Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Results
2.1. Effects of ZEA + DON and 4-PBA on Cell Viability
2.2. Effects of ZEA + DON and 4-PBA on Cell Growth
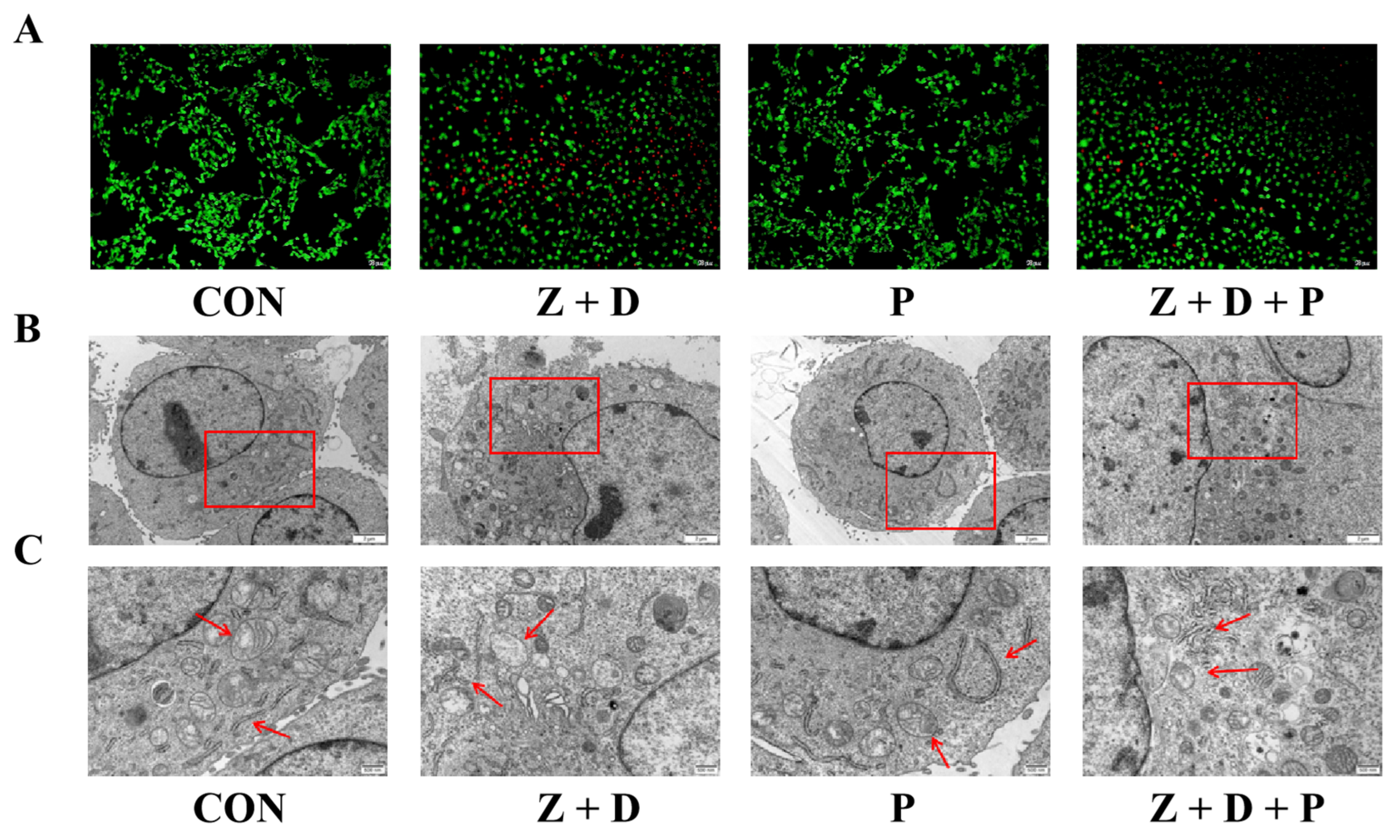
2.3. Effects of ZEA + DON and 4-PBA on Cell Ultrastructure
2.4. Effects of ZEA + DON and 4-PBA on Apoptosis Rate
2.5. Effects of ZEA + DON and 4-PBA on Mitochondrial Membrane Potential
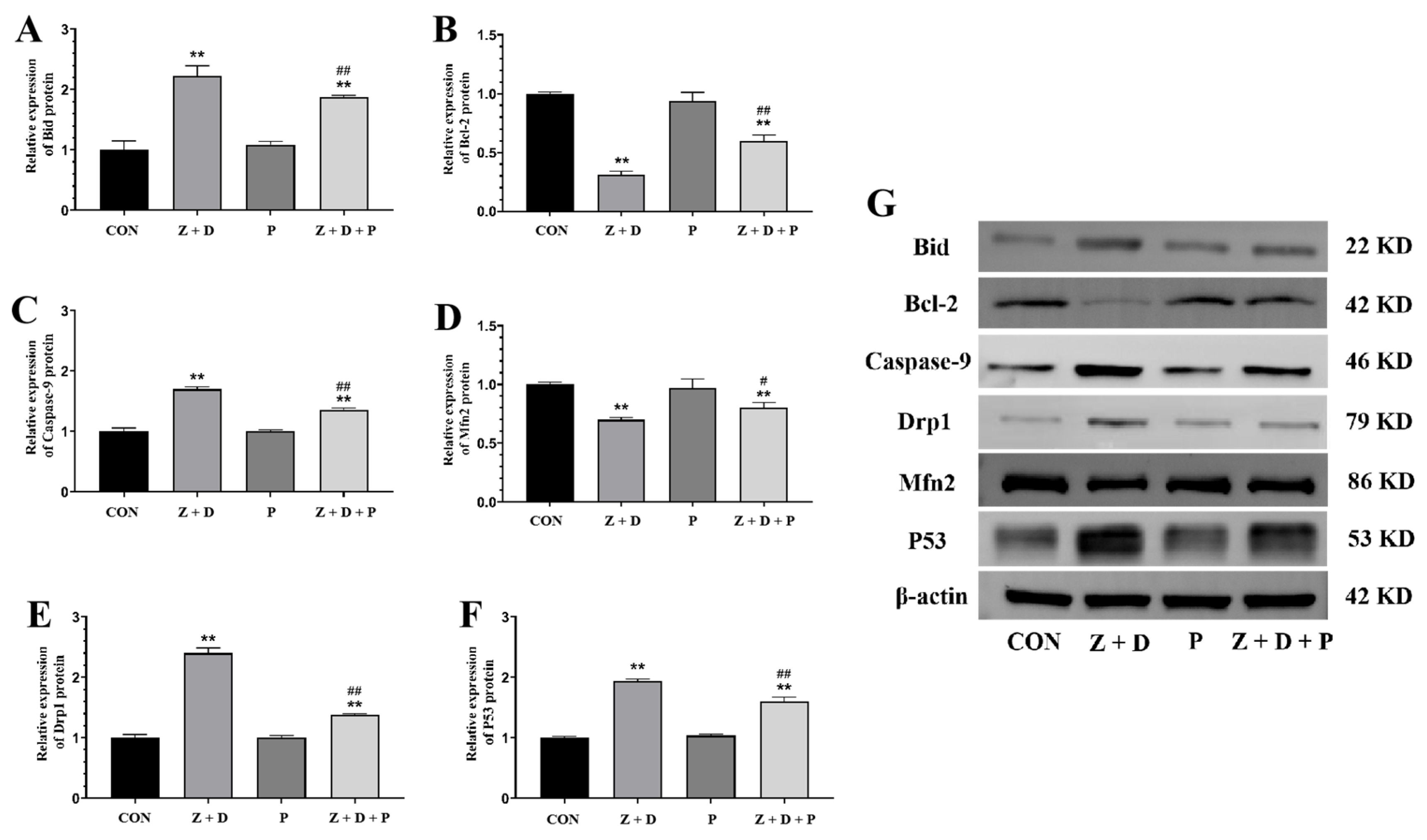
2.6. Effects of ZEA + DON and 4-PBA on Gene Expression
2.7. Effects of ZEA + DON and 4-PBA on Protein Expression
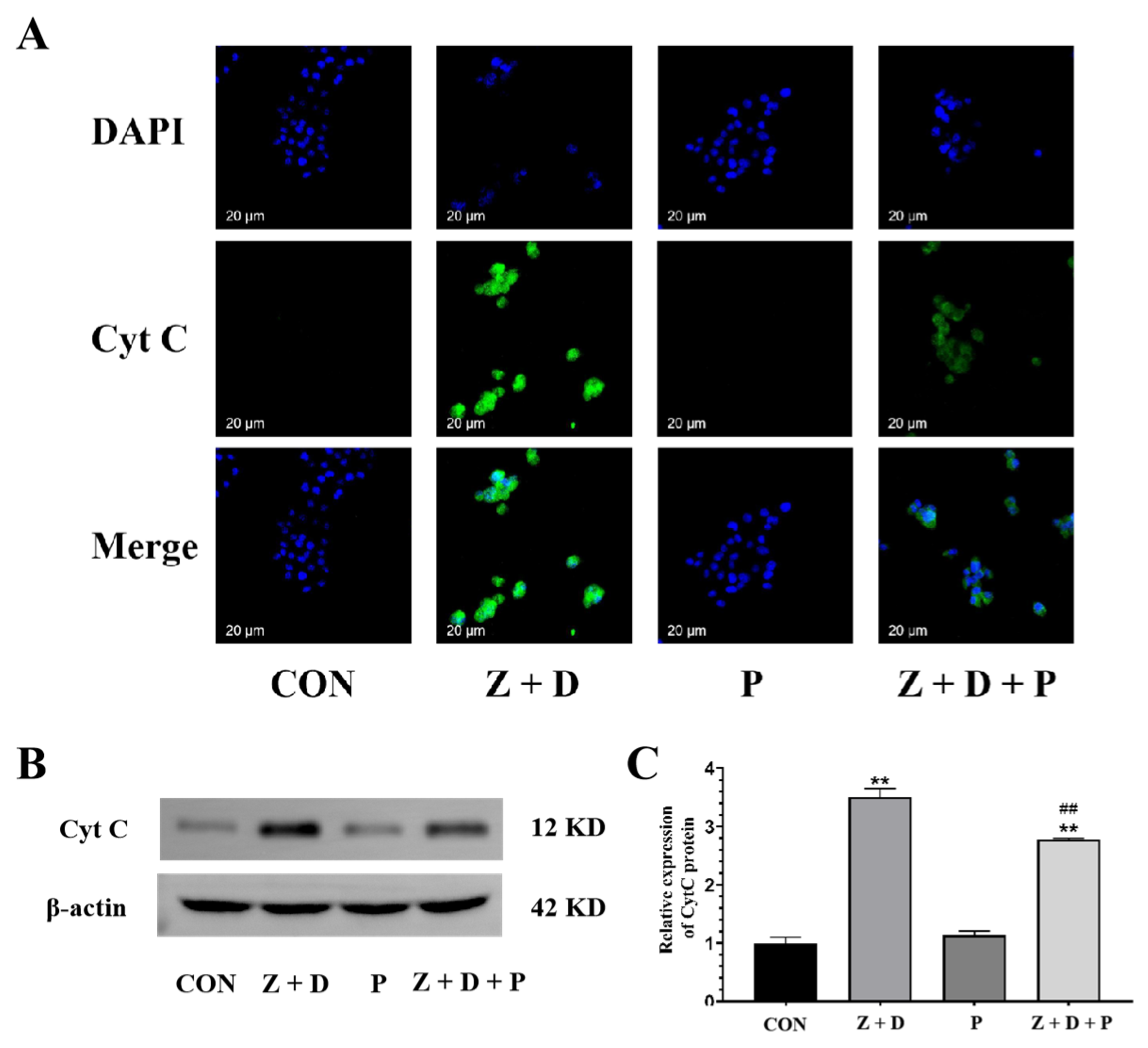
2.8. Effects of ZEA + DON and 4-PBA on CytC Expression and Distribution
3. Discussion
3.1. ZEA and DON Induce Cytotoxicity and Apoptosis through Mitochondrial-ER Pathway
3.2. How ZEA and DON Altered Gene and Protein Expression Related to Mitochondrial Apoptotic Pathway
3.3. Cell Homeostasis and Apoptosis Regulation Are Impacted by Mitochondrial Dynamics
3.4. The ER’s Role in Regulating ZEA- and DON-Induced Apoptosis in SCs through the Mitochondrial Pathway in MAMs
4. Conclusions
5. Materials and Methods
5.1. Chemical and Reagents
5.2. Cell Culture and Treatments
5.3. Cell Viability Assay
5.4. Detection of cell Survival Status
5.5. Changes in Cell Ultrastructure
5.6. Detection of Mitochondrial Membrane Potential
5.7. Determination of Apoptotic Cells
5.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
5.9. Determination of Protein Expression by Immunofluorescence
5.10. Western Blotting (WB)
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shkembi, X.; Svobodova, M.; Skouridou, V.; Bashammakh, A.S.; Alyoubi, A.O.; O’Sullivan, C.K. Aptasensors for mycotoxin detection: A review. Anal. Biochem. 2022, 644, 114156. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Ma, L.; Chen, J.; Xu, J.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 225, 112737. