Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide
Abstract
:1. Introduction
2. Results
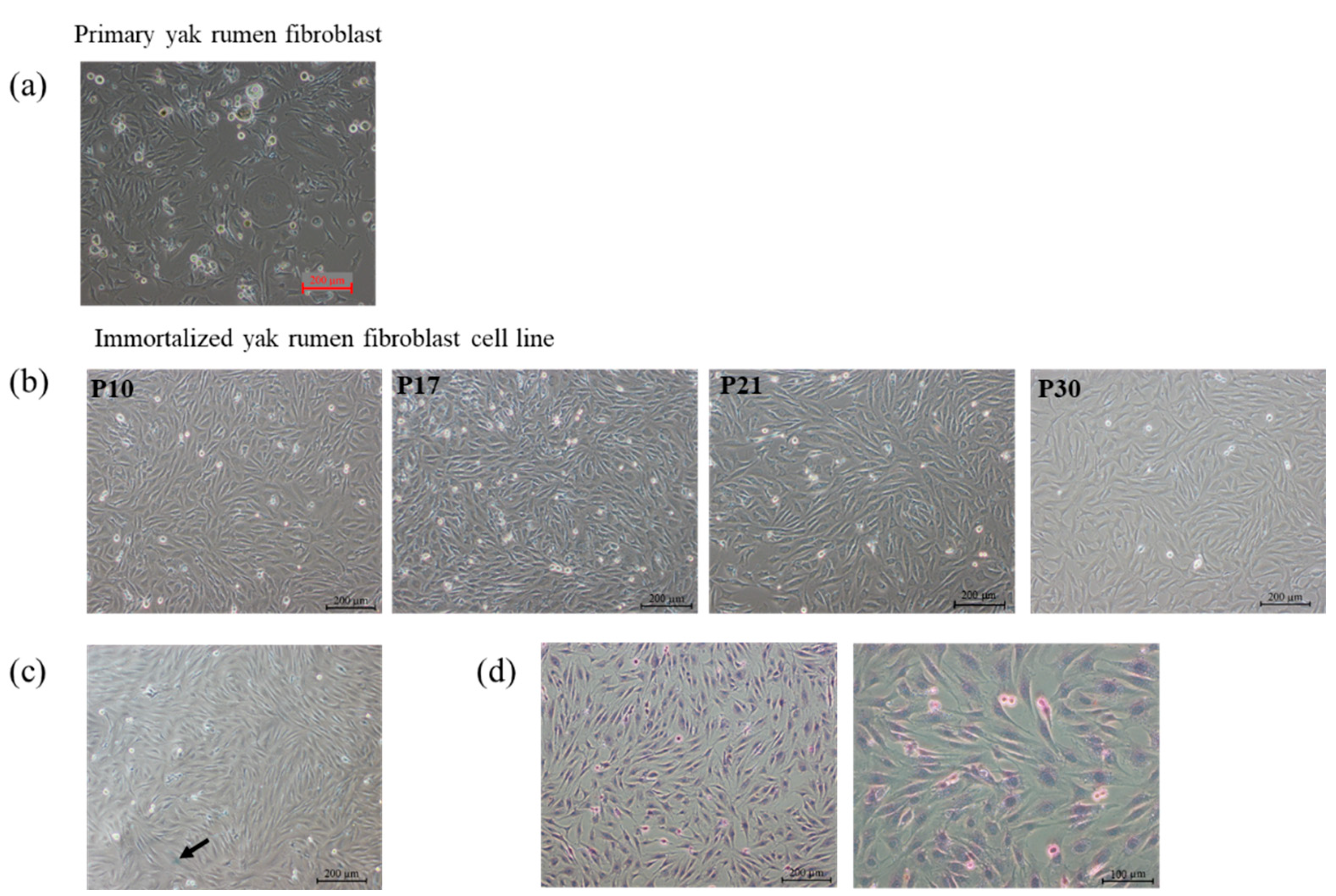
2.1. Establishment of Primary and Yak Rumen Fibroblast Cell Lines
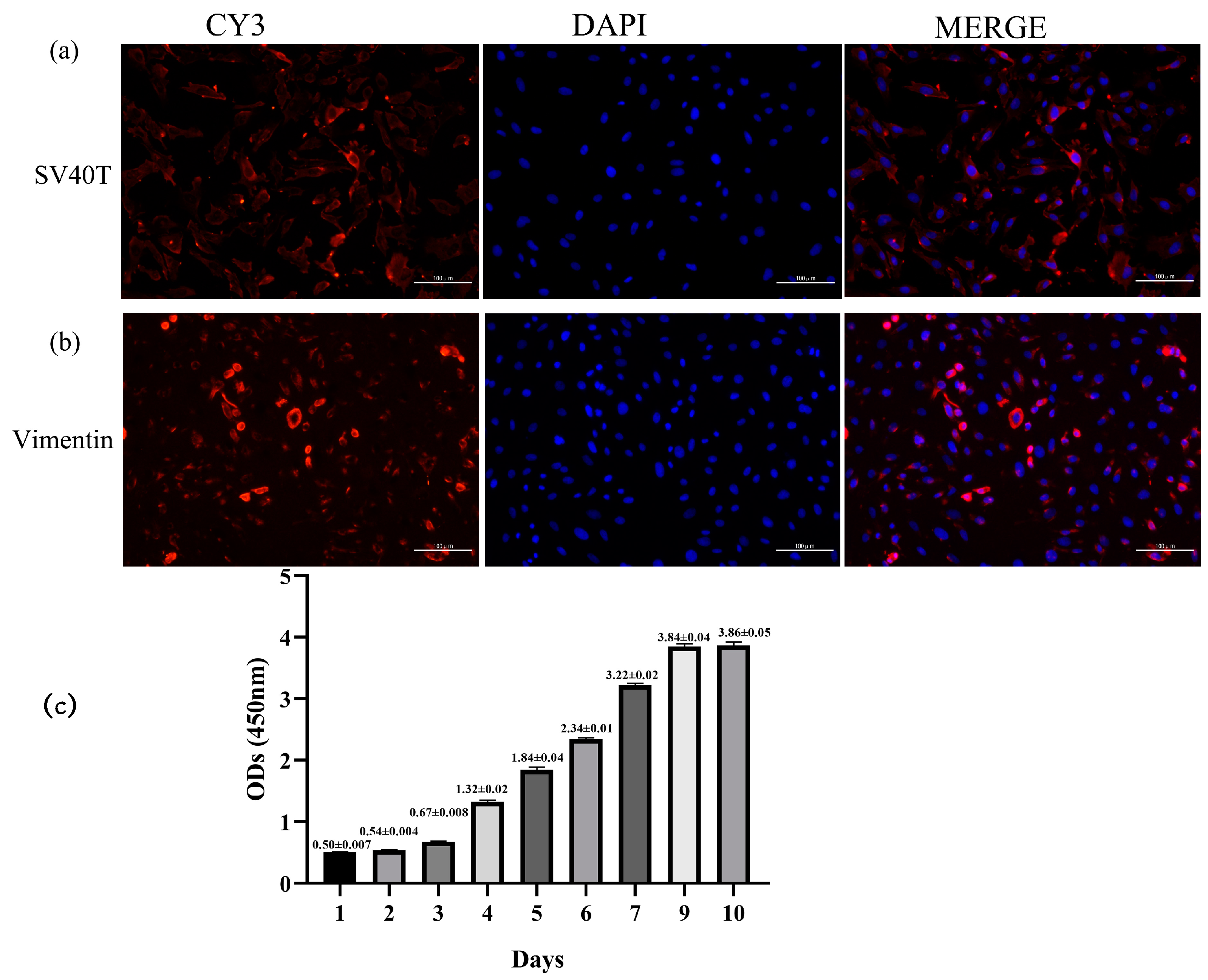
2.2. Transfection Efficiency of SV40T Gene, Immunofluorescence Identification, and Growth Curve in Yak Rumen Fibroblast Cell Lines
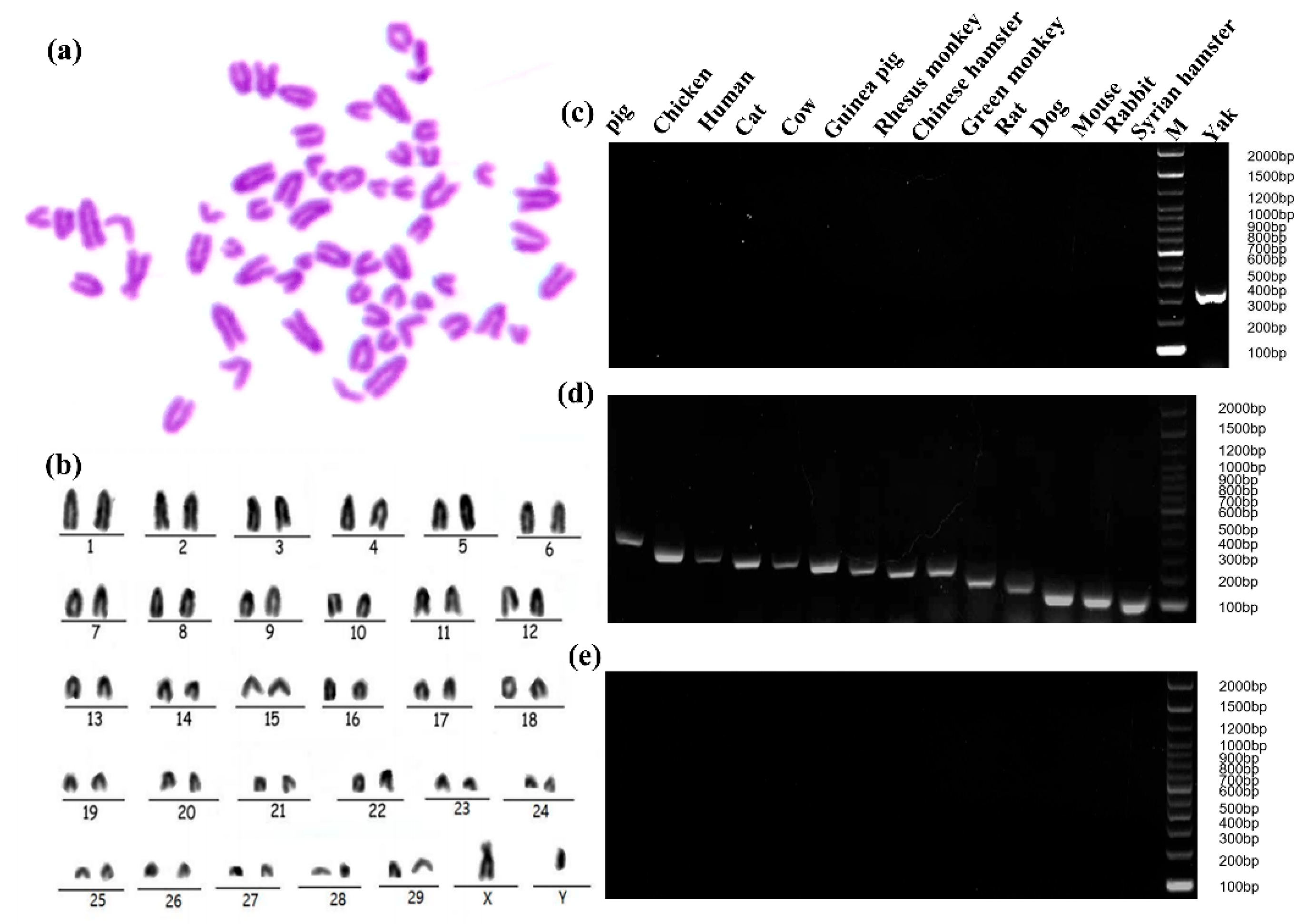
2.3. Specific Analysis and Identification of Cell Species
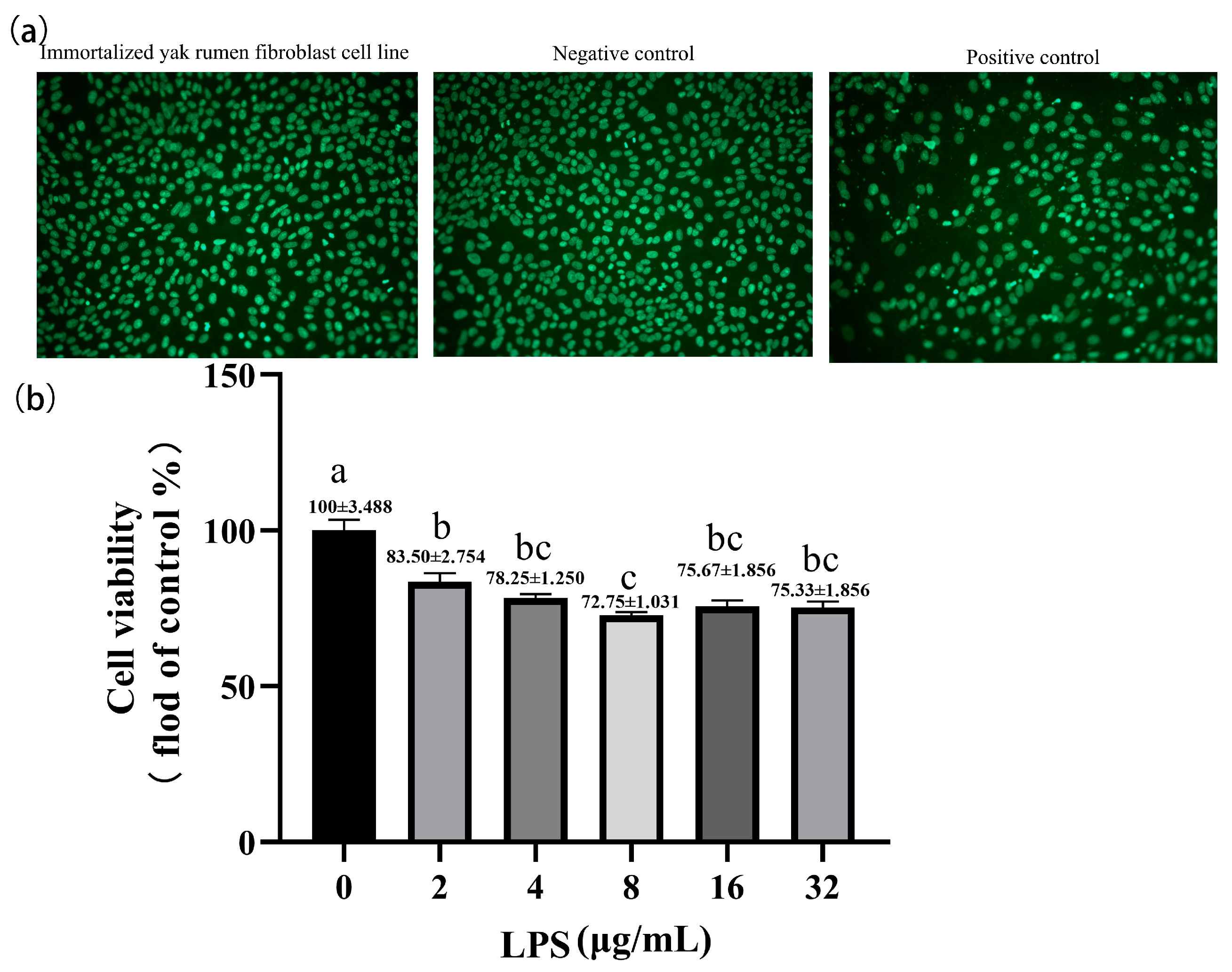
2.4. Effect of Lipopolysaccharide on the Cell Viability of Yak Rumen Fibroblasts
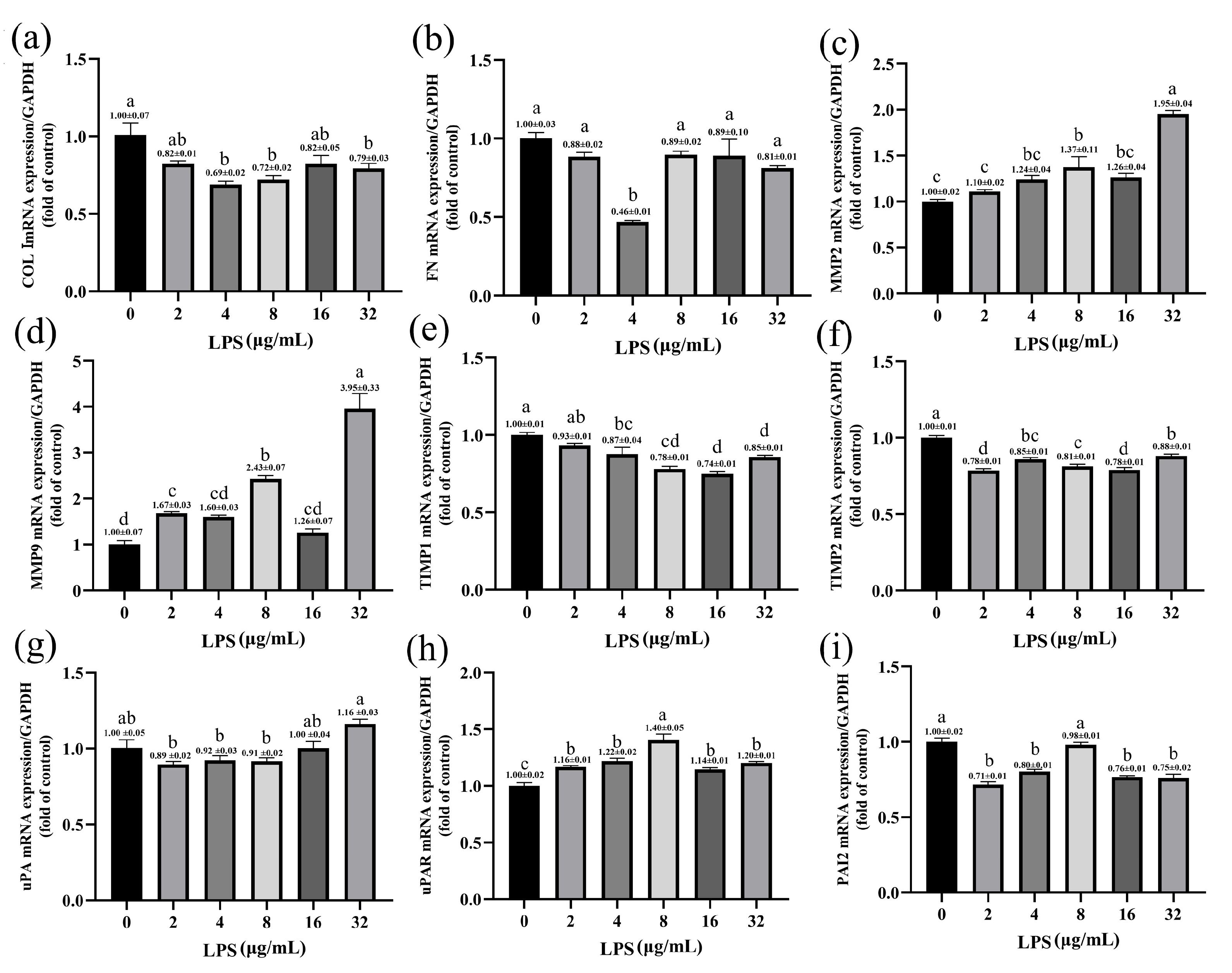
2.5. Effects of LPS on the Expression of ECM-Degradation-Related Genes in Yak Rumen Fibroblasts
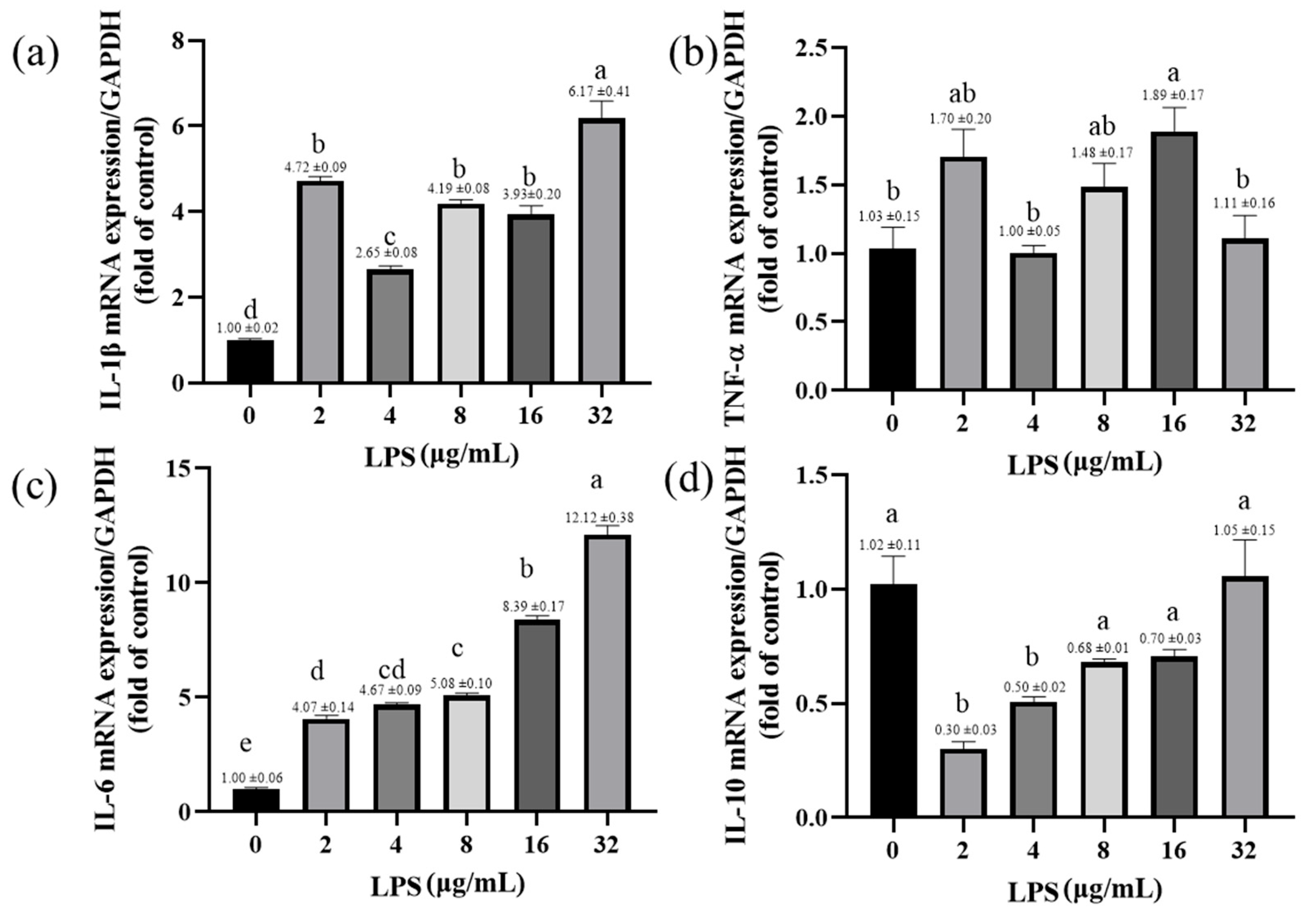
2.6. Effects of LPS on the Expression of Inflammatory-Response-Related Genes in Yak Rumen Fibroblasts
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolation and Cultivation of Primary Yak Rumen Fibroblasts
5.2. The Establishment of Yak Rumen Fibroblast Cell Line
5.3. Senescence-Associated β-Galactosidase (SA-β-Gal) and Hematoxylin–Eosin (HE) Staining
5.4. Cell Viability
5.5. Immunofluorescence Identification
5.6. Karyotype Analysis
5.7. Cell Species Identification
5.8. DNA Fluorescence Staining
5.9. RNA Was Extracted and Analyzed Using Quantitative Reverse Transcription Polymerase Chain Reaction (qRT–PCR) for Gene Expression
5.10. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Q.; Guo, Y.; Engelhardt, S.C.; Weladji, R.B.; Zhou, Y.; Long, M.; Meng, X. Endangered wild yak (Bos grunniens) in the Tibetan plateau and adjacent regions: Population size, distribution, conservation perspectives and its relation to the domestic subspecies. J. Nat. Conserv. 2016, 32, 35–43. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef]
- Shah, A.M.; Bano, I.; Qazi, I.H.; Matra, M.; Wanapat, M. “The Yak”—A remarkable animal living in a harsh environment: An overview of its feeding, growth, production performance, and contribution to food security. Front. Vet. Sci. 2023, 10, 1086985. [Google Scholar] [CrossRef]
