New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition
Abstract
:1. Introduction
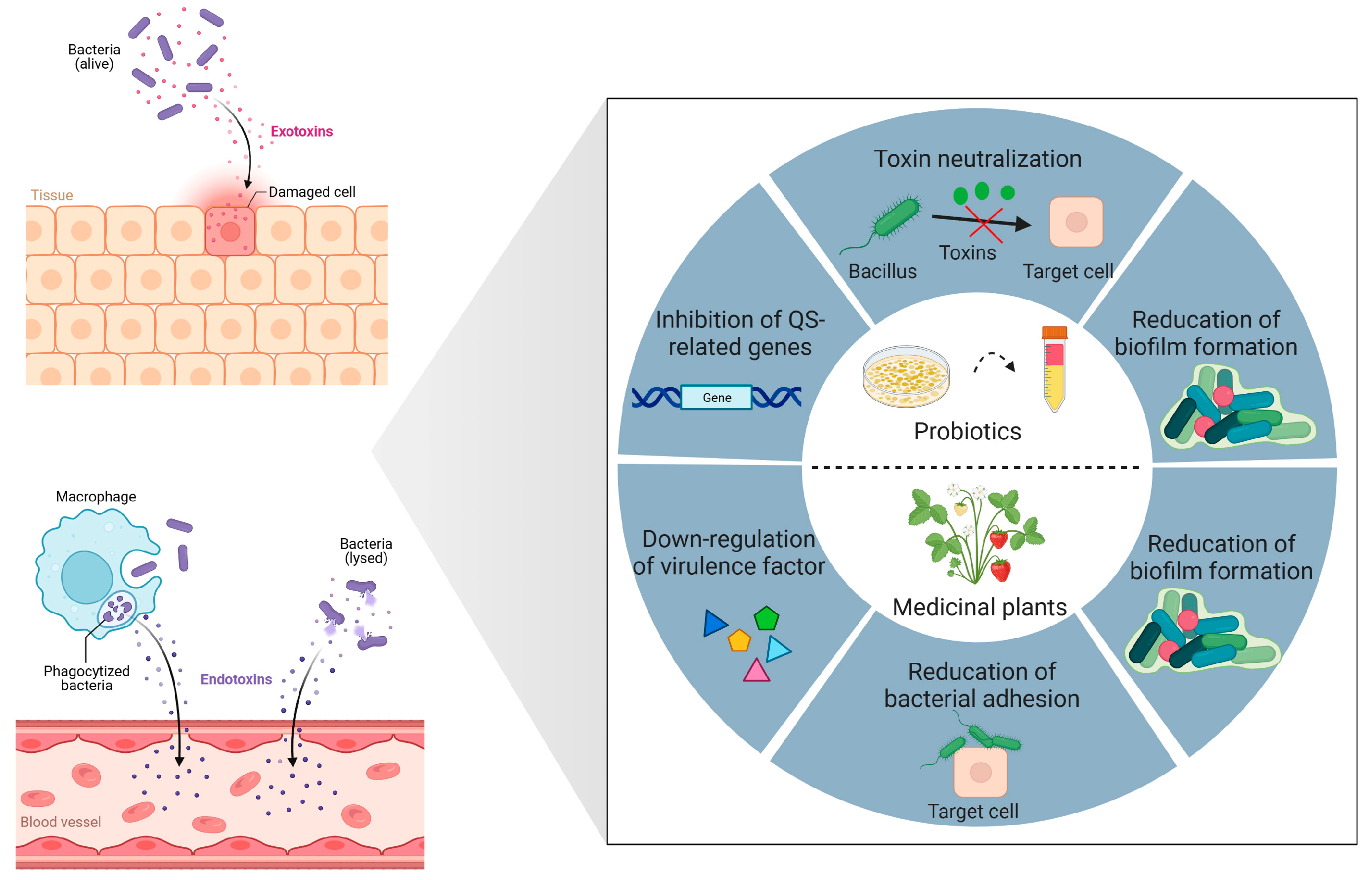
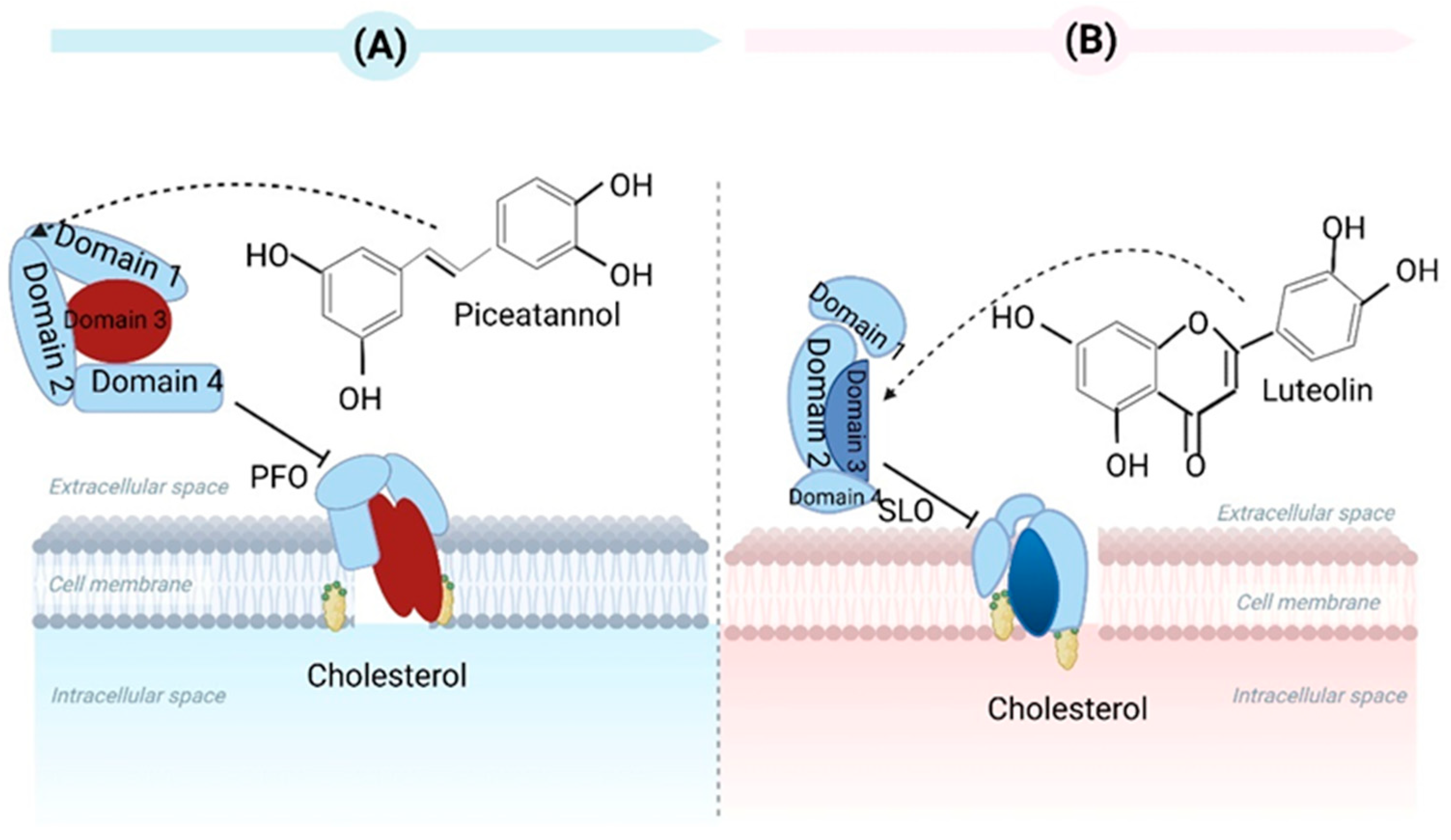
2. Bacterial Toxin Neutralization
3. Overview of Quorum-Sensing and Biofilm Formation
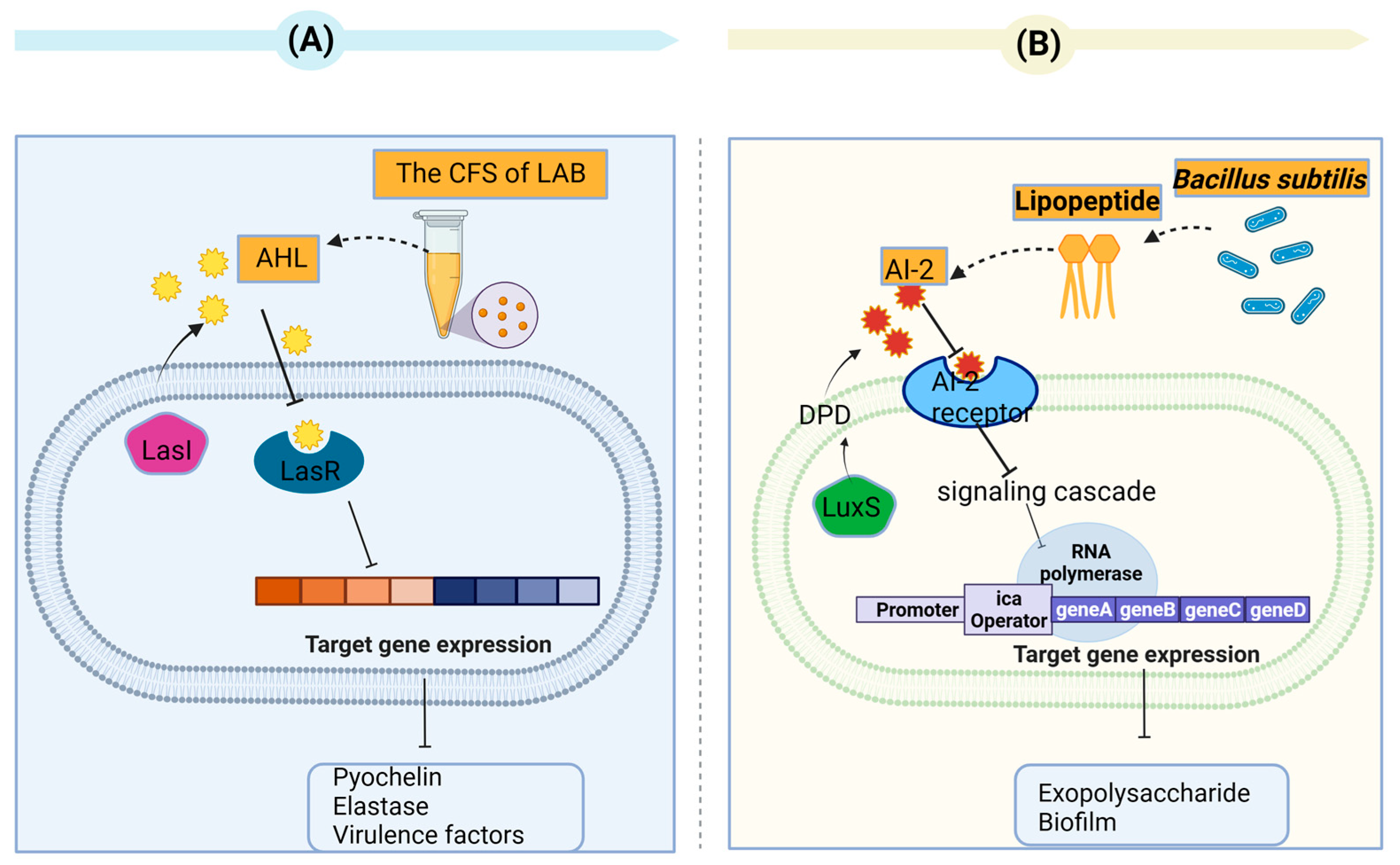
3.1. Quorum Sensing
3.2. Biofilm Formation
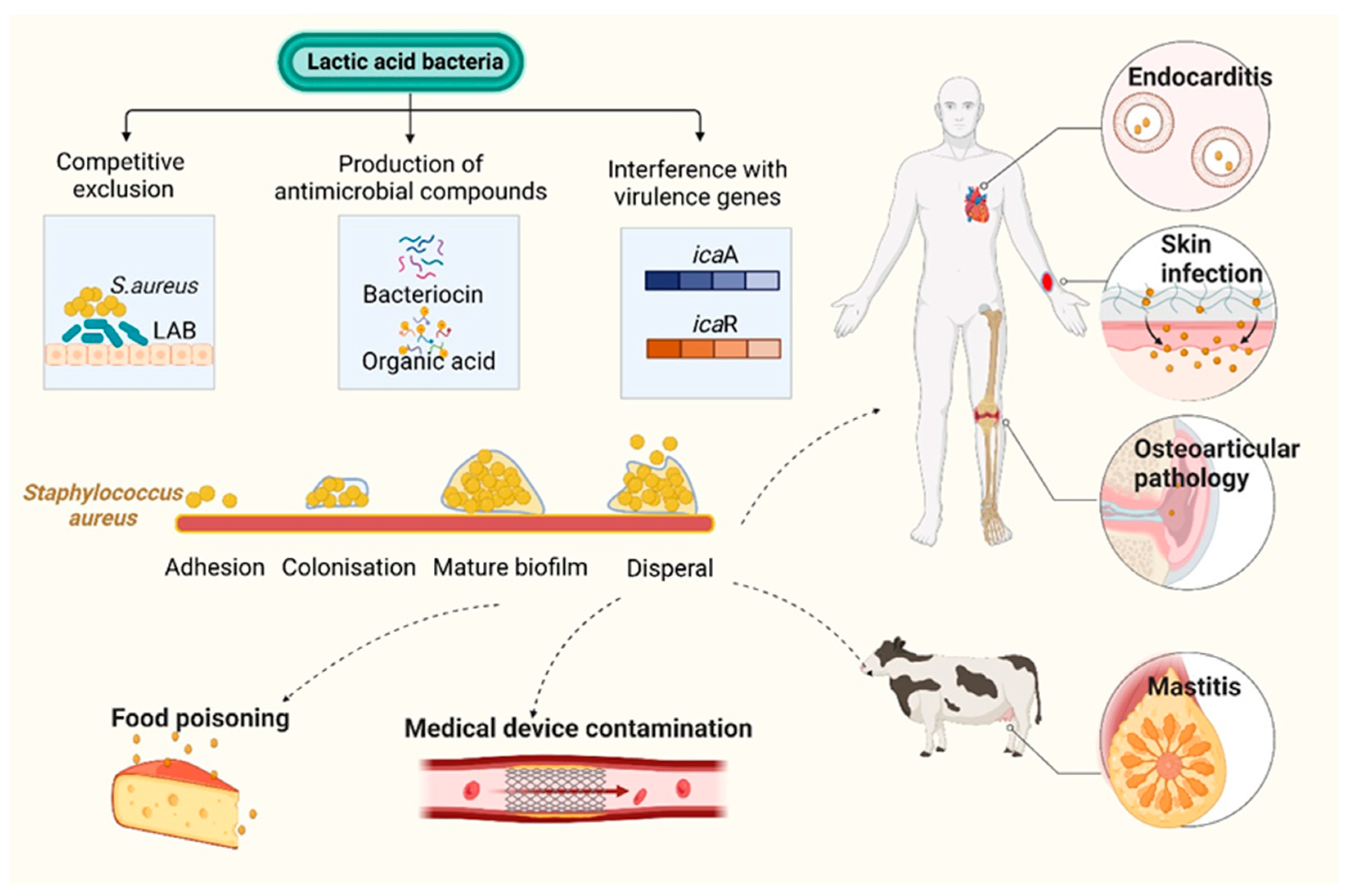
4. Anti-Virulence Treatment Strategies
4.1. Secondary Metabolites of Bacteria as Quorum-Quenching Agents
4.2. Secondary Metabolites of Medicinal Plants as Quorum-Quenching Agents
5. Conclusions and Discussion
- The determination of whether probiotics and medicinal plants can concurrently re-duce both pathogens and toxins produced by the pathogens.
