Antifungal and Antiaflatoxigenic Activities of Massoia Essential Oil and C10 Massoia Lactone against Aflatoxin-Producing Aspergillus flavus
Abstract
:1. Introduction
2. Results and Discussions
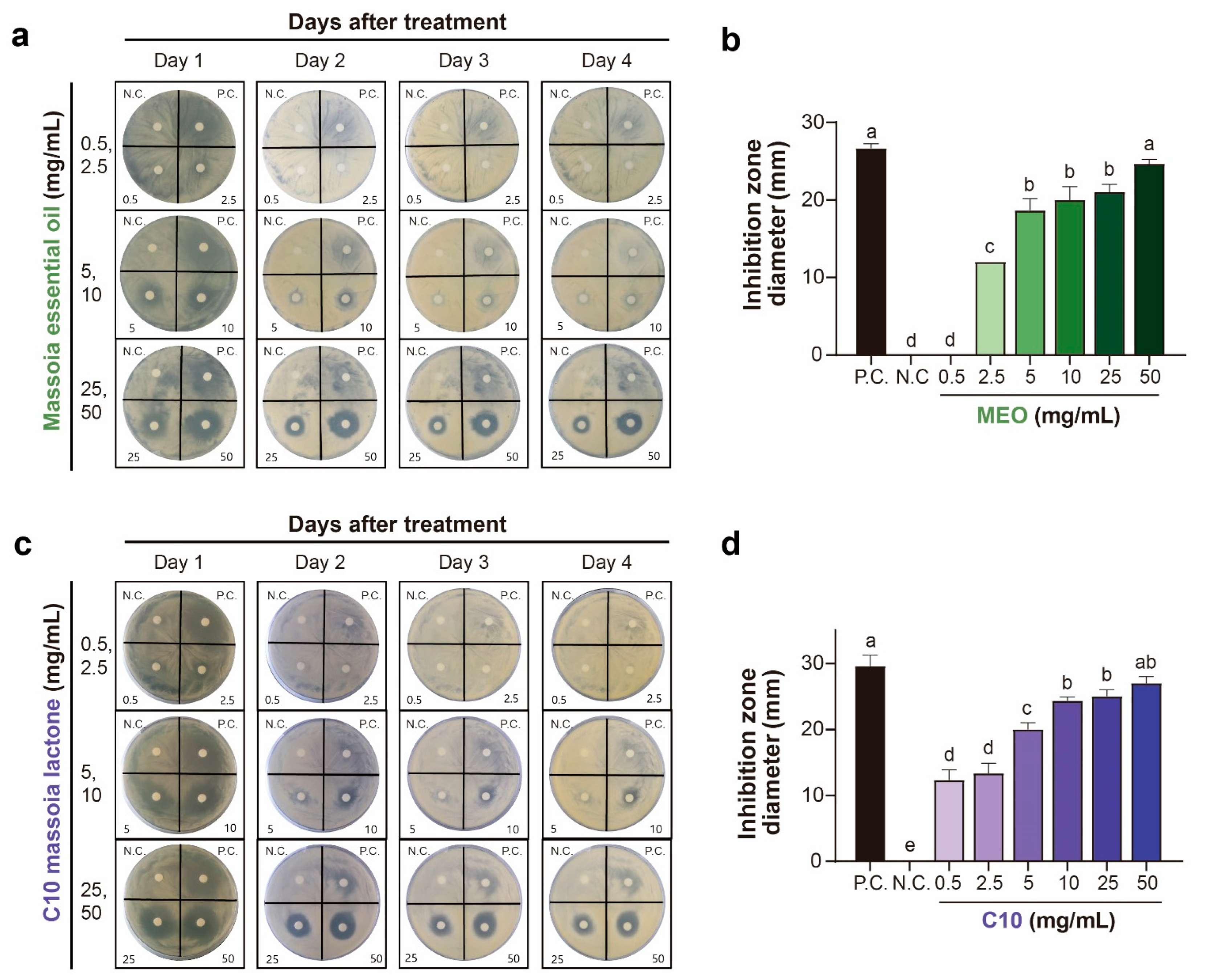
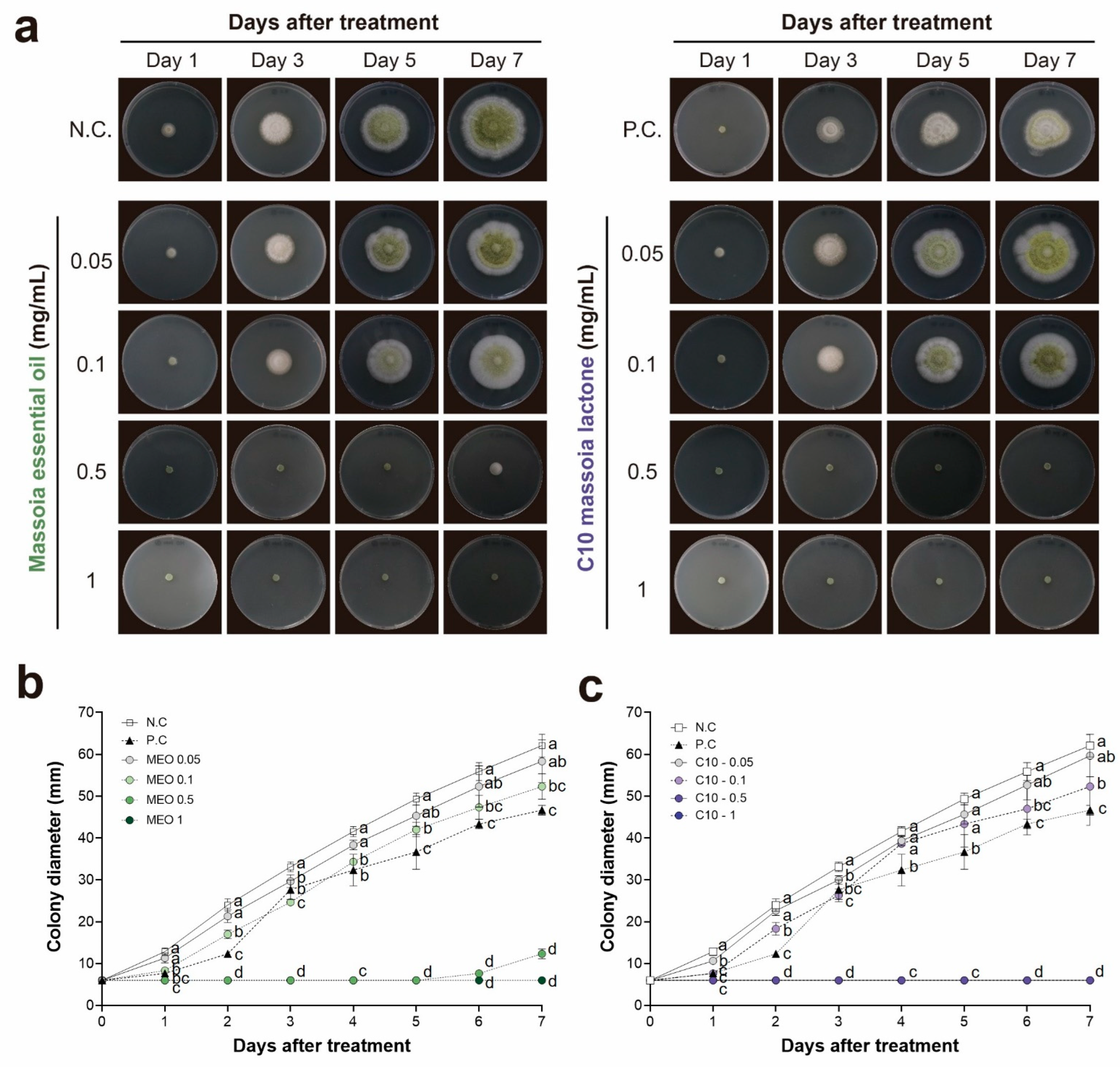
2.1. Antifungal Activities of MEO and C10 against A. flavus
2.2. GC–MS Analysis of MEO Constituents Using RI
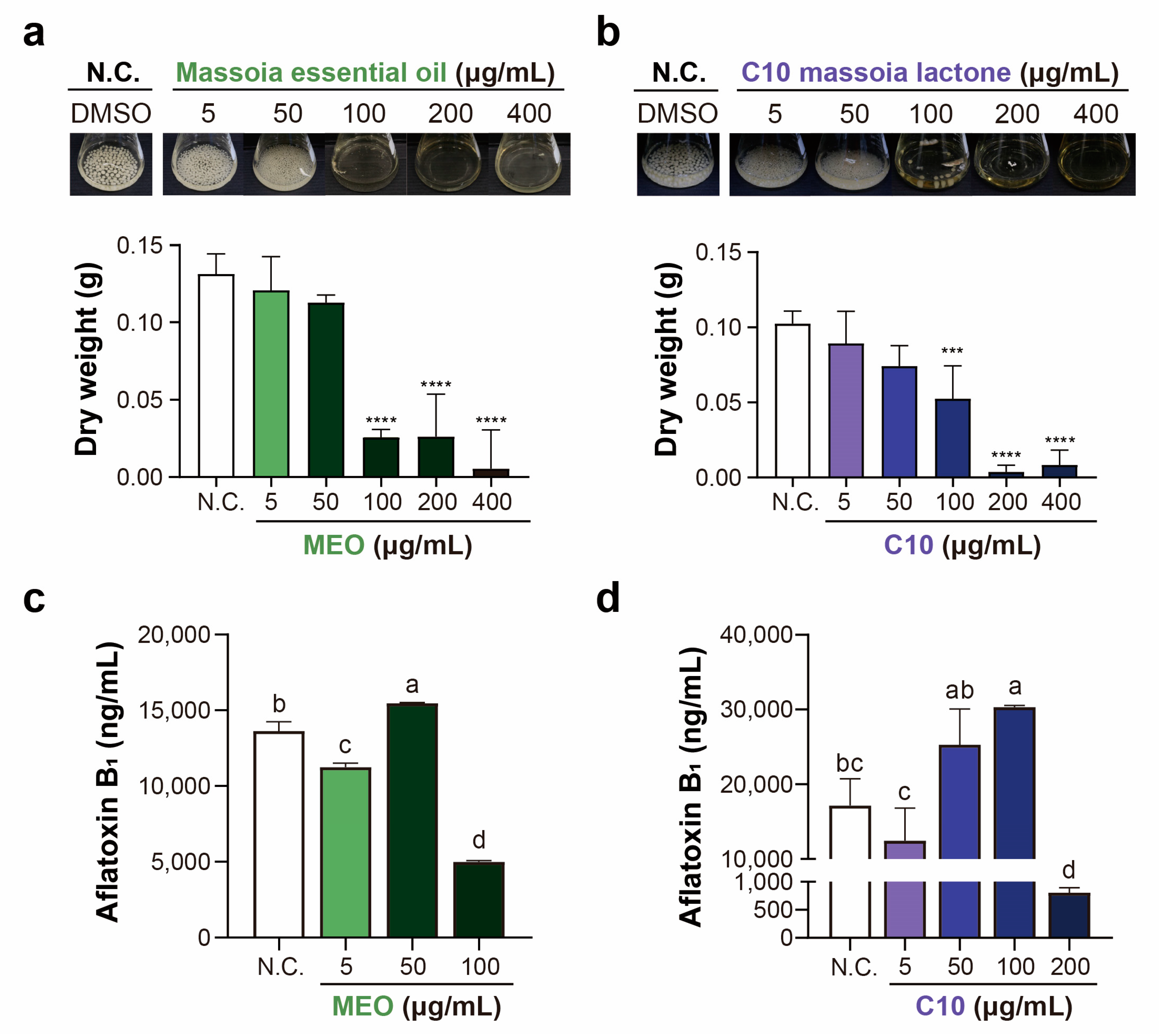
2.3. Aflatoxin Production after MEO and C10 Treatments in A. flavus
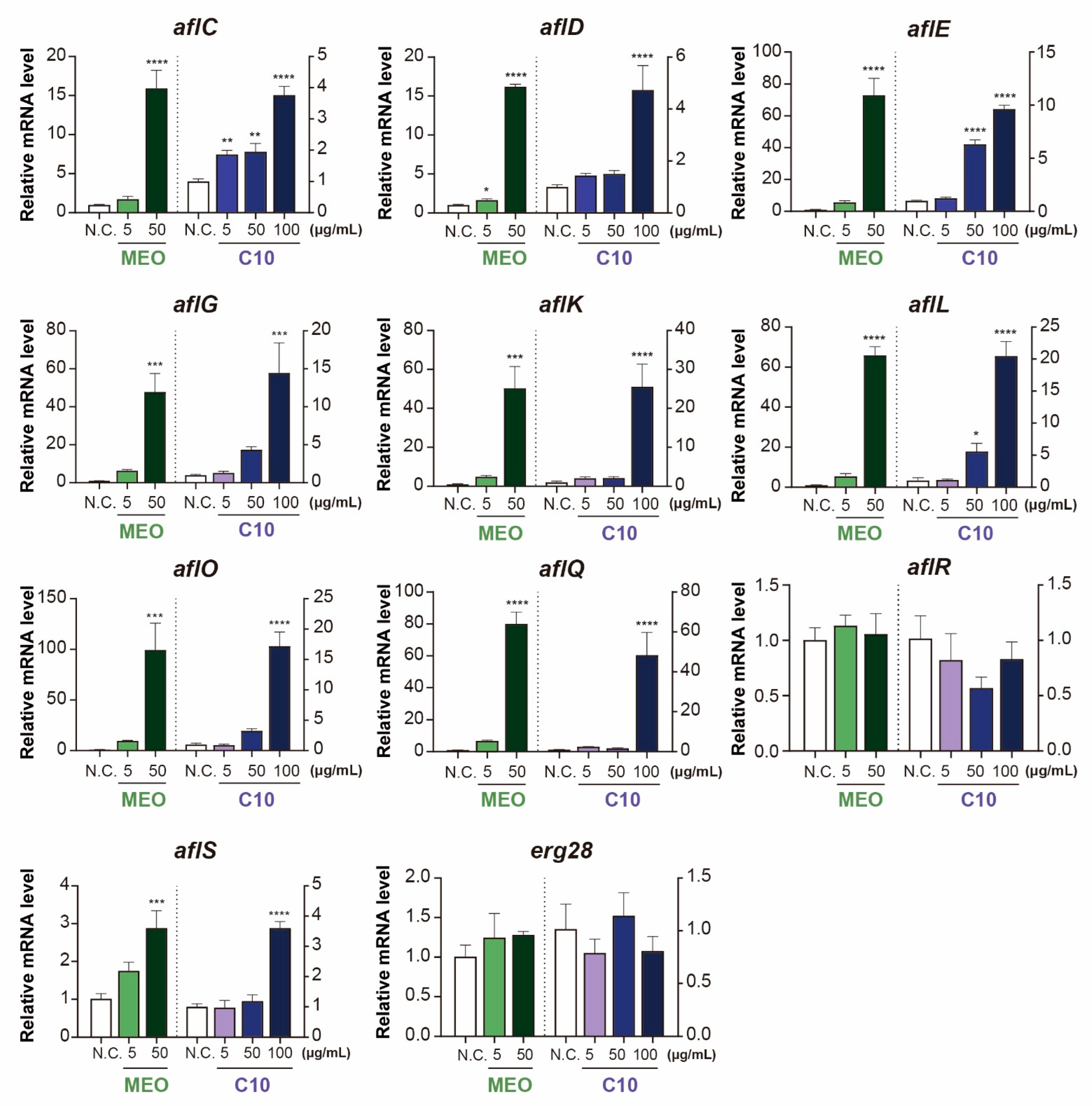
2.4. Differential Expression of Aflatoxin-Producing Genes after Chemical Treatments
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Fungal Cultures
4.3. Antifungal Disc Diffusion Assay
4.4. Agar Dilution Method
4.5. Mycelial Growth Assay
4.6. Aflatoxin Analysis Using Liquid Chromatography Triple Quadrupole Mass Spectrometry
4.7. GC–MS Analysis and Quantification of Massoia Essential Oil
4.8. Total RNA Isolation and qRT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the Risk of Aflatoxin-induced Liver Cancer in Tanzania based on Biomarker Data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef]