- Ayalew, W.; Chu, M.; Liang, C.; Wu, X.; Yan, P. Adaptation mechanisms of yak (Bos grunniens) to high-altitude environmental stress. Animals 2021, 11, 2344. [Google Scholar] [CrossRef]
- Jing, X.; Ding, L.; Zhou, J.; Huang, X.; Degen, A.; Long, R. The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. 2022, 9, 249–258. [Google Scholar] [CrossRef]
- Ma, G.; Cairang, D.; Zhao, Y.; Chen, S.; Ma, Z. Research progress of mineral nutrition of yak (Bos grunniens). J. Henan Agric. Sci. 2012, 41, 11–14. [Google Scholar]
- Ma, Z. Research progress of the yak (Bos grunniens) mitochondrial genome. J. China Agric. Univ. 2014, 19, 154–161. [Google Scholar]
- Bazhenova, B.; Zabalueva, Y.; Danilov, M.; Vtorushina, I.; Badmaeva, T. Yak meat as a lucrative raw material for meat products. Food Process. Tech. Technol. 2018, 48, 16–33. [Google Scholar] [CrossRef]
- Shah, M.A.; Xu, C.; Wu, S.; Zhao, W.; Luo, H.; Yi, C.; Liu, W.; Cai, X. Isolation and characterization of spermatogenic cells from cattle, yak and cattleyak. Anim. Reprod. Sci. 2018, 193, 182–190. [Google Scholar] [CrossRef]
- Wang, J.; Hu, R.; Wang, Z.; Guo, Y.; Wang, S.; Zou, H.; Peng, Q.; Jiang, Y. Establishment of Immortalized Yak Ruminal Epithelial Cell Lines by Lentivirus-Mediated SV40T and hTERT Gene Transduction. Oxidative Med. Cell. Longev. 2022, 2022, 8128028. [Google Scholar] [CrossRef]
- Wang, J.; Pan, Y.; Wang, M.; Xu, R.; Han, X.; Ma, R.; Zhao, L.; Zhang, T.; Wang, Y.; Zhao, T.; et al. Follicular fluid exosomes regulate OVGP1 secretion in yak oviduct epithelial cells via autophagy in vitro. J. Cell. Physiol. 2023, 238, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, H.; Hao, M.; Yu, S. Establishment and characterization of two fetal fibroblast cell lines from the yak. In Vitr. Cell. Dev. Biol. Anim. 2012, 48, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Ichim, T.E.; O’Heeron, P.; Kesari, S. Fibroblasts as a practical alternative to mesenchymal stem cells. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar]