- The development of probiotic-based oral vaccines to protect animals against contamination by toxins.
- The determination of whether the anti-toxin effects of probiotics and medicinal plants and extracts in vitro can be duplicated in vivo, especially in humans.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heine, H.; Rietschel, E.T.; Ulmer, A.J. The Biology of Endotoxin. Mol. Biotechnol. 2001, 19, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Schumann, R.R. Old and new findings on lipopolysaccharide-binding protein: A soluble pattern-recognition molecule. Biochem. Soc. Trans. 2011, 39, 989–993. [Google Scholar] [CrossRef]
- Schletter, J.; Heine, H.; Ulmer, A.J.; Rietschel, E.T. Molecular mechanisms of endotoxin activity. Arch. Microbiol. 1995, 164, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Kagan, J.C. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell 2014, 54, 212–223. [Google Scholar] [CrossRef]
- Balfanz, J.; Rautenberg, P.; Ullman, U. Molecular mechanisms of action of bacterial exotoxins. J. Med. Microbiol. 1996, 284, 170–206. [Google Scholar] [CrossRef]
- Xu, S.X.; McCormick, J.K. Staphylococcal superantigens in colonization and disease. Front. Cell. Infect. Microbiol. 2012, 2, 52. [Google Scholar] [CrossRef]
- Barnett, T.C.; Cole, J.N.; Rivera-Hernandez, T.; Henningham, A.; Paton, J.C.; Nizet, V.; Walker, M.J. Streptococcal toxins: Role in pathogenesis and disease. Cell. Microbiol. 2015, 17, 1721–1741. [Google Scholar] [CrossRef]
- Rossetto, O.; de Bernard, M.; Pellizzari, R.; Vitale, G.; Caccin, P.; Schiavo, G.; Montecucco, C. Bacterial toxins with intracellular protease activity. Clin. Chim. Acta 2000, 291, 189–199. [Google Scholar] [CrossRef]
- iDali, C.; Foged, N.T.; Frandsen, P.L.; Nielsen, M.H.; Elling, F. Ultrastructural localization of the Pasteurella multocida toxin in a toxin-producing strain. J. Gen. Microbiol. 1991, 137, 1067–1071. [Google Scholar] [CrossRef]
- Welch, R.A.; Forestier, C.; Lobo, A.; Pellett, S.; Thomas, W.; Rowe, G. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol. Immunol. 1992, 105, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, targets, and triggers: An overview of toxin-antitoxin biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W. Bacterial pathogenesis, 4th ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996; Chapter 7. [Google Scholar]
- Defoirdt, T. Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol. 2018, 26, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Empting, M. Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers. Beilstein J. Org. Chem. 2018, 14, 2627–2645. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, W.; Zheng, H.; Du, J.; Luo, H.; Wu, Q.; Ren, N. Inhibition of biofilm formation by chemical uncoupler, 3, 3′, 4′, 5-tetrachlorosalicylanilide (TCS): From the perspective of quorum sensing and biofilm related genes. Biochem. Eng. J. 2018, 137, 95–99. [Google Scholar] [CrossRef]
- Liu, X.; Peng, L.Y.; Meng, J.X.; Zhu, Z.P.; Han, B.; Wang, S.T. Protein-mediated anti-adhesion surface against oral bacteria. Nanoscale 2018, 10, 2711–2714. [Google Scholar] [CrossRef]
- Puga, C.; Rodríguez-López, P.; Cabo, M.L.; SanJose, C.; Orgaz, B. Enzymatic dispersal of dual-species biofilms carrying Listeria monocytogenes and other associated food industry bacteria. Food Control 2018, 94, 222–228. [Google Scholar] [CrossRef]
- Robinson, C.M.; Sinclair, J.F.; Smith, M.J.; O’Brien, A.D. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 2006, 103, 9667–9672. [Google Scholar] [CrossRef]
- Carey, C.M.; Kostrzynska, M.; Ojha, S.; Thompson, S. The effect of probiotics and organic acids on Shiga-toxin 2 gene expression in enterohemorrhagic Escherichia coli O157:H7. J. Microbiol. Methods 2008, 73, 125–132. [Google Scholar] [CrossRef]
- Hostetter, S.J.; Helgerson, A.F.; Paton, J.C.; Paton, A.W.; Cornick, N.A. Therapeutic use of a receptor mimic probiotic reduces intestinal shiga toxin levels in a piglet model of hemolytic uremic syndrome. BMC Res. Notes 2014, 7, 331. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiae IFST062013, a potential probiotic. BMC Compl. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Czerucka, D.; Rampal, P. Effect of Saccharomyces boulardii on cAMP- and Ca2+-dependent Cl− secretion in T84 cells. Dig. Dis. Sci. 1999, 44, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.L.; Castro, I.M.; Bambirra, E.A.; Amaral, S.C.; Fietto, L.G.; Tropia, M.J.M.; Neves, M.J.; Dos Santos, R.G.; Gomes, N.C.M.; Nicoli, J.R. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1998, 64, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Mühlen, S.; Dersch, P. Treatment strategies for infections with Shiga toxin-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2020, 10, 169. [Google Scholar] [CrossRef]
- Uzal, F.A.; Freedman, J.C.; Shrestha, A.; Theoret, J.R.; Garcia, J.; Awad, M.M.; Adams, V.; Moore, R.J.; Rood, J.I.; McClane, B.A. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014, 9, 361–377. [Google Scholar] [CrossRef]
- Kawarizadeh, A.; Tabatabaei, M.; Hosseinzadeh, S.; Farzaneh, M.; Pourmontaseri, M. The effects of probiotic Bacillus coagulans on the cytotoxicity and expression of alpha toxin gene of Clostridium perfringens Type A. Anaerobe 2019, 59, 61–67. [Google Scholar] [CrossRef]
- Wang, G.Z.; Liu, H.T.; Gao, Y.W.; Niu, X.D.; Deng, X.M.; Wang, J.F.; Feng, H.H.; Guo, Z.M.; Qiu, J.Z. Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O. Molecules 2022, 27, 5145. [Google Scholar] [CrossRef]
- Ruas-Madiedo, P.; Medrano, M.; Salazar, N.; de los Reyes-Gavilan, C.G.; Perez, P.F.; Abraham, A.G. Exopolysaccharides pro duced by Lactobacillus and Bifidobacterium strains abrogate in vitro the cytotoxic effect of bacterial toxins on eukaryotic cells. J. Appl. Microbiol. 2010, 109, 2079–2086. [Google Scholar] [CrossRef]