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of zearalenone-induced ferroptosis on mice spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, P.; Liu, M.; Liu, S.; Huo, L.; Ding, Z.; Liu, M.; Wang, S.; Lv, C.; Wu, H.; et al. Deoxynivalenol exposure inhibits biosynthesis of milk fat and protein by impairing tight junction in bovine mammary epithelial cells. Ecotoxicol. Environ. Saf. 2022, 237, 113504. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Mo, Y.; Li, J.; Wang, J.; Karrow, N.A.; Wu, H.; Sun, L. Ferroptosis is involved in deoxynivalenol-induced intestinal damage in pigs. J. Anim. Sci. Biotechnol. 2023, 14, 29. [Google Scholar] [CrossRef]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and zearalenone-synergistic or antagonistic agri-food chain co-contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Xu, J.; Zhu, L.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. N-acetylcysteine ameliorate cytotoxic injury in piglets sertoli cells induced by zearalenone and deoxynivalenol. Environ. Sci. Pollut. Res. Int. 2021, 28, 60276–60289. [Google Scholar] [CrossRef]
- Alm, H.; Brüssow, K.P.; Torner, H.; Vanselow, J.; Tomek, W.; Dänicke, S.; Tiemann, U. Influence of fusarium-toxin contaminated feed on initial quality and meiotic competence of gilt oocytes. Reprod. Toxicol. 2006, 22, 44–50. [Google Scholar] [CrossRef]
- Herrera-Cruz, M.S.; Simmen, T. Over Six Decades of Discovery and Characterization of the Architecture at Mitochondria-Associated Membranes (MAMs). Adv. Exp. Med. Biol. 2017, 997, 13–31. [Google Scholar] [CrossRef]
- Ma, L.; Chen, C.; Hai, S.; Wang, C.; Rahman, S.U.; Hang, W.; Zhao, C.; Feng, S.; Wang, X. Inhibition of mitochondrial fission alleviates zearalenone-induced mitochondria-associated endoplasmic reticulum membrane dysfunction in piglet sertoli cells. Toxins 2023, 15, 253. [Google Scholar] [CrossRef]