- Miao, Z.; Ding, Y.; Bi, Y.; Chen, M.; Cao, X.; Wang, F. Staphylococcus aureus on the effect of expression of MMPs/TIMPs and uPA system in bovine mammary fibroblasts. J. Microbiol. Immunol. Infect. 2021, 54, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ueha, S.; Shand, F.H.; Matsushima, K. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front. Immunol. 2012, 3, 71. [Google Scholar] [CrossRef]
- Bush, S.J.; McCulloch, M.E.; Muriuki, C.; Salavati, M.; Davis, G.M.; Farquhar, I.L.; Lisowski, Z.M.; Archibald, A.L.; Hume, D.A.; Clark, E.L. Comprehensive transcriptional profiling of the gastrointestinal tract of ruminants from birth to adulthood reveals strong developmental stage specific gene expression. G3 Genes Genomes Genet. 2019, 9, 359–373. [Google Scholar] [CrossRef]
- Czerkawski, J.W. An Introduction to Rumen Studies; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.S.; The, T.H.; Jahn, G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 1995, 76, 741–750. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Monteiro, H.F.; Faciola, A.P. Ruminal acidosis, bacterial changes, and lipopolysaccharides. J. Anim. Sci. 2020, 98, skaa248. [Google Scholar] [CrossRef]
- Aschenbach, J.; Gäbel, G. Effect and absorption of histamine in sheep rumen: Significance of acidotic epithelial damage. J. Anim. Sci. 2000, 78, 464–470. [Google Scholar] [CrossRef]
- Yang, J.X.; Li, M.; Hu, X.; Lu, J.C.; Wang, Q.; Lu, S.Y.; Gao, F.; Jin, S.W.; Zheng, S.X. Protectin DX promotes epithelial injury repair and inhibits fibroproliferation partly via ALX/PI3K signalling pathway. J. Cell. Mol. Med. 2020, 24, 14001–14012. [Google Scholar] [CrossRef]
- He, G.; Ma, M.; Yang, W.; Wang, H.; Zhang, Y.; Gao, M.-Q. SDF-1 in mammary fibroblasts of bovine with mastitis induces EMT and inflammatory response of epithelial cells. Int. J. Biol. Sci. 2017, 13, 604. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Neilson, E.G. Origin and functional heterogeneity of fibroblasts. FASEB J. 2020, 34, 3519–3536. [Google Scholar] [CrossRef] [PubMed]