- Guo, T.T.; Liu, P.; Wang, Z.Y.; Zheng, Y.L.; Huang, W.H.; Kong, D.C.; Ding, L.Z.; Lv, Q.Y.; Wang, Z.T.; Jiang, H.; et al. Luteolin Binds Streptolysin O Toxin and Inhibits Its Hemolytic Effects and Cytotoxicity. Front. Pharmacol. 2022, 13, 942180. [Google Scholar] [CrossRef]
- Lam, T.I.; Tam, C.C.; Stanker, L.H.; Cheng, L.W. Probiotic Microorganisms Inhibit Epithelial Cell Internalization of Botulinum Neurotoxin Serotype A. Toxins 2016, 8, 377. [Google Scholar] [CrossRef]
- Umeda, J.E.; Demuth, D.R.; Ando, E.S.; Faveri, M.; Mayer, M.P.A. Signaling transduction analysis in gingival epithelial cells after infection with Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2012, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Boesze-Battaglia, K.; Scuron, M.D.; Walker, L.P.; Zekavat, A.; Dlakić, M. The toxicity of the Aggregatibacter actinomycetemcomitans cytolethal distending toxin correlates with its phosphatidylinositol-3,4,5-triphosphate phosphatase activity. Cell. Microbiol. 2016, 18, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Sgorbati, B.; Biavati, B.; Belibasakis, G.N. Lactobacillus salivarius and L. gasseri down-Regulate Aggregatibacter actinomycetemcomitans exotoxins expression. Ann. Microbiol. 2014, 64, 611–617. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli postbiotics reduce biofilm for- mation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef]
- Zhong, S.; He, S.Z. Quorum sensing inhibition or quenching in Acinetobacter baumannii: The novel therapeutic strategies for new drug development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Elgaml, A.; Habib, E.E. Biotechnological applications of quorum sensing inhibition as novel therapeutic strategies for multidrug resistant pathogens. Microb. Pathog. 2019, 127, 138–143. [Google Scholar] [CrossRef]
- Elgaml, A.; Higaki, K.; Miyoshi, S. Effects of temperature, growth phase and luxO-disruption on regulation systems of toxin production in Vibrio vulnificus strain L-180, a human clinical isolate. World J. Microbiol. Biotechnol. 2014, 30, 681–691. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial virulence factors: Secreted for survival. Indian J. Microbiol. 2017, 57, 1–10. [Google Scholar] [CrossRef]
- Asfour, H. Anti-Quorum Sensing Natural Compounds. J. Microsc. Ultrastruct. 2018, 6, 1. [Google Scholar] [CrossRef]
- Heilmann, S.; Krishna, S.; Kerr, B. Why do bacteria regulate public goods by quorum sensing?—How the shapes of cost and benefit functions determine the form of optimal regulation. Front. Microbiol. 2015, 6, 767. [Google Scholar] [CrossRef] [PubMed]
- Finch, R.G.; Pritchard, D.I.; Bycroft, B.W.; Williams, P.; Stewart, G.S. Quorum sensing: A novel target for anti-infective therapy. J. Antimicrob. Chemother. 1998, 42, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Hadi, N. Quorum-Quenching Activity of Some Iranian Medicinal Plants. New Microbes New Infect. 2021, 42, 100882. [Google Scholar] [CrossRef] [PubMed]
- Weiland-Bräuer, N. Friends or Foes—Microbial Interactions in Nature. Biology 2021, 10, 496. [Google Scholar] [CrossRef]
- Choo, J.H.; Rukayadi, Y.; Hwang, J.K. Inhibition of bacterial quorum sensing by vanilla extract. Lett. Appl. Microbiol. 2006, 42, 637–641. [Google Scholar] [CrossRef]
- Chan, K.G.; Atkinson, S.; Mathee, K.; Sam, C.K.; Chhabra, S.R.; Camara, M.; Koh, C.L.; Williams, P. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011, 11, 51. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Tracing the origins of extracellular DNA in bacterial biofilms: Story of death and predation to community benefit. Biofouling 2021, 37, 1022–1039. [Google Scholar] [CrossRef]
- Das, T.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter 2011, 7, 2927–2935. [Google Scholar] [CrossRef]
- Preda, V.G.; Săndulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Filloux, A. Biofilms and Cyclic di-GMP (c-di-GMP) Signaling: Lessons from Pseudomonas aeruginosa and Other Bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef] [PubMed]
- Markova, J.A.; Anganova, E.V.; Turskaya, A.L.; Bybin, V.A.; Savilov, E.D. Regulation of Escherichia coli Biofilm Formation. Appl. Biochem. Microbiol. 2018, 54, 1–11. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Thakur, A.; Sharma, S.; Kumar, M. aBiofilm: A resource of anti-biofilm agents and their potential implications in targeting antibiotic drug resistance. Nucleic Acids Res. 2018, 46, D894–D900. [Google Scholar] [CrossRef]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Keshavan, N.D. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 2006, 70, 859–875. [Google Scholar] [CrossRef]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef]
- Inglin, R.C.; Stevens, M.J.A.; Meile, L.; Lacroix, C.; Meile, L. High-throughput screening assays for antibacterial and antifungal activities of Lactobacillus species. J. Microbiol. Methods 2015, 114, 26–29. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.C.; Peral, M.C.; Rachid, M.; Santana, M.; Perdigon, G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect. 2005, 11, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Chandla, S.; Harjai, K.; Shukla, G. Combinatorial Therapeutic Strategy of Biogenics Derived from Lactobacillus fermentum PUM and Zingerone Against Pseudomonas aeruginosa PAO1-Induced Surgical Site Infection: An Experimental Study. Probiotics Antimicro. Prot. 2022, 14, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Bhawal, S.; Kumari, A.; Kapila, S.; Kapila, R. pH-dependent inhibition of AHL-mediated quorum sensing by cell-free supernatant of lactic acid bacteria in Pseudomonas aeruginosa PAO1. Microb. Pathog. 2020, 142, 104105. [Google Scholar] [CrossRef]
