Antagonistic Activity of Oroxylin A against Fusarium graminearum and Its Inhibitory Effect on Zearalenone Production
Abstract
:1. Introduction
2. Results
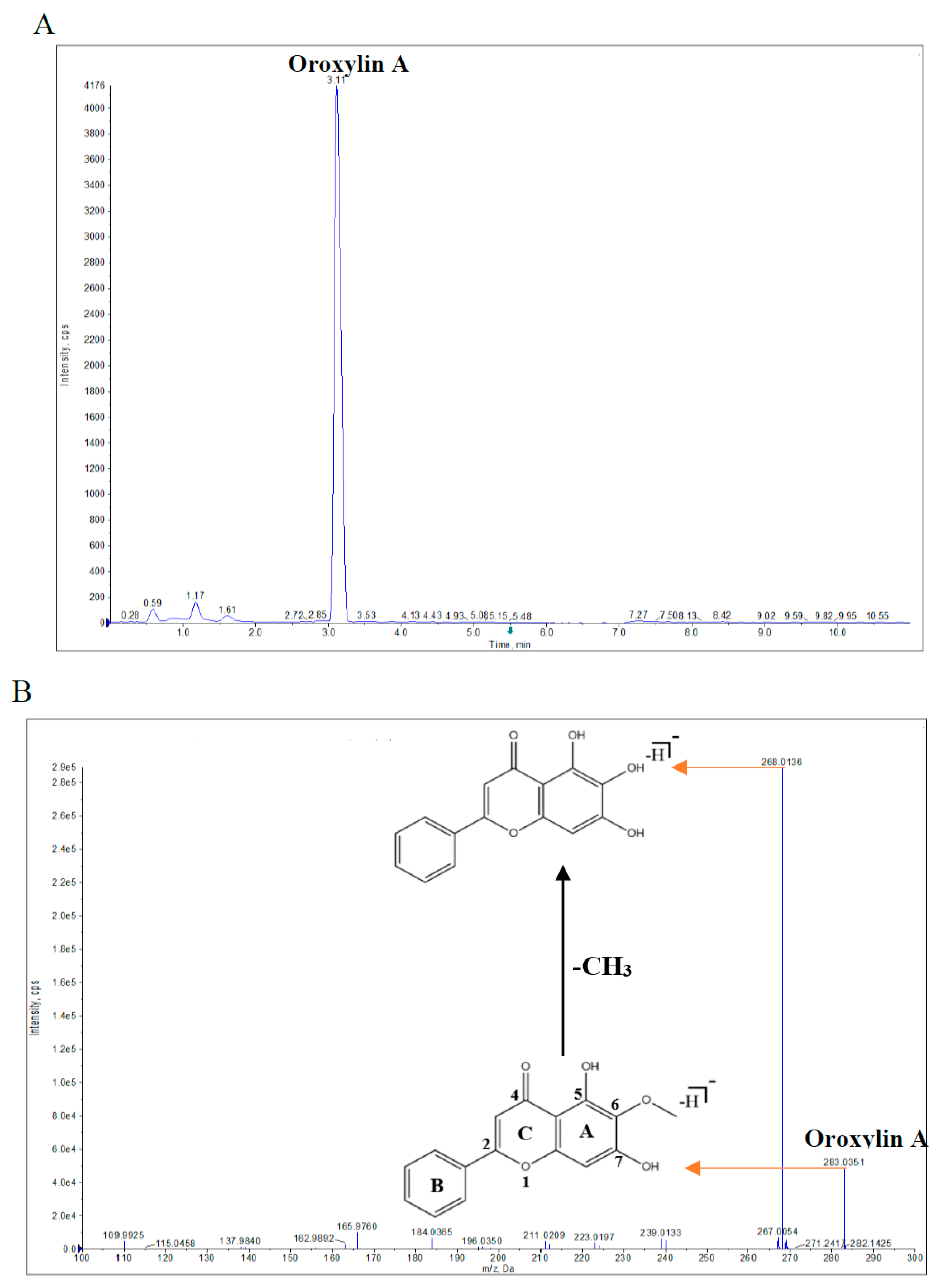
2.1. Qualitative and Quantitative Analyses of Oroxylin A in Piper Sarmentosum Extract (PSE)
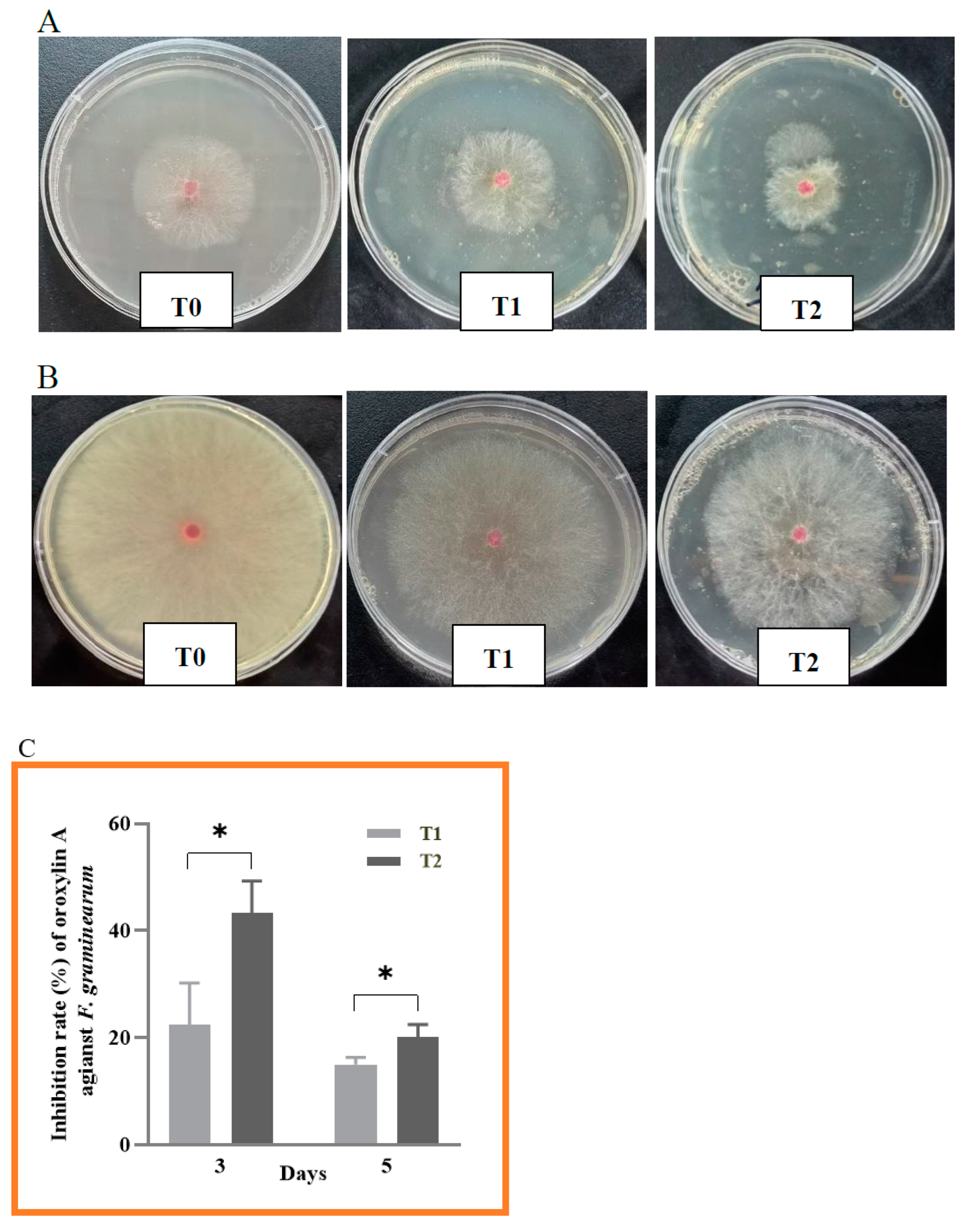
2.2. Antifungal Activity of Oroxylin A against F. graminearum
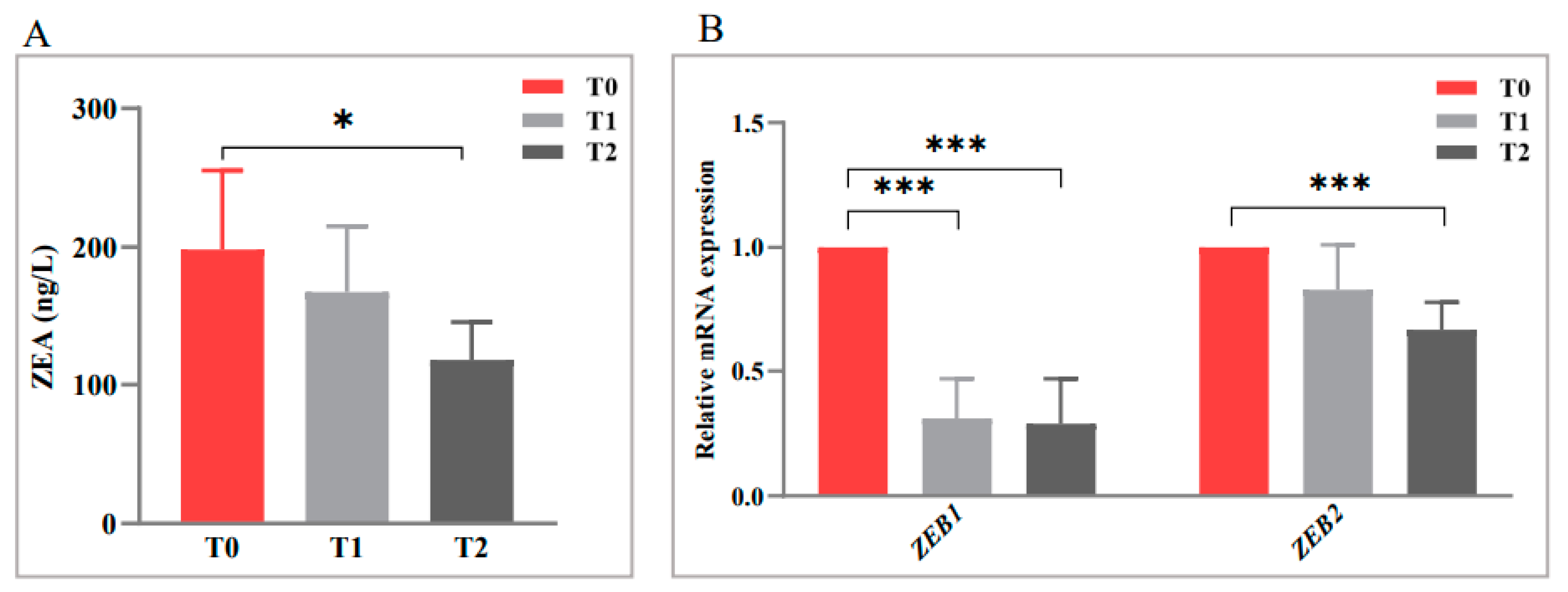
2.3. Anti-Mycotoxin Activity of Oroxylin A against F. graminearum
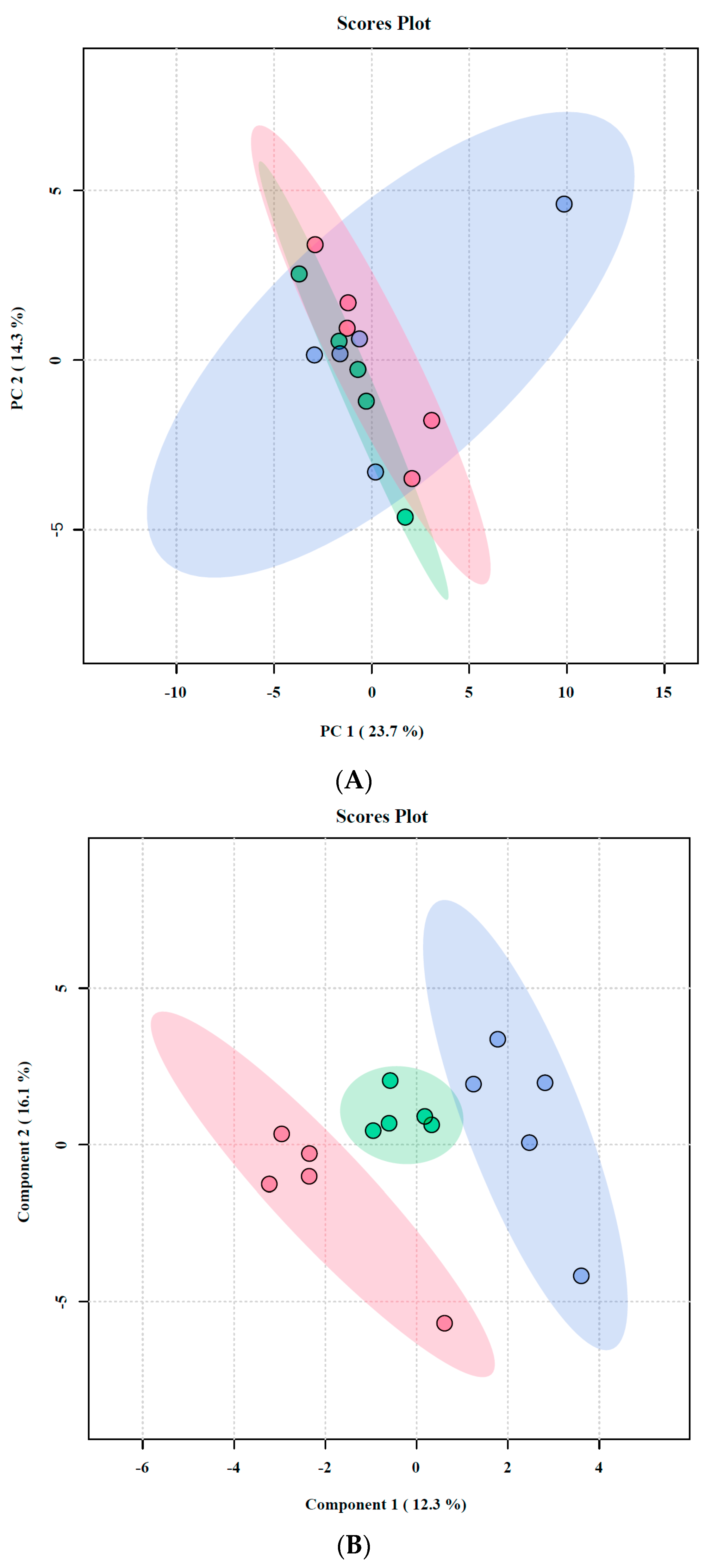
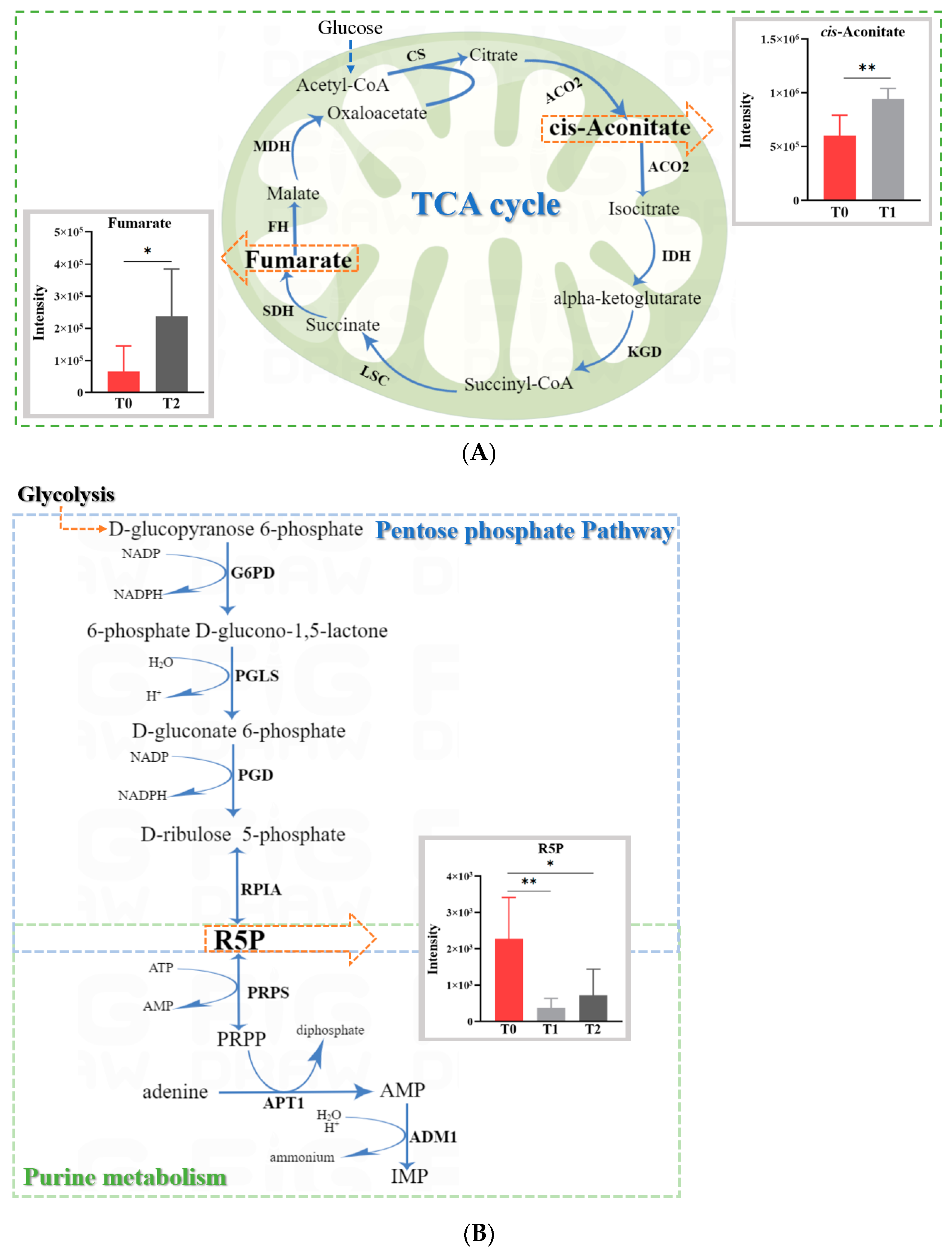
2.4. Untargeted Metabolomic Analysis of Oroxylin A-Treated F. graminearum
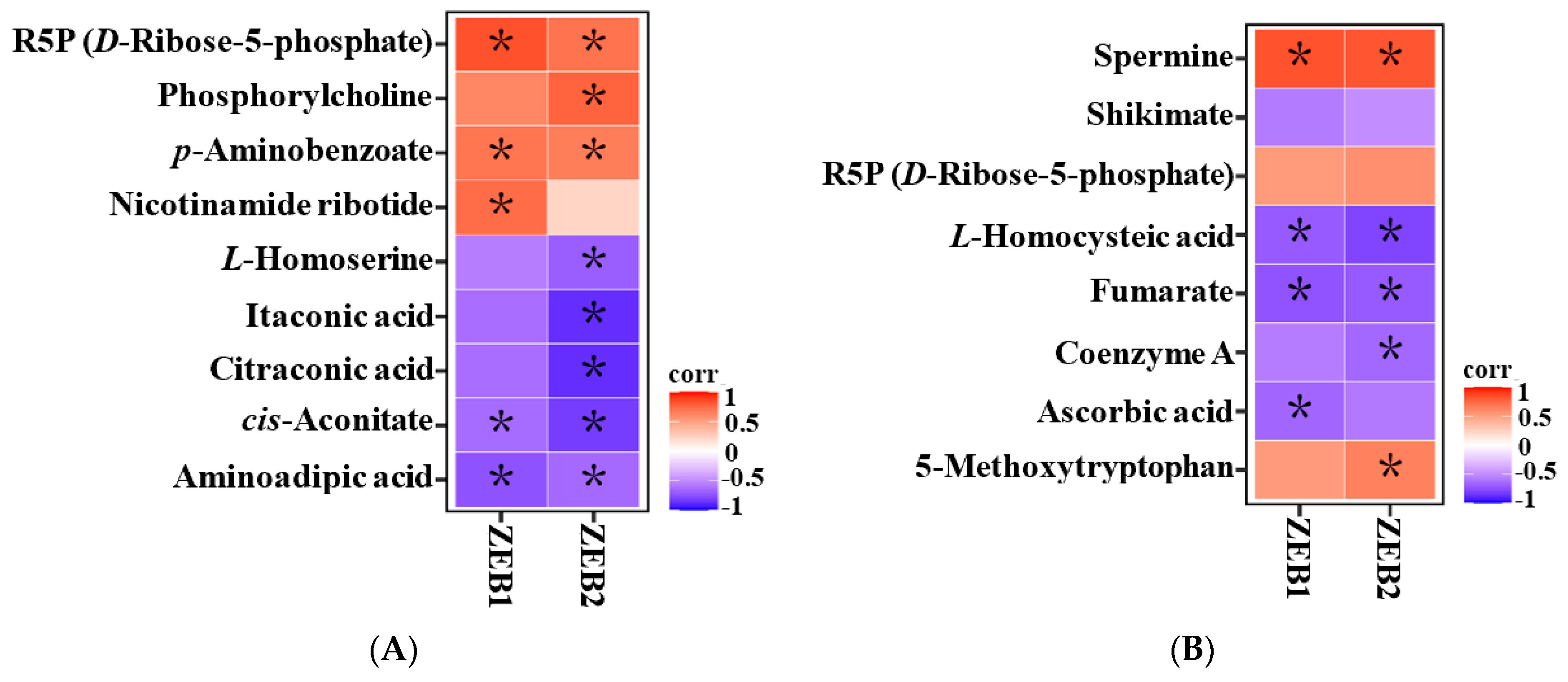
2.5. Correlation Analysis between Differential Metabolites and Expression Changes of ZEB1 and ZEB2 Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Fungal Culture
5.3. Analysis of Oroxylin A in PSE via UPLC-QTOF-MS/MS
5.3.1. Preparation of Oroxylin A Standard Solution and PSE
5.3.2. UPLC-QTOF-MS/MS Analysis
5.4. Experimental Design
5.4.1. Antifungal Activity of Oroxylin A against F. graminearum
5.4.2. Anti-Mycotoxin Activity of Oroxylin A against F. graminearum
UPLC Analysis of ZEA
mRNA Expression Analysis of ZEA Biosynthesis Genes via RT-PCR
Metabolomic Analysis
