CB), expressed as percent of control in conditions of high vWF concentrations. Figure 5 highlights the contrasting effect of B. lanceolatus venom on collagen type I and collagen type III vWF binding properties versus collagen type VI. No comparisons between collagen type I, type III, and type VI vWF:CB were performed.
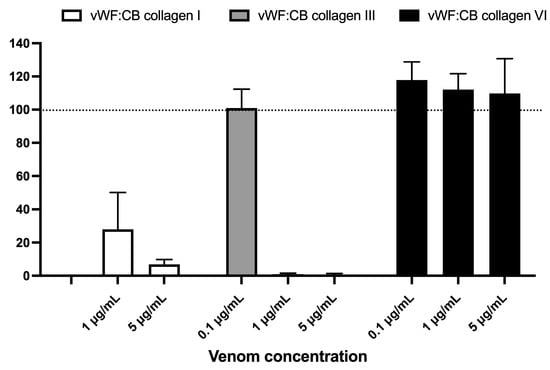
Figure 5.
Effects of varying concentrations of B. lanceolatus venom (0.1, 1, and 5 µg/mL) on vWF collagen type I, III, and VI binding activities (vWF:CB collagen binding), expressed as percent of control without venom in conditions of high vWF concentrations (vWFhigh). Data are displayed as box plots (median, whiskers min–max) of 5–8 independent experiments.
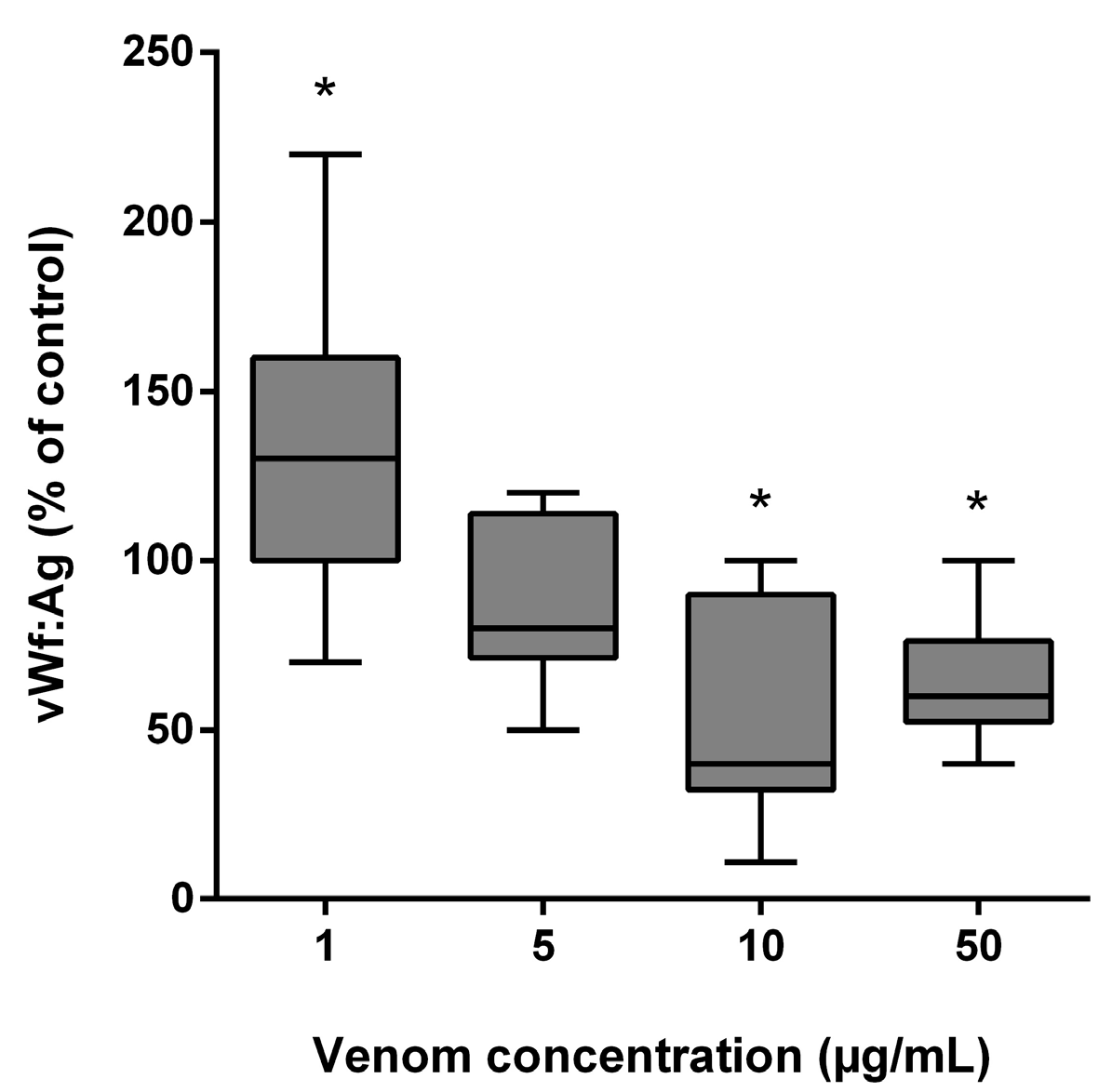
B. lanceolatus venom at the concentration of 1 µg/mL elicited a significant increase in vWF antigen (vWF:Ag) levels, while incubation with higher B. lanceolatus venom concentrations (10 and 50 µg/mL) was associated with reduced vWF:Ag levels (Figure 6).