- Neilson, E.G. Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat. Clin. Pract. Nephrol. 2006, 2, 101–108. [Google Scholar] [CrossRef]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Wang, J.H.-C.; Thampatty, B.P.; Lin, J.-S.; Im, H.-J. Mechanoregulation of gene expression in fibroblasts. Gene 2007, 391, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ergashev, U.; Malikov, N.; Yakubov, D.; Abdusalomov, B.; Gafurov, B. Use of Collagen and Fibroblasts in Modern Medicine (Review of Literature). Eurasian Res. Bull. 2023, 17, 78–84. [Google Scholar]
- Zou, M.-L.; Teng, Y.-Y.; Wu, J.-J.; Liu, S.-Y.; Tang, X.-Y.; Jia, Y.; Chen, Z.-H.; Zhang, K.-W.; Sun, Z.-L.; Li, X.; et al. Fibroblasts: Heterogeneous cells with potential in regenerative therapy for scarless wound healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Piwocka, O.; Musielak, M.; Piotrowski, I.; Suchorska, W.M.; Trzeciak, T. From donor to the lab: A fascinating journey of primary cell lines. Front. Cell Dev. Biol. 2021, 9, 711381. [Google Scholar] [CrossRef]
- Stacey, G.; MacDonald, C. Immortalisation of primary cells. In Cell Culture Methods for In Vitro Toxicology; Springer: Dordrecht, The Netherlands, 2001; pp. 27–42. [Google Scholar] [CrossRef]
- Meunier, V.; Bourrie, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hendijani, F. Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Prolif. 2017, 50, e12334. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E.; Werbin, H. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta (BBA)/Rev. Cancer 1991, 1072, 1–7. [Google Scholar] [CrossRef]
- Holliday, R. The limited proliferation of cultured human diploid cells: Regulation or senescence? J. Gerontol. 1990, 45, B36–B41. [Google Scholar] [CrossRef]
- Yeager, T.R.; Reddel, R.R. Constructing immortalized human cell lines. Curr. Opin. Biotechnol. 1999, 10, 465–469. [Google Scholar] [CrossRef]
- Lundberg, A.S.; Hahn, W.C.; Gupta, P.; Weinberg, R.A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 2000, 12, 705–709. [Google Scholar] [CrossRef]
- Bryan, T.; Redder, R.R. SV40-lnduced Immortalization of Human Cells. Crit. Rev. Oncog. 1994, 5, 331–357. [Google Scholar] [CrossRef]