- Magnussen, A.; Parsi, M.A. Aflatoxins, Hepatocellular Carcinoma and Public Health. World J. Gastroenterol. 2013, 19, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Al Khoury, A.; El Khoury, R.; Lorber, S.; Oswald, I.P.; El Khoury, A.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin Biosynthesis and Genetic Regulation: A review. Toxins 2021, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin Contamination in Food Crops: Causes, Detection, and Management: A Review. Food Prod. Process. Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Sserumaga, J.P.; Ortega-Beltran, A.; Wagacha, J.M.; Mutegi, C.K.; Bandyopadhyay, R. Aflatoxin-producing Fungi Associated with Pre-harvest Maize Contamination in Uganda. Int. J. Food Microbiol. 2020, 313, 108376. [Google Scholar] [CrossRef]
- Taherabadi, M.S.; Gharavi, M.J.; Javadi, I.; Alimohammadi, M.; Moghadamnia, H.; Mosleh, N.; Farajollahi, M.M.; Sharif, M. The Level of Aflatoxin M1 in Raw and Pasteurized Milk Produced in Alborz Province, Iran. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e31708. [Google Scholar] [CrossRef]
- Fakhri, Y.; Ghorbani, R.; Taghavi, M.; Keramati, H.; Amanidaz, N.; Moradi, B.; Nazari, S.H.; Shariatifar, N.; Khaneghah, M. Concentration and Prevalence of Aflatoxin M1 in Human Breast Milk in Iran: Systematic Review, Meta-Analysis, and Carcinogenic Risk Assessment: A Review. J. Food Prot. 2019, 82, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Anukul, N.; Vangnai, K.; Mahakarnchanakul, W. Significance of Regulation Limits in Mycotoxin Contamination in Asia and Risk Management Programs at the National Level. J. Food Drug Anal. 2013, 21, 227–241. [Google Scholar] [CrossRef]
- Ali, N. Aflatoxin in Rice: Worldwide Occurrence and Public Health Perspective. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary Aflatoxin Exposure and Impaired Growth in Young Children from Benin and Togo: Cross Sectional Study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in Dairy Cows: Toxicity, Occurrence in Feedstuffs and Milk and Dietary Mitigation Strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef]