- Kampouris, I.D.; Karayannakidis, P.D.; Banti, D.C.; Sakoula, D.; Konstantinidis, D.; Yiangou, M.; Samaras, P.E. Evaluation of a Novel Quorum Quenching Strain for MBR Biofouling Mitigation. Water Res. 2018, 143, 56–65. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, Y.; Oh, S.; Song, M.; Choe, J.H.; Whang, K.Y.; Kim, K.H.; Oh, S. Influences of Quorum-Quenching Probiotic Bacteria on the Gut Microbial Community and Immune Function in Weaning Pigs. Anim. Sci. J. 2018, 89, 412–422. [Google Scholar] [CrossRef]
- Han, X.P.; Chen, Q.Y.; Zhang, X.G.; Chen, X.L.; Luo, D.S.; Zhong, Q.P. Antibiofilm and Antiquorum Sensing Potential of Lactiplantibacillus plantarum Z057 against Vibrio parahaemolyticus. Foods 2022, 11, 2230. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.S.; Cui, X.Y.; Shi, Y.J.; Wen, S.S.; Chen, H.Y.; Ge, J.W. Antimicrobial, Anti-Adhesive and Anti-Biofilm Potential of Biosurfactants Isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus Aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhu, X.Y.; Zhao, H.Z.; Lu, Y.J.; Zhang, C.; Lu, Z.X. Surfactin effectively inhibits Staphylococcus aureus adhesion and biofilm formation on surfaces. Appl. Microbiol. Biotechnol. 2019, 103, 4565–4574. [Google Scholar] [CrossRef]
- Yong, C.C.; Lim, J.; Kim, B.-K.; Park, D.-J.; Oh, S. Suppressive Effect of Lactobacillus fermentum Lim2 on Clostridioides difficile 027 Toxin Production. Lett. Appl. Microbiol. 2019, 68, 386–393. [Google Scholar] [CrossRef]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2014, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.A.; dos Santos, T.F.; de Almeida, M.E.; Fontes, L.A.; Andrade, E.F.; Rezende, R.P.; Marques, L.M.; Romano, C.C. Inhibition of Staphylococcus aureus biofilm by Lactobacillus isolated from fine cocoa. BMC Microbiol. 2016, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Squarzanti, D.F.; Zanetta, P.; Ormelli, M.; Manfredi, M.; Barberis, E.; Vanella, V.V.; Amoruso, A.; Pane, M.; Azzimonti, B. An animal derivative-free medium enhances Lactobacillus johnsonii LJO02 supernatant selective efficacy against the methicillin (oxacillin)-resistant Staphylococcus aureus virulence through key-metabolites. Sci. Rep. 2022, 12, 8666. [Google Scholar] [CrossRef] [PubMed]
- Sevin, S.; Karaca, B.; Haliscelik, O.; Kibar, H.; OmerOglou, E.; Kiran, F. Postbiotics secreted by Lactobacillus sakei EIR/CM-1 isolated from cow milk microbiota, display antibacterial and antibiofilm activity against ruminant mastitis-causing pathogens. Ital. J. Anim. Sci. 2021, 20, 1302–1316. [Google Scholar] [CrossRef]
- Tahmourespour, A.; Kasra-Kermanshahi, R.; Salehi, R. Lactobacillus rhamnosus biosurfactant inhibits biofilm formation and gene expression of caries-inducing Streptococcus mutans. Dent. Res. J. 2019, 16, 87–94. [Google Scholar] [CrossRef]
- OmerOglou, E.; Karaca, B.; Kibar, H.; Haliscelik, O.; Kiran, F. The role of microbiota-derived postbiotic mediators on biofilm formation and quorum sensing-mediated virulence of Streptococcus mutans: A perspective on preventing dental caries. Microb. Pathog. 2022, 164, 105390. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic Lactobacillu ssp. inhibit growth, biofilm formation and gene expression of caries-inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Saber, I.I.; Yassin, M.H.; Ibrahim, H.F. Probiotics: Lactic Acid Bacteria have Antibacterial Activity and Down- regulate Biofilm Genes of Uropathogenic E. coli. J. Pure Appl. Microbiol. 2022, 16, 1834–1843. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.Y.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Vivek, J.M. Anti quorum sensing activities of medicinal plant extracts against quorum sensing medi- ated virulence factors of Pseudomonas aeruginosa. Pharm. Lett. 2016, 8, 412–423. [Google Scholar]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.R.; Qian, L.; Cao, L.X.; Tan, H.M.; Huang, Y.L.; Xue, X.L.; Shen, Y.; Zhou, S.N. Virtual screening for novel quorum sensing inhibitors to eradicate biofilm formation of Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2008, 79, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Ciofu, O.; Molin, S.; Givskov, M.; Høiby, N. Applying insights from biofilm biology to drug development—Can a new approach be developed? Nat. Rev. Drug Discov. 2013, 12, 791–808. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.R.; Pillai, S.D.; Patil, B.S. Suppression of bacterial cell-cell signalling, biofilm formation and type III secretion system by citrus flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Saei, H.D.; Ahmadi, M.; Zahraei-Salehi, T. Anti-Quorum Sensing Effects of Licochalcone A and Epigallocate chin-3-Gallate against Salmonella typhimurium Isolates from Poultry Sources. Vet. Res. Forum 2020, 11, 273–279. [Google Scholar]
- Nyila, M.A.; Leonard, C.M.; Hussein, A.A.; La, N. Activity of South African medicinal plants against Listeria monocytogenes biofilms, and isolation of active compounds from Acacia karroo. S. Afr. J. Bot. 2012, 78, 220–227. [Google Scholar] [CrossRef]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-Sensing. Z. Naturforschung C 2003, 58, 879–884. [Google Scholar] [CrossRef]
- Matsunaga, T.; Nakahara, A.; Minnatul, K.M.; Noiri, Y.; Ebisu, S.; Kato, A.; Azakami, H. The Inhibitory Effects of Catechins on Biofilm Formation by the Periodontopathogenic Bacterium, Eikenella corrodens. Biosci. Biotechnol. Biochem. 2010, 74, 2445–2450. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Ahmed, A.F.; Al-Shabib, N.A.; Laeeq, S.; Khan, R.A.; Rehman, M.T.; Alsalme, A.; Al-Ajmi, M.F.; Khan, M.S.; Husain, F.M. Onion Peel Ethylacetate Fraction and Its Derived Constituent Quercetin 4′-O-β-D Glucopyranoside Attenuates Quorum Sensing Regulated Virulence and Biofilm Formation. Front. Microbiol. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Ouyang, J.; Sun, F.; Feng, W.; Sun, Y.; Qiu, X.; Xiong, L.; Liu, Y.; Chen, Y. Quercetin is an effective inhibitor of quorum sensing, biofilm formation and virulence factors in Pseudomonas aeruginosa. J. Appl. Microbiol. 2016, 120, 966–974. [Google Scholar] [CrossRef]