- Shay, J.W.; Werbin, H.; Wright, W. Telomere shortening may contribute to aging and cancer: A perspective. Mol. Cell Diff. 1994, 2, 1–21. [Google Scholar]
- Bryan, T.; Reddel, R. Telomere dynamics and telomerase activity in in vitro immortalised human cells. Eur. J. Cancer 1997, 33, 767–773. [Google Scholar] [CrossRef]
- Colgin, L.M.; Reddel, R.R. Telomere maintenance mechanisms and cellular immortalization. Curr. Opin. Genet. Dev. 1999, 9, 97–103. [Google Scholar] [CrossRef]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Simmons, N.L. Functional organization of the bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R173–R181. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, M.; Kam, N.-W.; Brentano, F.; Choi, K.; Filer, A.; Kyburz, D.; McInnes, I.B.; Gay, S.; Buckley, C.; Pitzalis, C. A BAFF/APRIL-dependent TLR3-stimulated pathway enhances the capacity of rheumatoid synovial fibroblasts to induce AID expression and Ig class-switching in B cells. Ann. Rheum. Dis. 2011, 70, 1857–1865. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Hung, S.-L.; Lee, Y.-Y.; Ho, Y.-C.; Yang, S.-F. The role of fibroblasts in the modulation of dental pulp inflammation. J. Formos. Med. Assoc. 2022, 121, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, J.; Wang, X.; Zheng, M.; Luan, Q. P53 mediates lipopolysaccharide—Induced inflammation in human gingival fibroblasts. J. Periodontol. 2018, 89, 1142–1151. [Google Scholar] [CrossRef]
- Chakravortty, D.; KS, N.K. Modulation of barrier function of small intestinal epithelial cells by lamina propria fibroblasts in response to lipopolysaccharide: Possible role of tnfα in inducing barrier dysfunction. Microbiol. Immunol. 1999, 43, 527–533. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Płóciennikowska, A.; Hromada-Judycka, A.; Borzęcka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Ingber, D.E. Extracellular Matrix: A Solid-State Regulator of Cell form, Function, and Tissue Development. Compr. Physiol. 2010, 541–556. [Google Scholar] [CrossRef]