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Cela, E.; Kiaitsi, E.; Sulyok, M.; Medina, A.; Magan, N. Fusarium graminearum in stored wheat: Use of CO2 production to quantify dry matter losses and relate this to relative risks of zearalenone contamination under interacting environmental conditions. Toxins 2018, 10, 86. [Google Scholar] [CrossRef]
- Ma, T.; Liu, K.; Yang, X.; Yang, J.; Pan, M.; Wang, S. Development of indirect competitive ELISA and visualized multicolor ELISA based on gold nanorods growth for the determination of Zearalenone. Foods 2021, 10, 2654. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Wang, L.; Li, L.; Liu, J.; Liu, S.; Zhao, X. Bacillus licheniformis CK1 alleviates the toxic effects of zearalenone in feed on weaned female Tibetan piglets. J. Anim. Sci. 2018, 96, 4471–4480. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, S.; Demay, F.; Pham, T.H.; Moulis, S.; Efstathiou, T.; Chalmel, F.; Pakdel, F. Deciphering the molecular mechanisms sustaining the estrogenic activity of the two major dietary compounds zearalenone and apigenin in ER-positive breast cancer cell lines. Nutrients 2019, 11, 237. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Cabral, L.; Pinto, V.F.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef]
- Webster, D.; Taschereau, P.; Belland, R.J.; Sand, C.; Rennie, R.P. Antifungal activity of medicinal plant extracts; preliminary screening studies. J. Ethnopharmacol. 2008, 115, 140–146. [Google Scholar] [CrossRef]
- Abdali, E.; Javadi, S.; Akhgari, M.; Hosseini, S.; Dastan, D. Chemical composition and biological properties of Satureja avromanica Maroofi. J. Food Sci. Technol. 2017, 54, 727–734. [Google Scholar] [CrossRef]
- Dikhoba, P.M.; Mongalo, N.I.; Elgorashi, E.E.; Makhafola, T.J. Antifungal and anti-mycotoxigenic activity of selected South African medicinal plants species. Heliyon 2019, 5, e02668. [Google Scholar] [CrossRef]
- Heard, S.C.; Wu, G.; Winter, J.M. Antifungal natural products. Curr. Opin. Biotechnol. 2021, 69, 232–241. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Zhou, H.; Hou, G. Chemical composition and anti-inflammatory activity of n-butanol extract of Piper sarmentosum Roxb. In the intestinal porcine epithelial cells (IPEC-J2). J. Ethnopharmacol. 2021, 269, 113723. [Google Scholar] [CrossRef] [PubMed]
- Zaidan, M.R.; Noor Rain, A.; Badrul, A.R.; Adlin, A.; Norazah, A.; Zakiah, I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop. Biomed. 2005, 22, 165–170. [Google Scholar]