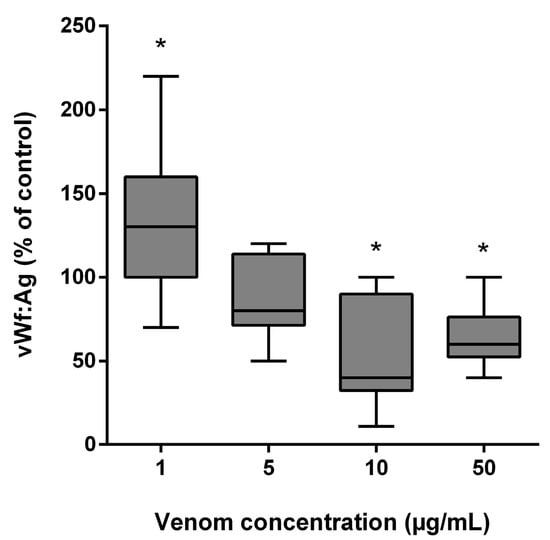
Figure 6.
Effects of B. lanceolatus venom on vWF:Ag levels. Data are displayed as box plots (median, whiskers min–max) of 5 independent experiments. * indicates statistical difference (p < 0.05) with control.
3. Discussion
Despite initial local damage similar to that described after B. atrox snakebites, B. lanceolatus snakebites are infrequently responsible for systemic bleeding and anticoagulability. Instead, B. lanceolatus snakebites are commonly complicated by multiple systemic macro-thrombosis within 48 h after the bite, if no rapid antivenom administration is initiated [37,38,39,40]. The proposed mechanisms of these thrombotic events are related to the switch of the endothelium to a prothrombotic phenotype. The activation of vWF can play a critical role in these prothrombotic conditions by facilitating platelet tethering to injured sub-endothelium through binding sites for collagen(s) and the platelet GPIb receptor.
The potential role of B. lanceolatus venom in inducing changes in vWF collagen binding activity has not been previously investigated. In the present in vitro study, vWF to collagen type I and type III binding activities, which are determined by vWF A3 domain interactions, were fully inhibited by B. lanceolatus venom. In sharp contrast, B. lanceolatus venom increased vWF to collagen type VI binding, which is determined by vWF A1 domain interactions. Pre-incubation with the monospecific antivenom Bothrofav reversed the inhibitory effects of B. lanceolatus venom on vWF collagen type I and type III binding activities. Of note, we observed an increase in vWF:Ag levels in conditions of low B. lanceolatus venom concentrations, possibly due to vWF proteolysis. Overall, our results are consistent with previous studies showing that many components of Bothrops spp. venom interact with the vWF A1 and A3 domains for collagen binding, to regulate platelet adhesion and aggregation [3,5,7,27,28]. For example, C-type lectin-like proteins, botrocetin and bitiscetin, previously known as coagglutinins, induce platelet agglutination via the enhancement of vWF A1 domain binding sites [5,18,19,41]. Other molecular forms of bitiscetin, such as bitiscetin-2 and bitiscetin-3, interact with the vWF A3 domain binding site [42,43]. Jararhagin, a PIII SVMP containing a metalloproteinase domain followed by disintegrin-like and cysteine-rich domains, interacts with the vWF A1 domain [27,28,30]. Atroxlysins (P-I and P-III hemorrhagic metalloproteinases) isolated from B. atrox, can dose-dependently inhibit ADP- or collagen-triggered platelet aggregation via the vWF A1 domain [25,44].
In our study, we found that B. lanceolatus venom fully inhibited vWF to collagen type I and type III binding. This is consistent with previous studies indicating that interactions between platelets, vWF, and collagen are reduced, owing to the direct inhibitory properties of Bothrops spp. venom on glycoprotein Ib (GPIb) and α2β integrin platelet receptors [18,22,23,44,45,46,47]. In line, human victims of B. jararaca envenomation, who present systemic bleeding, display vWF collagen binding inhibition as evidenced by hypoaggregation to ADP, ristocetin, and collagen [21]. Interestingly, we found that the monospecific antivenom Bothrofav was able to fully reverse vWF-collagen binding activity (vWF:CB) inhibition induced by B. lanceolatus venom. Indeed, the Bothrofav antivenom was able to fully reverse vWF collagen type I and type III binding inhibition. The effects of Bothrofav on vWF:CB inhibition was obtained using a co-incubation procedure with B. lanceolatus venom. The procedure is the gold standard protocol to test antivenom efficacy in preclinical studies. Previous clinical studies have shown that antivenom therapy was able to partially restore platelet hypoaggregation for ADP and ristocetin in Bothrops spp. envenomation [21]. Thereon, further studies are required to address whether Bothrofav is able to reverse vWF:CB inhibition in human venom envenomation by B. lanceolatus.