- Klaas, M.; Kangur, T.; Viil, J.; Mäemets-Allas, K.; Minajeva, A.; Vadi, K.; Antsov, M.; Lapidus, N.; Järvekülg, M.; Jaks, V. The alterations in the extracellular matrix composition guide the repair of damaged liver tissue. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dass, K.; Ahmad, A.; Azmi, A.S.; Sarkar, S.H.; Sarkar, F.H. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008, 34, 122–136. [Google Scholar] [CrossRef]
- Lijnen, H. Elements of the fibrinolytic system. Ann. N. Y. Acad. Sci. 2001, 936, 226–236. [Google Scholar] [CrossRef]
- Loskutoff, D.J.; Quigley, J.P. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J. Clin. Investig. 2000, 106, 1441–1443. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y.; Zhang, Y.; Harris, D.C.; Zheng, G. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. World J. Nephrol. 2013, 2, 84. [Google Scholar] [CrossRef]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Ren. Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef]
- Ogura, N.; Tobe, M.; Tamaki, H.; Nagura, H.; Abiko, Y. IL-β Increases uPA and uPA Receptor Expression in Human Gingival Fibroblasts. IUBMB Life 2001, 51, 381–385. [Google Scholar]
- Zou, M.-L.; Teng, Y.-Y.; Chen, Z.-H.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Wu, J.-J.; Yuan, Z.-D.; Tang, X.-Y.; Yu, S.; et al. The uPA System Differentially Alters Fibroblast Fate and Profibrotic Ability in Skin Fibrosis. Front. Immunol. 2022, 13, 845956. [Google Scholar] [CrossRef]
- Horowitz, J.C.; Tschumperlin, D.J.; Kim, K.K.; Osterholzer, J.J.; Subbotina, N.; Ajayi, I.O.; Teitz-Tennenbaum, S.; Virk, A.; Dotson, M.; Liu, F.; et al. Urokinase plasminogen activator overexpression reverses established lung fibrosis. Thromb. Haemost. 2019, 119, 1968–1980. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Wu, B.; Xia, F. Semaphorin 3A mitigates lipopolysaccharide-induced chondrocyte inflammation, apoptosis and extracellular matrix degradation by binding to Neuropilin-1. Bioengineered 2021, 12, 9641–9654. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qu, Y.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Wang, H. Cortisol inhibits NF-κB and MAPK pathways in LPS activated bovine endometrial epithelial cells. Int. Immunopharmacol. 