- Grabski, H.; Hunanyan, L.; Tiratsuyan, S.; Vardapetyan, H. Interaction of Quercetin with Transcriptional Regulator LasR of Pseudomonas aeruginosa: Mechanistic Insights of the Inhibition of Virulence through Quorum Sensing. bioRxiv 2017. bioRxiv:239996. [Google Scholar] [CrossRef]
- Chemmugil, P.; Lakshmi, P.T.V.; Annamalai, A. Exploring Morin as an Anti-Quorum Sensing Agent (Anti-QSA) against Re- sistant Strains of Staphylococcus aureus. Microb. Pathog. 2019, 127, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.C.; Cao, F.J.; Xiang, H.; Mu, D.; Cao, J.J.; Li, B.B.; Zhong, L.; Dong, X.Y.; Zhong, X.B.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stévigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The Flavanone Naringenin Reduces the Production of Quorum Sensing-Controlled Virulence Factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and Cancer: Current Status, Challenges, and Future Directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, D.F.; Li, J.R.; Liu, Y.T.; Zhou, S.; Yang, Y.B.; Xu, N.; Yang, Q.H.; Ai, X.H. Genistein Inhibits the Pathogenesis of Aeromonas hydrophila by Disrupting Quorum Sensing Mediated Biofilm Formation and Aerolysin Production. Front. Pharmacol. 2021, 12, 753581. [Google Scholar] [CrossRef]
- Sun, B.; Luo, H.Z.; Jiang, H.; Wang, Z.N.; Jia, A.Q. Inhibition of Quorum Sensing and Biofilm Formation of Esculetin on Aeromonas Hydrophila. Front. Microbiol. 2021, 12, 737626. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, L.S.; Liu, Y.T.; Xu, N.; Zhou, S.; Yang, Y.B.; Yang, Q.H.; Ai, X.H. Luteolin decreases the pathogenicity of Aeromonas hydrophila via inhibiting the activity of aerolysin. Virulence 2021, 12, 165–176. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolome, B. Antibacterial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Joshi, J.R.; Burdman, S.; Lipsky, A.; Yariv, S.; Yedidia, I. Plant phenolic acids affect the virulence of Pectobacterium aroidearum and P. carotovorum ssp. brasiliense via quorum sensing regulation. Mol. Plant Pathol. 2016, 17, 487–500. [Google Scholar] [PubMed]
- Su, M.M.; Liu, F.; Luo, Z.; Wu, H.H.; Zhang, X.X.; Wang, D.Y.; Zhu, Y.Z.; Sun, Z.L.; Xu, W.M.; Miao, Y. The antibacterial activity and mechanism of chlorogenic acid against foodborne pathogen pseudomonas aeruginosa. Foodb. Pathog. Dis. 2019, 16, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, M.; Zuo, J.; He, X.X.; Lu, P.; Li, Y.Y.; Zhao, Y.N.; Xia, F. Vanillic acid combats Vibrio alginolyticus by cell membrane damage and biofilm reduction. J. Fish. Dis. 2021, 44, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.R.; Srinivasan, R.; Kannappan, A.; Santhakumari, S.; Bhuvaneswari, M.; Rajasekar, P.; Prabhu, N.M.; Ravi, A.V. In vitro and in vivo efficacy of rosmarinic acid on quorum sensing mediated biofilm formation and virulence factor production in Aeromonas hydrophila. Biofouling 2016, 32, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Jailani, A.; Ahmed, B.; Lee, J.H.; Lee, J. Inhibition of Agrobacterium tumefaciens Growth and Biofilm Formation by Tannic Acid. Biomedicines 2022, 10, 1619. [Google Scholar] [CrossRef]
- Hancock, V.; Dahl, M.; Vejborg, R.M.; Klemm, P. Dietary plant components ellagic acid and tannic acid inhibit Escherichia coli biofilm formation. J. Med. Microbiol. 2010, 59, 496–498. [Google Scholar] [CrossRef]
- Payne, D.E.; Martin, N.R.; Parzych, K.R.; Rickard, A.H.; Underwood, A.; Boles, B.R. Tannic acid inhibits Staphylococcus aureus surface colonization in an IsaA-dependent manner. Infect. Immun. 2013, 81, 496–504. [Google Scholar] [CrossRef]
- Sukumar, M.R.; Konig, B. Pomegranate extract specifically inhibits Clostridium difficile growth and toxin production without disturbing the beneficial bacteria in Vitro. Infect. Drug Resist. 2018, 11, 2357–2362. [Google Scholar] [CrossRef]
- Li, G.H.; Yan, C.H.; Xu, Y.F.; Feng, Y.Q.; Wu, Q.; Lv, X.Y.; Yang, B.W.; Wang, X.; Xia, X.D. Punicalagin Inhibits Salmonella Virulence Factors and Has Anti-Quorum-Sensing Potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- de Freitas, L.L.; da Silva, F.P.; Fernandes, K.M.; Carneiro, D.G.; de Oliveira, L.L.; Martins, G.F.; Dantas Vanetti, M.C. The virulence of Salmonella Enteritidis in Galleria mellonella is improved by N-dodecanoyl-homoserine lactone. Microb. Pathog. 2021, 152, 104730. [Google Scholar] [CrossRef]
- Sivasankar, C.; Jha, N.K.; Ghosh, R.; Shetty, P.H. Anti quorum sensing and anti virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi / Paratyphi A clinical isolates. Microb. Pathog. 2020, 138, 103813. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.; Sperandio, V. Quorum sensing in Escherichia coli and Salmonella. Int. J. Med. Microbiol. 2006, 296, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Che, G.Y.; Pang, H.; Liao, B.; Zhang, J.C.; Liu, J.L. Analysis of the Volatile Components in Lavender from Supercritical CO2 Extraction by GC-MS. Se Pu 2005, 23, 322. [Google Scholar]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre Hemp Inflorescences: From Crop-residues to Essential Oil Production. Ind. Crop. Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Chen, J.L.; Tang, C.L.; Zhang, R.F.; Ye, S.X.; Zhao, Z.M.; Huang, Y.Q.; Xu, X.J.; Lan, W.J.; Yang, D.P. Metabolomics Analysis to Evaluate the Antibacterial Activity of the Essential Oil from the Leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef]
- Pontes, E.K.U.; Melo, H.M.; Araujo Nogueira, J.W.; Sa Firmino, N.C.; de Carvalho, M.G.; Aragao Catunda Junior, F.E.; Arruda Cavalcante, T.T. Antibiofilm Activity of the Essential Oil of Citronella (Cymbopogon nardus) and Its Major Component, Geraniol, on the Bacterial Biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2019, 28, 633–639. [Google Scholar] [CrossRef]