- Syed Ab Rahman, S.F.; Sijam, K.; Omar, D. Chemical composition of Piper sarmentosum extracts and antibacterial activity against the plant pathogenic bacteria Pseudomonas fuscovaginae and Xanthomonas oryzae pv. oryzae. J. Plant Dis. Prot. 2014, 121, 237–242. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, D.; Zhou, H. Metabolic profiling of two medicinal Piper species. S. Afr. J. Bot. 2021, 139, 281–289. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Hou, G.; Ji, F.; Wang, D. Antifungal activity and metabolomics analysis of Piper sarmentosum extracts against Fusarium graminearum. J. Appl. Microbiol. 2023, 134, lxad019. [Google Scholar] [CrossRef]
- Lu, L.; Guo, Q.; Zhao, L. Overview of Oroxylin A: A promising flavonoid compound. Phytother. Res. 2016, 30, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Hegde, M.; Girisa, S.; Devanarayanan, T.N.; Alqahtani, M.S.; Abbas, M.; Sil, S.K.; Sethi, G.; Chen, J.T.; Kunnumakkara, A.B. Oroxylin A: A promising flavonoid for prevention and treatment of chronic diseases. Biomolecules 2022, 12, 1185. [Google Scholar] [CrossRef]
- Sajeev, A.; Hegde, M.; Daimary, U.D.; Kumar, A.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Modulation of diverse oncogenic signaling pathways by Oroxylin A: An important strategy for both cancer prevention and treatment. Phytomedicine 2022, 105, 154369. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Atoui, A. Current status on the molecular biology of zearalenone: Its biosynthesis and molecular detection of zearalenone producing Fusarium species. Eur. J. Plant Pathol. 2021, 159, 247–258. [Google Scholar] [CrossRef]
- Kai, J.; Yang, X.; Wang, Z.; Wang, F.; Jia, Y.; Wang, S.; Tan, S.; Chen, A.; Shao, J.; Zhang, F.; et al. Oroxylin a promotes PGC-1α/Mfn2 signaling to attenuate hepatocyte pyroptosis via blocking mitochondrial ROS in alcoholic liver disease. Free Radic. Biol. Med. 2020, 153, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.S.; Babu, T.H.; Srinivas, P.V.; Sastry, B.S.; Kishore, K.H.; Murty, U.S.N.; Rao, J.M. Synthesis and in vitro study of novel 7-O-acyl derivatives of Oroxylin A as antibacterial agents. Bioorganic Med. Chem. Lett. 2005, 15, 3953–3956. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.Y.; Lu, A.R.; Li, J.X.; Liu, J.; Fang, Y.M.; Pei, T.L.; Zhong, X.; Wei, Y.K.; Kong, Y.; Qiu, W.Q.; et al. Two types of O-methyltransferase are involved in biosynthesis of anticancer methoxylated 4′-deoxyflavones in Scutellaria baicalensis Georgi. Plant Biotechnol. J. 2022, 20, 129–142. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Park, S.B.; Im, J.H.; Chun, H.S. Plant-based natural flavonoids show strong inhibition of aflatoxin production and related gene expressions correlated with chemical structure. Food Microbiol. 2023, 109, 10414. [Google Scholar] [CrossRef] [PubMed]
- Riyanti; Balansa, W.; Liu, Y.; Sharma, A.; Mihajlovic, S.; Hartwig, C.; Leis, B.; Rieuwpassa, F.J.; Gruber Ljong, F.; Wägele, H.; et al. Selection of sponge-associated bacteria with high potential for the production of antibacterial compounds. Sci. Rep. 2020, 10, 19614. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Lone, A.; Betts, D.H.; Cumming, R.C. Lactate preconditioning promotes a HIF-1α-mediated metabolic shift from OXPHOS to glycolysis in normal human diploid fibroblasts. Sci. Rep. 2020, 10, 8388. [Google Scholar] [CrossRef]
- Aziz, I.; Bibi, T.; Rashid, N.; Aono, R.; Atomi, H.; Akhtar, M. A phosphofructokinase homolog from Pyrobaculum calidifontis displays kinase activity towards pyrimidine nucleosides and ribose 1-phosphate. J. Bacteriol. 2018, 200, 10–1128. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, R.; Xia, M.; Bai, X.; Mou, J.; Zheng, Y.; Wang, M. Effect of aspartic acid and glutamate on metabolism and acid stress resistance of Acetobacter pasteurianus. Microb. Cell Factories 2017, 16, 109. [Google Scholar] [CrossRef]