2018, 56, 71–77. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Quaggin, S.E.; Vaughan, D.E. Molecular basis of organ fibrosis: Potential therapeutic approaches. Exp. Biol. Med. 2013, 238, 461–481. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1. [Google Scholar] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Sun, B. The mechanics of fibrillar collagen extracellular matrix. Cell Rep. Phys. Sci. 2021, 2, 100515. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Yin, C.; Dong, N. Comparison of murine lymphocyte proliferation response as measured by thiazolyl blue and cell counting kit-8 assays. Infect. Inflamm. Repair 2012, 13, 199–202. [Google Scholar]
- Kent-Dennis, C.; Aschenbach, J.; Griebel, P.; Penner, G. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J. Dairy Sci. 2020, 103, 9587–9603. [Google Scholar] [CrossRef]
- Zhou, B.; Song, W.; Zhu, L.; Deng, Z.; Qian, G.; Li, C. The anti-lipopolysaccharide effect of glycine via fibroblastic cell culture from the embryonic calipash tissue of Chinese soft-shelled turtle Pelodiscus sinensis. Aquac. Rep. 2023, 30, 101575. [Google Scholar] [CrossRef]
- Bickmore, W.A. Karyotype analysis and chromosome banding. e LS 2001. [Google Scholar] [CrossRef]
- Wang, J.; Hu, G.; Lin, Z.; He, L.; Xu, L.; Zhang, Y. Characteristic and functional analysis of a newly established porcine small intestinal epithelial cell line. PLoS ONE 2014, 9, e110916. [Google Scholar] [CrossRef]
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R.; Valipour, B. Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit+ hematopoietic stem cells. Int. J. Biol. Macromol. 2021, 177, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Zakrajsek, B.A.; Mills, A.G.; Gorn, V.; Singer, M.J.; Reed, M.W. Quantitative reverse transcription–polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal. Biochem. 2000, 285, 194–204. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Gene | Forward (5′→3′) | Reverse (5′→3′) | Accessin No. |
|---|---|---|---|