- Ma, L.; Hai, S.; Wang, C.; Chen, C.; Rahman, S.U.; Zhao, C.; Bazai, M.A.; Feng, S.; Wang, X. Zearalenone induces mitochondria-associated endoplasmic reticulum membranes dysfunction in piglet Sertoli cells based on endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2023, 254, 114710. [Google Scholar] [CrossRef]
- Simmen, T.; Aslan, J.E.; Blagoveshchenskaya, A.D.; Thomas, L.; Wan, L.; Xiang, Y.; Feliciangeli, S.F.; Hung, C.H.; Crump, C.M.; Thomas, G. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005, 24, 717–729. [Google Scholar] [CrossRef]
- Iwasawa, R.; Mahul-Mellier, A.L.; Datler, C.; Pazarentzos, E.; Grimm, S. Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J. 2011, 30, 556–568. [Google Scholar] [CrossRef]
- Lee, R.; Kim, D.W.; Lee, W.Y.; Park, H.J. Zearalenone induces apoptosis and autophagy in a spermatogonia cell line. Toxins 2022, 14, 148. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Ni, C.; Dai, Y.; Guo, Y. Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol. Med. Rep. 2018, 17, 7797–7806. [Google Scholar] [CrossRef]
- Tiemann, U.; Dänicke, S. In vivo and in vitro effects of the mycotoxins zearalenone and deoxynivalenol on different non-reproductive and reproductive organs in female pigs: A review. Food Addit. Contam. 2007, 24, 306–314. [Google Scholar] [CrossRef]
- Dai, Y.; Xie, H.; Xu, Y. Evaluation of deoxynivalenol-induced toxic effects on mouse endometrial stromal cells: Cell apoptosis and cell cycle. Biochem. Biophys. Res. Commun. 2017, 483, 572–577. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Zong, Q.; Hu, P.; Bao, W.; Liu, H.; Cai, D. Lactoferrin Restores the Deoxynivalenol-Impaired Spermatogenesis and Blood-Testis Barrier Integrity via Improving the Antioxidant Capacity and Modifying the Cell Adhesion and Inflammatory Response. Antioxidants 2023, 12, 152. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Wang, J.; Han, S.; Zhang, Y.; Ma, M.; Zhu, Q.; Zhang, K.; Yin, H. Zearalenone induces apoptosis and cytoprotective autophagy in chicken granulosa cells by PI3K-AKT-mTOR and MAPK signaling pathways. Toxins 2021, 13, 199. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. The toxicity mechanisms of DON to humans and animals and potential biological treatment strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 790–812. [Google Scholar] [CrossRef]