Nonspecific proteolytical vWF degradation by different venom enzymes can also indirectly contribute the to vWF-to-collagen binding deficit [26,27,28,29,30]. For example, the inhibition of ristocetin-induced aggregation by jararhagin has been attributed to a direct effect on vWF, rather than to its action on the GP Ib-receptors [23]. In our study, the effects of B. lanceolatus venom on vWF:Ag levels were dose-dependent. Low venom concentration (1 µg/mL) increased vWF:Ag levels, which might be related to the proteolytic action of B. lanceolatus venom leading to newly exposed epitopes in cleaved vWF molecules, thereby amplifying the final signal as detected by the ELISA technique [28,30]. This has already been observed in B. jararaca envenomation in rodents, where, despite an observed general decrease in vWF:Ag levels in the overall rodent sample, some animal subjects nevertheless displayed elevated plasma vWF antigen [18]. Proteolytical vWF degradation may be due to the large predominance of SVMPs in B. lanceolatus venom (74%) [48,49]. In contrast, high venom concentrations (10 and 50 µg/mL) reduced vWF:Ag levels, which may suggest vWF conformation changes in terms of multimerization and masking epitopes, thereby reducing the final signal as detected by the ELISA technique. The effects of B. lanceolatus venom on ADAMTS13 activity and the accumulation of ultra high molecular weight multimers of vWF need to be further explored.
In sharp contrast with its inhibitory effect on vWF to collagen type I and type III binding, B. lanceolatus venom enhanced vWF to collagen type VI binding activity. This result is consistent with studies showing that botrocetin and bitiscetin, two venom C-type lectin-like proteins, induce platelet agglutination via the enhancement of vWF to collagen binding site affinity via the A1 domain [5,19,41]. The presence of C-type lectin-like protein(s) in B. lanceolatus venom has been demonstrated by proteomic studies, while the precise identity of these proteins is not yet known [48]. The respective roles of vWF A1 and A3 domains and their interactions in collagen binding processes have been extensively investigated. In static conditions, vWF with deleted A3 domain binds poorly to collagen fibrils, in comparison to vWF with a deleted A1 domain and intact VWF, which both bind equally well to collagen [46,47,48,49]. In flow conditions, platelet adhesion to collagen was only observed when vWF was allowed to first bind to collagen through the A3 domain, then subsequently to the A1 domain responsible for platelet recruitment via the GP Ibα receptor [50,51,52,53]. In line with the prominent role of the vWF A1 domain in platelet aggregation via the GP Ibα receptor and its binding to collagen VI of the sub-endothelium, we propose that increased vWF to collagen type VI binding could be a major pathophysiological mechanism leading to multiple thrombotic events described in B. lanceolatus envenomation in humans. Indeed, while the localization of collagen types I and III in the connective tissues, below the interfacing zone between the sub-endothelial basement membrane and the interstitium, raises some doubts about their physiological relevance in the early phases of vascular repair, the intimate association of collagen type VI with the basement membrane strongly supports an important role for this collagen type in binding circulating vWF at the site of vascular injury [54,55,56]. Furthermore, the co-localization of collagen type VI and vWF in the vascular sub-endothelium also supports that collagen type VI may represent a central component of the sub-endothelial matrix contributing to platelet aggregation upon rupture of the blood vessel wall [54,55,56]. Increased vWF to collagen binding has been previously reported in pathological conditions, such as inflammation related to infection and cardiovascular diseases (coronary heart diseases and cerebral stroke) [57,58,59]. Hence, it may be hypothesized that the macro-thrombotic events associated with human B. lanceolatus envenomation [37,38,39,40] might be related at least in part to increased vWF to collagen type VI binding.
In our study, Bothrofav, a monospecific antivenom against B. lanceolatus, was able to prevent the inhibitory effects of B. lanceolatus venom on vWF collagen type I and type III binding activities. Since 1991, a highly purified and monospecific antivenom against B. lanceolatus has been manufactured by Aventis-Pasteur (Lyon, France) as Bothrofav [60]. This antivenom is an F(ab′)2 preparation obtained by pepsin digestion and ammonium sulfate fractionation of hyperimmune plasma from horses immunized with the venom of B. lanceolatus. Bothrofav is highly effective in reducing mortality and preventing the development of thrombosis and other systemic disturbances in these envenomations [60,61]. The preclinical assessment of the neutralizing capacity of Bothrofav has corroborated its efficacy in the inhibition of lethal activities, and, most importantly, of toxic and enzymatic activities of the B. lanceolatus venom [62]. Third-generation antivenomics analysis has further proven Bothrofav’s high neutralizing efficacy of all the major immunogenic protein components of B. lanceolatus venom (i.e., metalloproteinases, phospholipases A2, serine proteinases, C-type lectin-like proteins, and disintegrins), with the exception of poor immunogenic peptides [63]. In our study, the Bothrovav-induced prevention of the inhibitory effects of B. lanceolatus venom on vWF collagen type I and type III binding may be related to the reported beneficial effects of the antivenom on coagulopathic activities [64,65].
4. Conclusions
Our in vitro study suggests that B. lanceolatus venom alters the adhesive properties of vWF on collagen and increases vWF:Ag levels, possibly due to vWF cleavage. B. lanceolatus venom seemed to fully inhibit vWF to collagen type I and type III binding, which was reversed by the monospecific antivenom Bothrofav. In contrast, B. lanceolatus venom increased vWF to collagen type VI binding. In light of the respective distribution of collagen type III and collagen type VI in perivascular connective tissue and the sub-endothelium, a plausible association between increased vWF:CB activity for collagen type VI and the onset of thrombotic events in human B. lanceolatus envenomation might be considered.