| MMP2 | GATAACCTGGATGCTGTGGTGGAC | TGCTTCCGAACTTCACGCTCTTC | XM_005890934 |
| MMP9 | CTGGCTTGCTGCTCTGCTGTC | CTGGCTTGCTGCTCTGCTGTC | XM_005901514 |
| uPA | CAGGTCACCAACGCCGAGAAC | GATGAGGCTGCCACCACACAAG | XM_005901514 |
| uPAR | GCTTCAAAACCTGCCACCAAACG | GTCCTTTAGTGCCTGTCGCTTCC | XM_005899359 |
| TIMP-1 | CCAGAACCGCAGTGAGGAGTTTC | CAGCAGCATAGGTCTTGGTGAATCC | XM_005899359 |
| TIMP-2 | GCTGGACATTGGAGGAAAGAAGGAG | CAGGGCACGATGAAGTCACAGAG | XM_014483144 |
| PAI-2 | AGGCGGTGGACTTCCTAGAACG | GTAGACAGCATTCACCAGGACCATC | XM_005906498 |
| COL-Ι | CCGAGGGCAACAGCAGATTCAC | TCAAGGATAGGCAGGCGAGATGG | XM_005909695 |
| FN | TGGCGAGTGGAAGTGTGAGAGG | ATACGGAGGCGGCTGAGGATG | XM_014482895 |
| IL-1β | CTCCGACGAGTTTCTGTGTGACG | GAGAGGAGGTGGAGAGCCTTCAG | NM_174093.1 |
| IL-6 | CACTGACCTGCTGGAGAAGATGC | CCGAATAGCTCTCAGGCTGAACTG | XM_005901249.2 |
| TNF-α | TGAAGGAAGAGGAGAGGCTCATCG | GTGGTCATCGGAGTTGCTGGTG | XM_005904178.1 |
| IL-10 | GAACCACGGGCCTGACATCAAG | CTTCTCCACCGCCTTGCTCTTG | XM_005891650 |
| GAPDH | GGGTCATCATCTCTGCACCT | GGTCATAAGTCCCTCCACGA | XM_014482068.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yue, Z.; Che, L.; Li, H.; Hu, R.; Shi, L.; Zhang, X.; Zou, H.; Peng, Q.; Jiang, Y.; et al. Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide. Toxins 2023, 15, 537. https://doi.org/10.3390/toxins15090537
Wang J, Yue Z, Che L, Li H, Hu R, Shi L, Zhang X, Zou H, Peng Q, Jiang Y, et al. Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide. Toxins. 2023; 15(9):537. https://doi.org/10.3390/toxins15090537
Chicago/Turabian StyleWang, Junmei, Ziqi Yue, Li Che, Hui Li, Rui Hu, Liyuan Shi, Xiaohong Zhang, Huawei Zou, Quanhui Peng, Yahui Jiang, and et al. 2023. "Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide" Toxins 15, no. 9: 537. https://doi.org/10.3390/toxins15090537
APA StyleWang, J., Yue, Z., Che, L., Li, H., Hu, R., Shi, L., Zhang, X., Zou, H., Peng, Q., Jiang, Y., & Wang, Z. (2023). Establishment of SV40 Large T-Antigen-Immortalized Yak Rumen Fibroblast Cell Line and the Fibroblast Responses to Lipopolysaccharide. Toxins, 15(9), 537. https://doi.org/10.3390/toxins15090537