- Hasnat, M.; Yuan, Z.; Ullah, A.; Naveed, M.; Raza, F.; Baig, M.M.F.A.; Khan, A.; Xu, D.; Su, Y.; Sun, L.; et al. Mitochondria-dependent apoptosis in triptolide-induced hepatotoxicity is associated with the Drp1 activation. Toxicol. Mech. Methods 2020, 30, 124–133. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, C.; Han, J.; Zhu, J.; Liu, Q.; Sun, D.; Xia, X.; Peng, X. Inhibition of ER stress attenuates kidney injury and apoptosis induced by 3-MCPD via regulating mitochondrial fission/fusion and Ca2+ homeostasis. Cell Biol. Toxicol. 2021, 37, 795–809. [Google Scholar] [CrossRef]
- Dromparis, P.; Paulin, R.; Sutendra, G.; Qi, A.C.; Bonnet, S.; Michelakis, E.D. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ. Res. 2013, 113, 126–136. [Google Scholar] [CrossRef]
- Lalier, L.; Mignard, V.; Joalland, M.P.; Lanoé, D.; Cartron, P.F.; Manon, S.; Vallette, F.M. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021, 12, 182. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef]
- Loncke, J.; Kaasik, A.; Bezprozvanny, I.; Parys, J.B.; Kerkhofs, M.; Bultynck, G. Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. 2021, 31, 598–612. [Google Scholar] [CrossRef]
- Çoku, J.; Booth, D.M.; Skoda, J.; Pedrotty, M.C.; Vogel, J.; Liu, K.; Vu, A.; Carpenter, E.L.; Ye, J.C.; Chen, M.A.; et al. Reduced ER-mitochondria connectivity promotes neuroblastoma multidrug resistance. EMBO J. 2022, 41, e108272. [Google Scholar] [CrossRef]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia induces endoplasmic reticulum stress in atrial cardiomyocytes, and mitofusin-2 downregulation prevents mitochondrial dysfunction and subsequent cell death. Oxid. Med. Cell Longev. 2020, 14, 6569728. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Guo, Y.; Liu, R.; Qi, F.; Li, X.; Yu, H.; Cheng, S.; Wang, Z. Circadian gene clock participates in mitochondrial apoptosis pathways by regulating mitochondrial membrane potential, mitochondria out membrane permeablization and apoptosis factors in AML12 hepatocytes. Mol. Cell Biochem. 2020, 467, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liao, Z. Mitochondrial apoptotic signaling pathway in neurons following brain injury induced by hypoxi. Fa Yi Xue Za Zhi 2004, 20, 178–182. [Google Scholar] [PubMed]
- Zhang, C.; Zhang, K.; Chen, F.; Chen, Y.; Yang, X.; Cai, Z.; Jiang, Y.; Wang, X.; Zhang, G.; Wang, F. Deoxynivalenol triggers porcine intestinal tight junction disorder: Insights from mitochondrial dynamics and mitophagy. Ecotoxicol. Environ. Saf. 2022, 248, 114291. [Google Scholar] [CrossRef] [PubMed]
- Korobova, F.; Ramabhadran, V.; Higgs, H.N. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science 2013, 339, 464–467. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.M.; Ke, H.L.; Ying, L.; Wu, Y.; Zhao, G.J.; Lu, Z.Q. Mdivi-1 Protects CD4+ T cells against apoptosis via balancing mitochondrial fusion-fission and preventing the induction of endoplasmic reticulum stress in sepsis. Mediat. Inflamm. 2019, 2019, 7329131. [Google Scholar] [CrossRef]
- Xie, N.; Wang, C.; Wu, C.; Cheng, X.; Gao, Y.; Zhang, H.; Zhang, Y.; Lian, Y. Mdivi-1 protects epileptic hippocampal neurons from apoptosis via inhibiting oxidative stress and endoplasmic reticulum stress in vitro. Neurochem. Res. 2016, 41, 1335–1342. [Google Scholar] [CrossRef]
- Gao, F.; Quan, J.; Lee, M.A.; Ye, W.; Yuk, J.M.; Cha, G.H.; Choi, I.W.; Lee, Y.H. Trichomonas vaginalis induces apoptosis via ROS and ER stress response through ER-mitochondria crosstalk in SiHa cells. Parasit. Vectors 2021, 14, 603. [Google Scholar] [CrossRef]