5. Material and Methods
5.1. Venom and Antivenom
For venom collection, we milked the front fangs of snakes. The animals were forced to bite through a thin membrane and release their venom into a clean glass vessel. The crude venom was obtained from adult wild B. lanceolatus specimens, captured in Martinique. Venom samples were pooled from the milking of five specimens. All venom samples were lyophilized (Freezone, Labconco, Kansas City, MO, USA) and stored at −80 °C until use (stock solution 10 mg/mL). B. lanceolatus venom was used in experiments at the final concentrations of 1, 5, 10, and 50 µg/mL. Bothrofav is a preparation containing F(ab’)2 fragments that have the property of neutralizing Bothrops lanceolatus venom. These equine F(ab’)2 fragments ligate venom antigens present in circulating blood to form inactive F(ab’)2-antigen complexes, in turn reducing the amount of free venom in circulation. The monospecific anti-venom Bothrofav (Sanofi Pasteur, Lyon, France) was used (batch J8216; protein concentration of 20.7 ± 0.05 g/dL). Bothrofav was used in experiments at the final concentrations of 0.21 mg/mL and 0.41 mg/mL.
5.2. Control Plasma and von Willebrand Factor
Lyophilized control plasmas with “low vWF level” (0.36 UI/mL, vWFlow) or “high level” (1.23 UI/mL, vWFhigh) included in ELISA assay kits were used for vWF:Ag and vWF:CB experiments (Technozym ELISA vWF:Ag and vWF:CB, Cryopep, Montpellier, France). For vWF multimer experiments, Cryocheck Pooled Normal Plasma and a highly purified plasma-derived von Willebrand factor Wilfactin, almost devoid of FVIII and containing high molecular weight (HMW) multimers with a distribution similar to normal plasma, were respectively purchased from Cryopep (Montpellier, France) and LFB-Biopharmaceuticals (Les Ulis, France).
5.3. Determination of vWF Antigen
vWF Ag ELISA kit was used for the quantitative determination of human von Willebrand factor (vWF) concentrations (Technozym ELISA assay kit, Cryopep, Montpellier, France) according to the manufacturer’s instructions. This assay employs the quantitative sandwich enzyme immunoassay technique utilizing a polyclonal anti-vWF Ag antibody and a vWF Ag-HRP conjugate. The assay sample and buffer were incubated together with vWF Ag-HRP conjugate in the pre-coated plate for one hour. After the incubation period, the wells were decanted and washed five times. The wells were then incubated with a substrate for HRP enzyme. The product of the enzyme-substrate reaction formed a blue-colored complex. Finally, a stop solution was added to stop the reaction, which then turned the solution yellow. Color intensity was measured spectrophotometrically at 450 nm in a microplate reader. Color intensity was inversely proportional to vWF Ag concentration, since vWF Ag from samples and vWF Ag-HRP conjugate compete for the anti-vWF Ag antibody binding site. As the number of sites is limited, the more sites that are occupied by vWF Ag from the sample, the fewer the sites that are free to bind the vWF Ag-HRP conjugate. A standard curve was plotted representing the color intensity (O.D.) according to the concentration of standards. The vWF Ag concentration in each sample was interpolated from this standard curve.
5.4. Determination of vWF Collagen Binding (Activity)
The vWF collagen binding assay (vWF:CB) was performed with an indirect ELISA, which involved two binding processes of primary antibody and labeled secondary antibody. vWF in patient plasma was captured in microtiter plates coated with human collagen(s). Unbound material was then washed away and a solution of antibody to human vWF, conjugated to an enzyme, was added to ‘tag’ onto any captured vWF. Unbound conjugate was washed off and a substrate for the enzyme was added, the product of the enzyme-substrate reaction being colored. Color intensity was in direct proportion to the degree of conjugate binding, itself proportional to the amount of vWF captured, and thus to vWF collagen binding activity. A standard curve was constructed from a pool of normal plasma donors. Bound vWF was detected using polyclonal anti-human vWF antibodies (anti-vWF-POX) labeled with horse radish peroxidase (HRP). Coloring reaction was performed with orthophenylenediamine and hydrogen peroxide. The reaction was stopped with sulfuric acid. Absorbance was measured at 450 nm. Specific vWF collagen binding assay kits were chosen for evaluating the functional activity of vWF, i.e., the interaction between vWF and the matrix collagen I, collagen III, and collagen VI (Technozym ELISA assay kit collagen type I, III, and VI purchased from Cryopep (Montpellier, France)).
5.5. Incubation Protocol
Microtiter plates coated with human collagen(s) were incubated with either phosphate saline buffer (PBS), B. lanceolatus venom in PBS, or a mixture of B. lanceoltaus venom plus Bothrofav in PBS at the indicated concentrations. Binding activity of vWF to collagen was studied after 45 min incubation of plasma samples with either low vWF level (vWFlow) or high vWF level (vWFhigh) included in ELISA assay kits.