- Yang, R.; Yang, C.; Ma, L.; Zhao, Y.; Guo, Z.; Niu, J.; Chu, Q.; Ma, Y.; Li, B. Identification of purine biosynthesis as an NADH-sensing pathway to mediate energy stress. Nat. Commun. 2022, 13, 7031. [Google Scholar] [CrossRef]
- Southall, T.D.; Gold, K.S.; Egger, B.; Davidson, C.M.; Caygill, E.E.; Marshall, O.J.; Brand, A.H. Cell-type-specific profiling of gene expression and chromatin binding without cell isolation: Assaying RNA Pol II occupancy in neural stem cells. Dev. Cell 2013, 26, 101–112. [Google Scholar] [CrossRef]
- Suzuki, C.; Hatayama, N.; Ogawa, T.; Nanizawa, E.; Otsuka, S.; Hata, K.; Seno, H.; Naito, M.; Hirai, S. Cardioprotection via metabolism for rat heart preservation using the high-pressure gaseous mixture of carbon monoxide and oxygen. Int. J. Mol. Sci. 2020, 21, 8858. [Google Scholar] [CrossRef] [PubMed]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Kalagatur, N.K.; Mudili, V.; Siddaiah, C.; Gupta, V.K.; Natarajan, G.; Sreepathi, M.H.; Vardhan, B.H.; Putcha, V.L.R. Antagonistic activity of Ocimum sanctum L. essential oil on growth and zearalenone production by Fusarium graminearum in maize grains. Front. Microbiol. 2015, 6, 892. [Google Scholar] [CrossRef]