- Zhao, J.; Hai, S.; Chen, J.; Ma, L.; Rahman, S.U.; Zhao, C.; Feng, S.; Li, Y.; Wu, J.; Wang, X. Zearalenone induces apoptosis in porcine endometrial stromal cells through JNK signaling pathway based on endoplasmic reticulum stress. Toxins 2022, 14, 758. [Google Scholar] [CrossRef]
- Hemann, M.T.; Lowe, S.W. The p53-Bcl-2 connection. Cell Death Differ. 2006, 13, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Zhu, L.; Cao, L.; Xu, W.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J. Autophagy protects PC12 cells against deoxynivalenol toxicity via the Class III PI3K/beclin 1/Bcl-2 pathway. J. Cell. Physiol. 2020, 235, 7803–7815. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, Y.; Zhu, L.; Xu, W.; Chu, X.; Zhang, Y.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; et al. Deoxynivalenol induces Caspase-8-mediated apoptosis through the mitochondrial pathway in hippocampal nerve cells of piglet. Toxins 2021, 13, 73. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, S.; Chen, J.; Ma, L.; Wang, C.; Chen, C.; Rahman, S.U.; Zhao, C.; Feng, S.; Wu, J.; Wang, X. Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress. Toxins 2023, 15, 471. https://doi.org/10.3390/toxins15070471
Hai S, Chen J, Ma L, Wang C, Chen C, Rahman SU, Zhao C, Feng S, Wu J, Wang X. Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress. Toxins. 2023; 15(7):471. https://doi.org/10.3390/toxins15070471
Chicago/Turabian StyleHai, Sirao, Jiawen Chen, Li Ma, Chenlong Wang, Chuangjiang Chen, Sajid Ur Rahman, Chang Zhao, Shibin Feng, Jinjie Wu, and Xichun Wang. 2023. "Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress" Toxins 15, no. 7: 471. https://doi.org/10.3390/toxins15070471
APA StyleHai, S., Chen, J., Ma, L., Wang, C., Chen, C., Rahman, S. U., Zhao, C., Feng, S., Wu, J., & Wang, X. (2023). Combination of Zearalenone and Deoxynivalenol Induces Apoptosis by Mitochondrial Pathway in Piglet Sertoli Cells: Role of Endoplasmic Reticulum Stress. Toxins, 15(7), 471. https://doi.org/10.3390/toxins15070471