5.6. Statistical Analysis
Quantitative data were presented as mean ± standard deviation (SD). The Shapiro–Wilk test was used to test for normal distribution of quantitative data. Data were analyzed by using analysis of variance ANOVA. When a significant difference was found, we identified specific differences between groups with a sequentially rejective Bonferroni procedure. After application of the Bonferroni correction, the level of statistical significance was set at p < 0.05. Results were analyzed with the Prism 6 for Windows software (Graphpad, Boston, MA, USA).
Author Contributions
Conceptualization, O.P.-L. and R.N.; methodology, F.D., R.B. and M.-D.D.; formal analysis, O.P.-L. and R.N.; data curation, C.A., M.-D.D., H.M., D.R. and R.B.; writing—original draft preparation, O.P.-L. and R.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agence Nationale de la Recherche (ANR), Mitobothrops Grant ANR-18-CE17-0026 (https://anr.fr/Project-ANR-18-CE17-0026 (accessed on 1 July 2023)).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable for studies not involving humans or animals.
Data Availability Statement
Data supporting reported results can be provided on request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Larreche, S.; Chippaux, J.P.; Chevillard, L.; Mathe, S.; Resiere, D.; Siguret, V.; Megarbane, B. Bleeding and Thrombosis: Insights into Pathophysiology of Bothrops Venom-Related Hemostasis Disorders. Int. J. Mol. Sci. 2021, 22, 9643. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Kamiguti, A.S.; Berlanga, O.; Leduc, M.; Theakston, R.D.; Watson, S.P. The use of snake venom toxins as tools to study platelet receptors for collagen and von Willebrand factor. Haemostasis 2001, 31, 155–172. [Google Scholar] [CrossRef]
- de Queiroz, M.R.; de Sousa, B.B.; da Cunha Pereira, D.F.; Mamede, C.C.N.; Matias, M.S.; de Morais, N.C.G.; de Oliveira Costa, J.; de Oliveira, F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon 2017, 133, 33–47. [Google Scholar] [CrossRef]
- Matsui, T.; Hamako, J. Structure and function of snake venom toxins interacting with human von Willebrand factor. Toxicon 2005, 45, 1075–1087. [Google Scholar] [CrossRef]
- Moore, G.W. Snake Venoms in Diagnostic Hemostasis and Thrombosis. Semin. Thromb. Hemost. 2022, 48, 145–160. [Google Scholar] [CrossRef]
- Matsui, T.; Hamako, J.; Titani, K. Structure and function of snake venom proteins affecting platelet plug formation. Toxins 2010, 2, 10–23. [Google Scholar] [CrossRef]
- Maxwell, M.J.; Westein, E.; Nesbitt, W.S.; Giuliano, S.; Dopheide, S.M.; Jackson, S.P. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood 2007, 109, 566–576. [Google Scholar] [CrossRef]
- Favaloro, E.J. An update on the von Willebrand factor collagen binding assay: 21 years of age and beyond adolescence but not yet a mature adult. Semin. Thromb. Hemost. 2007, 33, 727–744. [Google Scholar] [CrossRef]
- Yee, A.; Kretz, C.A. Von Willebrand factor: Form for function. Semin. Thromb. Hemost. 2014, 40, 17–27. [Google Scholar] [CrossRef]
- Henriksen, K.; Karsdal, M.A. Chapter 1, Type I collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: New York, NY, USA, 2019; pp. 1–12. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Villesen, I.F.; Sinkeviciute, D.; Bay-Jensen, A.C.; Karsdal, M.A. Chapter 3, Type III collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: New York, NY, USA, 2019; pp. 23–36. [Google Scholar] [CrossRef]
- Sun, S.; Genovese, F.; Karsdal, M.A. Chapter 6, Type VI collagen. In Biochemistry of Collagens, Laminins and Elastin; Karsdal, M.A., Ed.; Academic Press: New York, NY, USA, 2019; pp. 59–67. [Google Scholar] [CrossRef]
- Niewiarowski, S.; Kirby, E.P.; Brudzynski, T.M.; Stocker, K. Thrombocytin, a serine protease from Bothrops atrox venom. 2. Interaction with platelets and plasma-clotting factors. Biochemistry 1979, 18, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Fujimura, Y.; Miura, S.; Ozaki, Y.; Usami, Y.; Suzuki, M.; Titani, K.; Yoshida, E.; Sugimoto, M.; Yoshioka, A.; et al. Purification and characterization of bothrombin, a fibrinogen-clotting serine protease from the venom of Bothrops jararaca. Biochemistry 1994, 33, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Fuly, A.L.; Soares, A.M.; Marcussi, S.; Giglio, J.R.; Guimaraes, J.A. Signal transduction pathways involved in the platelet aggregation induced by a D-49 phospholipase A2 isolated from Bothrops jararacussu snake venom. Biochimie 2004, 86, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Hori, A.; Hamako, J.; Matsushita, F.; Ozeki, Y.; Sakurai, Y.; Hayakawa, M.; Matsumoto, M.; Fujimura, Y. Mutant botrocetin-2 inhibits von Willebrand factor-induced platelet agglutination. J. Thromb. Haemost. 2017, 15, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Thomazini, C.M.; Sachetto, A.T.A.; de Albuquerque, C.Z.; de Moura Mattaraia, V.G.; de Oliveira, A.K.; Serrano, S.M.T.; Lebrun, I.; Barbaro, K.C.; Santoro, M.L. Involvement of von Willebrand factor and botrocetin in the thrombocytopenia induced by Bothrops jararaca snake venom. PLoS Negl. Trop. Dis. 2021, 15, e0009715. [Google Scholar] [CrossRef]