- GB 5009.209–2016; Test of Zearalenone in Foods. National Health and Family Planning Commission of the People’s Republic of China (NHFPCRC): Beijing, China, 2016.
- Sim, J.H.; Tian, F.; Jung, S.Y.; Auh, J.H.; Chun, H.S. Multiplex polymerase chain reaction assays for the detection of the zearalenone chemotype of Fusarium species in white and brown rice. Int. J. Food Microbiol. 2018, 269, 120–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Hou, G.; Zhou, H.; Abouelezz, K.; Ye, Y.; Rao, J.; Guan, S.; Wang, D. Antagonistic Activity of Oroxylin A against Fusarium graminearum and Its Inhibitory Effect on Zearalenone Production. Toxins 2023, 15, 535. https://doi.org/10.3390/toxins15090535
Zhou L, Hou G, Zhou H, Abouelezz K, Ye Y, Rao J, Guan S, Wang D. Antagonistic Activity of Oroxylin A against Fusarium graminearum and Its Inhibitory Effect on Zearalenone Production. Toxins. 2023; 15(9):535. https://doi.org/10.3390/toxins15090535
Chicago/Turabian StyleZhou, Luli, Guanyu Hou, Hanlin Zhou, Khaled Abouelezz, Yuxiu Ye, Jun Rao, Song Guan, and Dingfa Wang. 2023. "Antagonistic Activity of Oroxylin A against Fusarium graminearum and Its Inhibitory Effect on Zearalenone Production" Toxins 15, no. 9: 535. https://doi.org/10.3390/toxins15090535