- Selvaraj, A.; Jayasree, T.; Valliammai, A.; Pandian, S.K. Myrtenol Attenuates MRSA Biofilm and Virulence by Suppressing sarA Expression Dynamism. Front. Microbiol. 2019, 10, 2027. [Google Scholar] [CrossRef]
- Yoo, H.J.; Jwa, S.K. Inhibitory Effects of β-Caryophyllene on Streptococcus mutans Biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.T.; Li, L.G.; Xie, R.J.; Xi, W.P.; Guan, Y.; Sumner, L.W.; Zhou, Z.Q. Antifungal activity of citrus essential oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Chakraborty, R. In-depth pharmacological and nutritional properties of bael (Aegle marmelos): A critical review. J. Agric. Food Res. 2020, 2, 100081. [Google Scholar] [CrossRef]
- Subramenium, G.A.; Vijayakumar, K.; Pandian, S.K. Limonene inhibits streptococcal biofilm formation by targeting surface-associated virulence factors. J. Med. Microbiol. 2015, 64, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Deak, E.; Tako, M.; Tserennadmid, R.; Petkovits, T.; Vagvolgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Duarte, A.; Gominho, J.; Domingues, F.; Duarte, A.P. Chemical composition, antioxidant, antibacterial and anti-quorum sensing activities of Eucalyptus globulus and Eucalyptus radiate essential oils. Ind. Crop. Prod. 2016, 79, 274–282. [Google Scholar] [CrossRef]
- Sepahi, E.; Tarighi, S.; Ahmadi, F.S.; Bagheri, A. Inhibition of quorum sensing in Pseudomonas aeruginosa by two herbal essential oils from Apiaceae family. J. Microbiol. 2015, 53, 176–180. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yao, X.; Zhu, Z.A.; Tang, T.T.; Dai, K.R.; Sadovskaya, I.; Flahaut, S.; Jabbouri, S. Effect of berberine on Staphylococcus epidermidis biofilm formation. Int. J. Antimicrob. Agents 2009, 34, 60–66. [Google Scholar] [CrossRef]

| Lactic Acid Bacteria | Mechanism of Action in QS Systems | Bacteria | Study | Reference |
|---|---|---|---|---|
| Lactobacillus plantarum ATCC 10241 | Inhibits acyl-homoserine lactone activity and decreased elastase and biofilm formation | Pseudomonas aeruginosa PA100 | in vitro and burned-mouse model | Valdez 2005 [63] |
| Lactobacillus rhamnosus MTCC 5897, Lactobacillus fermentum MTCC 5898, Lactococcus lactis NCDC-309 | Reduces levels of the auto-inducer AHL and causes a decline in elastase activity and a decrease in mRNA expression of lasI and rhlI | Pseudomonas aeruginosa PAO1 | in vitro | Rana 2020 [65] |
| Lactobacillus acidophilus strain 30SC | Inhibits autoinducer-2 (AI-2) activity and represses biofilm formation | Escherichia coli (EHEC) O157:H7 | in vitro | Kim 2018 [67] |
| Lactiplantibacillus plantarum Z057 | Disrupts the protective biofilm layer, suppresses the communication signal molecule AI-2 involved in quorum sensing, and reduces the activity of genes associated with quorum sensing (luxS, aphA, and opaR), as well as hemolysin-related genes (ToxS and ToxR) | Vibrio parahaemolyticus ATCC 17802 | in vitro | Han 2022 [68] |
| Pediococcus acidilactici 27167 Lactobacillus plantarum 27172 | Reduces expression levels of biofilm-related genes (cidA, icaA, dltB, agrA, sortaseA and sarA) and interferes with the release of signaling molecules (AI-2) in QS systems | Staphylococcus aureus CMCC 26003 | in vitro | Yan 2019 [69] |
| Lactobacillus fermentum Lim2 | Reduces autoinducer-2 (AI-2) activity and suppresses quorum-sensing (luxS) and virulence factors (tcdA, tcdB, and tcdE) | Clostridioides difficile 027 | in vitro | Yong 2019 [71] |
| Lactobacillus fermentum TCUESC01 | Reduces formation of biofilm, increases icaR gene, and reduces icaA gene expression | Staphylococcus aureus CCMB262 | in vitro | Melo 2016 [73] |
| Lactobacillus rhamnosus ATCC7469 | Reduces the expression level of gtfB, gtfC, and ftf genes | Streptococcus mutans ATCC 35668 and 22 | in vitro | Tahmourespour 2019 [76] |
| Lactiplantibacillus plantarum EIR/IF-1 Lactiplantibacillus curvatus EIR/DG-1 Lactiplantibacillus curvatus EIR/BG-2 | Inhibits biofilm formation and downregulates expression of gtfC, comA, and comX | Streptococcus mutans (ATCC 25175) | ex vivo human tooth surfaces | OmerOglou 2022 [77] |
| Lactobacillus reuteri (ATCC 23272) Lactobacillus plantarum subspecies plantarum (ATCC 14917) Lactobacillus salivarius (ATCC 11741) | Reduces adherence of preformed biofilm and gene expression of glucan (gtfB, gtfC, gtfD) and fructan (sacB) | Streptococcus mutans (ATCC 25175) | in vitro | Wasfi 2018 [78] |
| Lactobacillus acidophilus (ATCC 4356) Lactobacillus plantarum (ATCC 14917) | Inhibits ability to form biofilms and downregulates expression of biofilm genes (csgA, crl and csgD) | Uropathogenic E. coli | in vitro | Dawwam 2022 [79] |
| Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2 | Suppresses levels of QS (agrA) and expression of motility-related genes (flaA, fbp) and virulence-associated genes (hlyA, prfA) | Listeria monocytogenes ATCC 19113 | rubber gloves, plastic, silicon rubber surfaces and MBEC™ biofilm device | Hossain 2021 [80] |
| Class of Compound | Phytochemical | Mechanism of Action in QS Systems | Bacteria | Reference |
|---|---|---|---|---|
| Flavonoid | Baicalein | Reduces biofilm formation at 20 μM concentration | Pseudomonas aeruginosa PAO1 | Zeng 2008 [83] |
| Naringenin | Suppresses virulence genes (vopD, vscO and vcrD) and disrupts cell-cell signalling and biofilm formation | Escherichia coli O157:H7 ATCC 43895 and Vibrio harveyi BB120 | Vikram 2010 [85] | |
| Licochalcone A and epigallocatechin-3-gallate | Downregulates the expression of QS-associated genes (sdiA and luxS) at sub-MIC concentration | Salmonella Typhimurium | Hosseinzadeh 2020 [86] | |
| Epigallocatechin | Reduces biofilm formation | Listeria monocytogenes (LMG 21263) | Nyila 2012 [87] | |