- Yamamoto-Suzuki, Y.; Sakurai, Y.; Fujimura, Y.; Matsumoto, M.; Hamako, J.; Kokubo, T.; Kitagawa, H.; Kawsar, S.M.; Fujii, Y.; Ozeki, Y.; et al. Identification and recombinant analysis of botrocetin-2, a snake venom cofactor for von Willebrand factor-induced platelet agglutination. Biochemistry 2012, 51, 5329–5338. [Google Scholar] [CrossRef]
- Pinto, A.; Angulo, Y.; Jimenez, R.; Lomonte, B. Isolation of bothrasperin, a disintegrin with potent platelet aggregation inhibitory activity, from the venom of the snake Bothrops asper. Rev. Biol. Trop. 2003, 51, 253–259. [Google Scholar]
- Sano-Martins, I.S.; Santoro, M.L.; Castro, S.C.; Fan, H.W.; Cardoso, J.L.; Theakston, R.D. Platelet aggregation in patients bitten by the Brazilian snake Bothrops jararaca. Thromb. Res. 1997, 87, 183–195. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Baldo, C. Jararhagin, a hemorrhagic snake venom metalloproteinase from Bothrops jararaca. Toxicon 2012, 60, 280–289. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef]
- Loria, G.D.; Rucavado, A.; Kamiguti, A.S.; Theakston, R.D.; Fox, J.W.; Alape, A.; Gutierrez, J.M. Characterization of ‘basparin A’, a prothrombin-activating metalloproteinase, from the venom of the snake Bothrops asper that inhibits platelet aggregation and induces defibrination and thrombosis. Arch. Biochem. Biophys. 2003, 418, 13–24. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Schneider, F.S.; Yarleque, A.; Borges, M.H.; Richardson, M.; Figueiredo, S.G.; Evangelista, K.S.; Eble, J.A. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergon) snake venom acts both on blood vessel ECM and platelets. Arch. Biochem. Biophys. 2010, 496, 9–20. [Google Scholar] [CrossRef]
- Pereira, A.L.; Fritzen, M.; Faria, F.; Motta, G.; Chudzinski-Tavassi, A.M. Releasing or expression modulating mediator involved in hemostasis by Berythractivase and Jararhagin (SVMPs). Toxicon 2006, 47, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.; Kim, J.; Wang, D.; Dragulev, B.; Shannon, J.D.; Mann, H.H.; Veit, G.; Wagener, R.; Koch, M.; Fox, J.W. The cysteine-rich domain of snake venom metalloproteinases is a ligand for von Willebrand factor A domains: Role in substrate targeting. J. Biol. Chem. 2006, 281, 39746–39756. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Wang, D.; Shannon, J.D.; Pinto, A.F.M.; Polanowska-Grabowska, R.K.; Fox, J.W. Interaction of the cysteine-rich domain of snake venom metalloproteinases with the A1 domain of von Willebrand factor promotes site-specific proteolysis of von Willebrand factor and inhibition of von Willebrand factor-mediated platelet aggregation. FEBS J. 2007, 274, 3611–3621. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.; Alves, E.C.; Santos, A.S.; Pereira, J.P.T.; Sarraff, L.K.S.; Nascimento, E.F.; De-Brito-Sousa, J.D.; Sampaio, V.S.; Lacerda, M.V.G.; Sachett, J.A.G.; et al. Factors Associated with Systemic Bleeding in Bothrops Envenomation in a Tertiary Hospital in the Brazilian Amazon. Toxins 2019, 11, 22. [Google Scholar] [CrossRef]
- Thomazini, C.M.; Soares, R.P.S.; da Rocha, T.R.F.; Sachetto, A.T.A.; Santoro, M.L. Optimization of von Willebrand factor multimer analysis in vertical mini-gel electrophoresis systems: A rapid procedure. Thromb. Res. 2019, 175, 76–83. [Google Scholar] [CrossRef]
- Bucaretchi, F.; Pimenta, M.M.B.; Borrasca-Fernandes, C.F.; Prado, C.C.; Capitani, E.M.; Hyslop, S. Thrombotic microangiopathy following Bothrops jararaca snakebite: Case report. Clin. Toxicol. 2019, 57, 294–299. [Google Scholar] [CrossRef]
- Casamento, A.J.; Isbister, G.K. Thrombotic microangiopathy in two tiger snake envenomations. Anaesth. Intensiv. Care 2011, 39, 1124–1127. [Google Scholar] [CrossRef]
- Dineshkumar, T.; Dhanapriya, J.; Sakthirajan, R.; Thirumalvalavan, K.; Kurien, A.A.; Balasubramaniyan, T.; Gopalakrishnan, N. Thrombotic microangiopathy due to Viperidae bite: Two case reports. Indian J. Nephrol. 2017, 27, 161–164. [Google Scholar] [CrossRef]
- Noutsos, T.; Currie, B.J.; Lek, R.A.; Isbister, G.K. Snakebite associated thrombotic microangiopathy: A systematic review of clinical features, outcomes, and evidence for interventions including plasmapheresis. PLoS Negl. Trop. Dis. 2020, 14, e0008936. [Google Scholar] [CrossRef] [PubMed]
- Noutsos, T.; Currie, B.J.; Wijewickrama, E.S.; Isbister, G.K. Snakebite associated thrombotic microangiopathy and recommendations for clinical practice. Toxins 2022, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Priyankara, S.; Rathnasiri, V.; Mihiran, T.; Premawansa, G.; Isbister, G.K.; Silva, A. Mild venom-induced consumption coagulopathy associated with thrombotic microangiopathy following a juvenile Russell’s viper (Daboia russelii) envenoming: A case report. Toxicon 2022, 212, 8–10. [Google Scholar] [CrossRef]
- Malbranque, S.; Piercecchi-Marti, M.D.; Thomas, L.; Barbey, C.; Courcier, D.; Bucher, B.; Ridarch, A.; Smadja, D.; Warrell, D.A. Fatal diffuse thrombotic microangiopathy after a bite by the “Fer-de-Lance” pit viper (Bothrops lanceolatus) of Martinique. Am. J. Trop. Med. Hyg. 2008, 78, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Hossein, M.; Megarbane, B. Snake Bites by Bothrops lanceolatus in Martinique. Med. Sante Trop. 2018, 28, 37–43. [Google Scholar] [CrossRef]
- Thomas, L.; Chausson, N.; Uzan, J.; Kaidomar, S.; Vignes, R.; Plumelle, Y.; Bucher, B.; Smadja, D. Thrombotic stroke following snake bites by the “Fer-de-Lance” Bothrops lanceolatus in Martinique despite antivenom treatment: A report of three recent cases. Toxicon 2006, 48, 23–28. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Neviere, R. Inflammation and Oxidative Stress in Snakebite Envenomation: A Brief Descriptive Review and Clinical Implications. Toxins 2022, 14, 802. [Google Scholar] [CrossRef]
- Fukuda, K.; Doggett, T.; Laurenzi, I.J.; Liddington, R.C.; Diacovo, T.G. The snake venom protein botrocetin acts as a biological brace to promote dysfunctional platelet aggregation. Nat. Struct. Mol. Biol. 2005, 12, 152–159. [Google Scholar] [CrossRef]
- Obert, B.; Romijn, R.A.; Houllier, A.; Huizinga, E.G.; Girma, J.P. Characterization of bitiscetin-2, a second form of bitiscetin from the venom of Bitis arietans: Comparison of its binding site with the collagen-binding site on the von Willebrand factor A3-domain. J. Thromb. Haemost. 2006, 4, 1596–1601. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Matsushita, F.; Dijkstra, J.M.; Nakamura, Y.; Akiyama, H.; Hamako, J.; Morita, T.; Araki, S.; Matsui, T. Bitiscetin-3, a novel C-type lectin-like protein cloned from the venom gland of the viper Bitis arietans, induces platelet agglutination and inhibits binding of von Willebrand factor to collagen. Toxins 2022, 14, 236. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Estevao-Costa, M.I.; Alvarenga, V.G.; Vivas-Ruiz, D.E.; Yarleque, A.; Lima, A.M.; Cavaco, A.; Eble, J.A.; Sanchez, E.F. Atroxlysin-III, A Metalloproteinase from the Venom of the Peruvian Pit Viper Snake Bothrops atrox (Jergon) Induces Glycoprotein VI Shedding and Impairs Platelet Function. Molecules 2019, 24, 3489. [Google Scholar] [CrossRef]
- Andrews, R.K.; Gardiner, E.E.; Shen, Y.; Berndt, M.C. Structure-activity relationships of snake toxins targeting platelet receptors, glycoprotein Ib-IX-V and glycoprotein VI. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2003, 1, 143–149. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Alvarenga, V.G.; Oliveira, L.S.; Oliveira, D.L.; Estevao-Costa, M.I.; Flores-Ortiz, R.; Eble, J.A. A fibrinolytic snake venom metalloproteinase, mutalysin-II, with antiplatelet activity and targeting capability toward glycoprotein GPIbalpha and glycoprotein GPVI. Biochimie 2021, 184, 1–7. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. A novel fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (Barnett’s pitviper) snake venom with anti-platelet properties. Biochim. Biophys. Acta 2016, 1860, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Sanz, L.; Escolano, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Rucavado, A.; Warrell, D.A.; Calvete, J.J. Snake venomics of the lesser antillean pit vipers Bothrops caribbaeus and Bothrops lanceolatus: Correlation with toxicological activities and immunoreactivity of a heterologous antivenom. J. Proteome Res. 2008, 7, 4396–4408. [Google Scholar] [CrossRef]