| Epigallocatechin | Reduces biofilm formation at 40 µg/mL concentration | Burkholderia cepacia | Huber 2003 [88] | |
| Catechin | Affects autoinducer 2-mediated quorum sensing and inhibits biofilm formation | Eikenella corrodens 1073 | Matsunaga 2010 [89] | |
| Quercetin | Inhibits QS-controlled virulence factors such as violacein, elastase, and pyocyanin and biofilm formation at sub-MIC concentration | Pseudomonas aeruginosa PAO1 Pseudomonas aeruginosa PAF79 Aeromonas hydrophila WAF38 | Al-Yousef 2017 [90] | |
| Quercetin | Inhibits biofilm formation and suppresses the production of virulence factors, such as pyocyanin, protease, and elastase, at a concentration of 16 μg/mL. Additionally, there was a decrease in the expression levels of genes associated with quorum sensing (lasI, lasR, rhlI, and rhlR) | Pseudomonas aeruginosa PAO1 | Ouyang 2016 [91] | |
| Morin | Reduces biofilm formation, motility, and spreading and EPS production | Staphylococcus aureus | Chemmugil 2019 [93] | |
| Kaempferol | Prevents the formation of biofilms by inhibiting the activity of sortase A and downregulates the expression of adhesion-related genes (clfA, clfB, fnbA, and fnbB) | Staphylococcus aureus ATCC 29213™ | Ming 2017 [94] | |
| Taxifolin | Diminishes levels of QS-regulated genes, including lasI, lasR, rhlI, rhlR, lasA, lasB, phzA1, and rhlA. | Pseudomonas aeruginosa PAO1 | Vandeputte 2011 [95] | |
| Genistein | Decreases the production of aerolysin and biofilm formation at a dose-dependent manner and downregulates QS-related genes (ahyI and ahyR) and aerolysin encoding gene (aerA) | Aeromonas hydrophila XS-91-4-1 | Dong 2021 [97] | |
| Esculetin | Inhibits the production of protease and hemolysin, and the formation of biofilms, downregulates QS-related and biofilm formation-related genes (ahyI, ahyR, luxS, csgAB, and fleQ), and negatively upregulates biofilm formation-related gene (litR) | Aeromonas hydrophila SHAe 115 | Sun 2021 [98] | |
| Phenolic acids | Cinnamic acid and salicylic acid | Inhibits the expression of QS genes, including expI, expR, luxR, and luxS; reduces the level of the AHL signal | Pectobacterium aroidearum PC1 Pectobacterium carotovorum ssp. brasiliense Pcb1692 | Joshi 2016 [102] |
| Chlorogenic acid | Downregulates the expression of major genes (LPxB and LPxC) in LPS biosynthesis | Pseudomonas aeruginosa P1 | Su 2019 [103] | |
| Vanillic acid | Diminishes capacity to form biofilms, movement ability, and production of exotoxins (protease and exopolysaccharide), while suppressing the expression of genes associated with biofilm formation and virulence (sypG, fliS, fliK, lafA, lafK, asp, and luxR) when exposed to subinhibitory concentrations | Vibrio alginolyticus | Liu 2021 [104] | |
| Rosmarinic acid | Reduces QS-mediated hemolysin, lipase, and elastase production at 750 µg/mL concentration; downregulates virulence genes (ahh1, aerA, lip, and ahyB) | Aeromonas hydrophila | Devi 2016 [105] | |
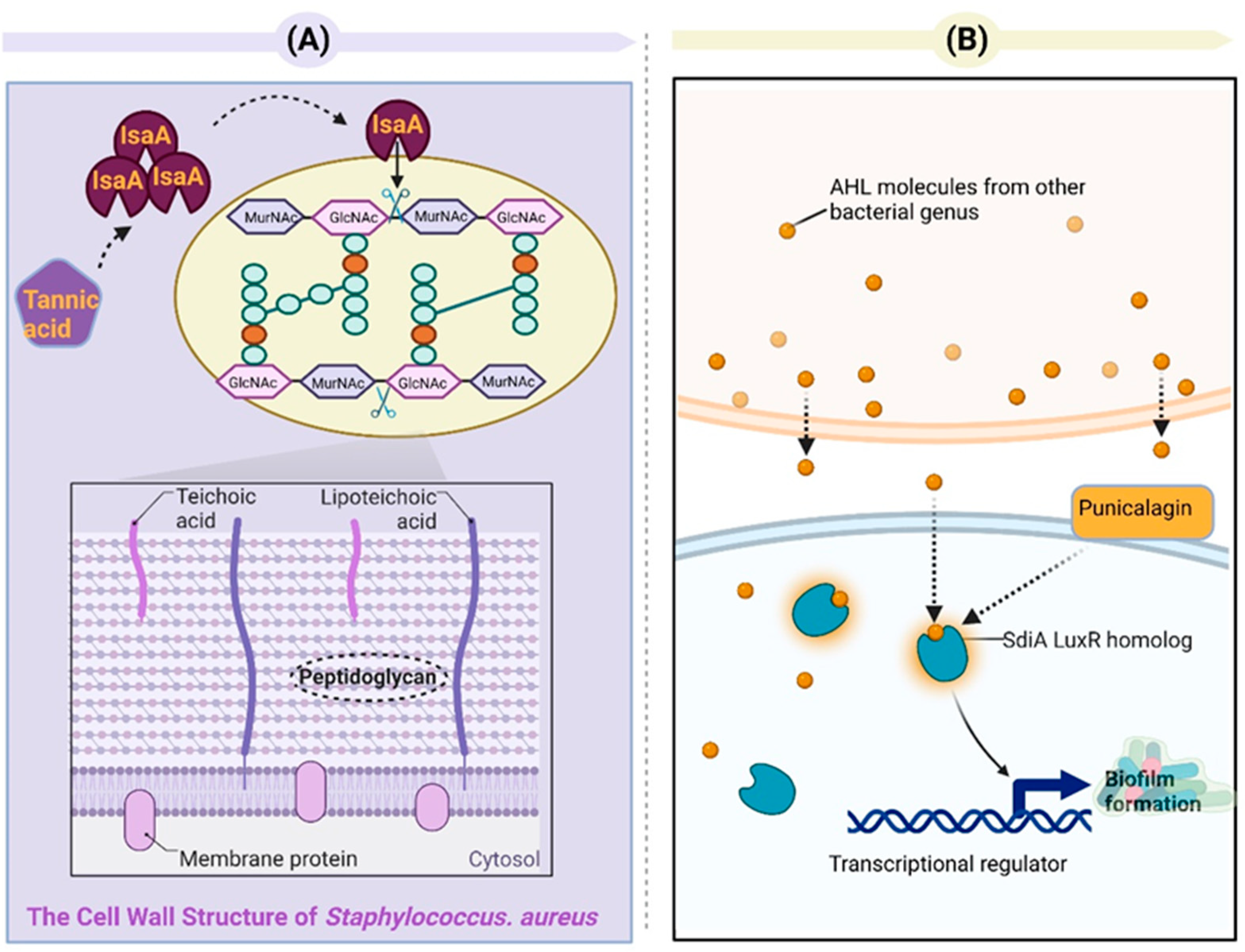
| Tannic | Tannic acid | Reduces biofilm formation; downregulates the adhesion-associated exoR gene, limited the iron supply | Agrobacterium tumefaciens GV2260 | Jailani 2022 [106] |
| Ellagic acid Tannic acid | Reduces biofilm formation | Escherichiacoli VR50 and F18 | Hancock 2010 [107] | |
| Tannic acid | Reduces biofilm formation | Staphylococcus aureus | Payne 2013 [108] | |
| Punicalagin | Represses the expression of QS-related genes (sdiA and srgE) | Salmonella Typhimurium SL1344 | Li 2014 [110] | |
| Terpenoid | Linalool | Inhibits the formation of biofilms and disrupts pre-existing biofilms, alters adhesion properties, and interferes with the quorum sensing mechanism | Acinetobacter baumannii | Alves 2016 [117] |
| Geraniol | Reduces biofilm biomass | Staphylococcus aureus ATCC 6538 | Pontes 2019 [118] | |
| Myrtenol | Reverses the formation of mature biofilm; inhibits the production of key virulence factors such as slime, lipase, alpha-hemolysin, staphyloxanthin, and autolysin. Reduces the activity of the global regulator sarA and its influence on virulence genes (agrA, icaA, icaD, fnbA, fnbB, clfA, cna, hla, hld, geh, altA, and crtM). | Methicillin-resistant Staphylococcus aureus | Selvaraj 2019 [119] | |
| β-caryophyllene | Inhibits biofilm formation and reduces the expression of gtf genes | Streptococcus mutans KCTC 3065 (ATCC 25175) | Yoo 2018 [120] | |
| Limonene | Inhibits adhesion and prevents biofilm formation cascade | Streptococcus pyogenes (SF370 and 5 clinical isolates) Streptococcus mutans (UA159) Streptococcus mitis (ATCC 6249) | Subramenium 2015 [124] | |
| Ferula Dorema | Decreases pyocyanin, pyoverdine, elastase, and biofilm production Reduces pyoverdine and elastase production | Pseudomonas aeruginosa PAO1 (ATTC 15692) | Sepahi 2015 [127] | |
| Alkaloid | Berberine | Inhibits biofilm formation at sub-MIC concentrations | Staphylococcus epidermidis 243 (SE243) Staphylococcus epidermidis ATCC 35984 and ATCC 12228 | Wang 2009 [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhang, Z.; Li, J.; Qin, G. New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins 2023, 15, 570. https://doi.org/10.3390/toxins15090570
Zhang H, Zhang Z, Li J, Qin G. New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins. 2023; 15(9):570. https://doi.org/10.3390/toxins15090570
Chicago/Turabian StyleZhang, Hua, Zhen Zhang, Jing Li, and Guangyong Qin. 2023. "New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition" Toxins 15, no. 9: 570. https://doi.org/10.3390/toxins15090570
APA StyleZhang, H., Zhang, Z., Li, J., & Qin, G. (2023). New Strategies for Biocontrol of Bacterial Toxins and Virulence: Focusing on Quorum-Sensing Interference and Biofilm Inhibition. Toxins, 15(9), 570. https://doi.org/10.3390/toxins15090570