- Terra, R.M.S.; Pinto, A.F.M.; Guimarães, J.A.; Fox, J.W. Proteomic Profiling of Snake Venom Metalloproteinases (SVMPs): Insights into venom induced pathology. Toxicon 2009, 54, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.F.; Fallah, M.A.; Mess, C.; Obser, T.; Schneppenheim, R.; Alexander-Katz, A.; Schneider, S.W.; Huck, V. Platelet adhesion and aggregate formation controlled by immobilised and soluble VWF. BMC Mol. Cell Biol. 2020, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Sixma, J.J.; Schiphorst, M.E.; Verweij, C.L.; Pannekoek, H. Effect of deletion of the A1 domain of von Willebrand factor on its binding to heparin, collagen and platelets in the presence of ristocetin. Eur. J. Biochem. 1991, 196, 369–375. [Google Scholar] [CrossRef]
- Obert, B.; Houllier, A.; Meyer, D.; Girma, J.P. Conformational changes in the A3 domain of von Willebrand factor modulate the interaction of the A1 domain with platelet glycoprotein Ib. Blood 1999, 93, 1959–1968. [Google Scholar] [CrossRef]
- Bonnefoy, A.; Romijn, R.A.; Vandervoort, P.A.H.; Van Rompaey, I.; Vermylen, J.; Hoylaerts, M.F. von Willebrand factor A1 domain can adequately substitute for A3 domain in recruitment of flowing platelets to collagen. J. Thromb. Haemost. 2006, 4, 2151–2161. [Google Scholar] [CrossRef]
- Kuo, H.J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.H.; Wu, X.X.; Potter, B.J.; Uson, R.R.; Gordon, R.E. Co-localization of von Willebrand factor and type VI collagen in human vascular subendothelium. Am. J. Pathol. 1993, 142, 843–850. [Google Scholar] [PubMed]
- Wu, X.X.; Gordon, R.E.; Glanville, R.W.; Kuo, H.J.; Uson, R.R.; Rand, J.H. Morphological relationships of von Willebrand factor, type VI collagen, and fibrillin in human vascular subendothelium. Am. J. Pathol. 1996, 149, 283–291. [Google Scholar]
- Yan, B.; Wang, Q.; Du, W.; Zhai, S.; Gou, C.; Hu, T.; Xia, L.; Ruan, C.; Zhao, Y. Elevated Plasma von Willebrand Factor Antigen and Activity Levels Are Associated With the Severity of Coronary Stenosis. Clin. Appl. Thromb. Hemost 2020, 26, 1076029619900552. [Google Scholar] [CrossRef] [PubMed]
- Philippe, A.; Gendron, N.; Bory, O.; Beauvais, A.; Mirault, T.; Planquette, B.; Sanchez, O.; Diehl, J.L.; Chocron, R.; Smadja, D.M. Von Willebrand factor collagen-binding capacity predicts in-hospital mortality in COVID-19 patients: Insight from VWF/ADAMTS13 ratio imbalance. Angiogenesis 2021, 24, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Jiang, M.; Qiu, W.; Lu, S.; Zhao, Y.; Xia, L.; Ruan, C.; Zhao, Y. Assessment of the Diagnostic Value of Plasma Levels, Activities, and Their Ratios of von Willebrand Factor and ADAMTS13 in Patients with Cerebral Infarction. Clin. Appl. Thromb. Hemost. 2016, 22, 252–259. [Google Scholar] [CrossRef]
- Thomas, L.; Tyburn, B.; Lang, J.; Ketterle, J. Early infusion of a purified monospecific F(ab’)2 antivenom serum for Bothrops lanceolatus bites in Martinique. Lancet 1996, 347, 406. [Google Scholar] [CrossRef]
- Bogarín, G.; Romero, M.; Rojas, G.; Lutsch, C.; Casadamont, M.; Lang, J.; Otero, R.; Gutiérrez, J.M. Neutralization, by a monospecific Bothrops lanceolatus antivenom, of toxic activities induced by homologous and heterologous Bothrops snake venoms. Toxicon 1999, 37, 551–557. [Google Scholar] [CrossRef]
- Resiere, D.; Arias, A.S.; Villalta, M.; Rucavado, A.; Brouste, Y.; Cabié, A.; Nevière, R.; Césaire, R.; Kallel, H.; Mégarbane, B.; et al. Preclinical evaluation of the neutralizing ability of a monospecific antivenom for the treatment of envenomings by Bothrops lanceolatus in Martinique. Toxicon 2018, 148, 50–55. [Google Scholar] [CrossRef]
- Pla, D.; Rodríguez, Y.; Resiere, D.; Mehdaoui, H.; Gutiérrez, J.M.; Calvete, J.J. Third-generation antivenomics analysis of the preclinical efficacy of Bothrofav® antivenom towards Bothrops lanceolatus venom. Toxicon X 2018, 1, 100004. [Google Scholar] [CrossRef]
- Bourke, L.A.; Zdenek, C.N.; Neri-Castro, E.; Bénard-Valle, M.; Alagón, A.; Gutiérrez, J.M.; Sanchez, E.F.; Aldridge, M.; Fry, B.G. Pan-American Lancehead Pit-Vipers: Coagulotoxic Venom Effects and Antivenom Neutralisation of Bothrops asper and B. atrox Geographical Variants. Toxins 2021, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Alsolaiss, J.; Alomran, N.; Hawkins, L.; Casewell, N.R. Commercial Antivenoms Exert Broad Paraspecific Immunological Binding and In Vitro Inhibition of Medically Important Bothrops Pit Viper Venoms. Toxins 2022, 15, 1. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).