- Wangia, R.N.; Githanga, D.P.; Wang, J.S.; Anzala, O.A. Aflatoxin Exposure in Children Age 6-12 Years: A Study Protocol of a Rrandomixed Comparative Cross-sectional Study in Kenya, East Africa. Pilot Feasibility Stud. 2019, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Ayelign, A.; Alemu, T.; Saeger, S.D. Validation of a HACCP Community-based Infants’ Complementary Food Safety Assurance Method in Cash Crop Producing Communities in Gedeo zone, Southern Ethiopia. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 1311–1320. [Google Scholar] [CrossRef]
- Kim, K.H.; Nam, M.S. Decrease of Aflatoxin M1 Level in Raw Cow’s Milk using the Hazard Analysis and Critical Control Points (HACP) System. J. Life Sci. 2016, 26, 190–197. [Google Scholar] [CrossRef]
- Jeong, S.E.; Chung, S.H.; Hong, S.Y. Natural Occurrence of Aflatoxins and Ochratoxins A in Meju and Soybean Paste Produced in South Korea. Appl. Biol. Chem. 2019, 62, 65. [Google Scholar] [CrossRef]
- Lee, S.Y.; Woo, S.Y.; Tian, F.; Jeong, A.Y.; Park, S.B.; Chun, H.S. Contamination Characteristics and Risk Assessment of Aflatoxins in Homemade Soybean Paste, a Traditional Fermented Soybean Food, in South Korea. J. Hazard. Mat. 2022, 424, 127576. [Google Scholar] [CrossRef]
- Akbar, N.; Nasir, M.; Naeem, N.; Ahmad, M.; Saeed, F.; Anjum, F.M.; Iqbal, S.; Imran, M.; Tufail, T.; Shah, F.; et al. Assessment of Aflatoxin in Milk and Feed Samples and Impact of Seasonal Variations in the Punjab, Pakistan. Food Sci. Nutr. 2020, 8, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Schamann, A.; Schmidt-Heydt, M.; Geisen, R.; Kulling, S.E.; Soukup, S.T. Formation of B- and M-group Aflatoxins and Precursors by Aspergillus flavus on Maize and its Implication for Food Safety. Mycotoxin Res. 2022, 38, 79–92. [Google Scholar] [CrossRef]
- Sun, X.D.; Su, P.; Shan, H. Mycotoxin Contamination of Maize in China. Compr. Rev. Food Sci. Food Saf. 2017, 16, 835–849. [Google Scholar] [CrossRef]
- Peles, F.; Sipos, P.; Kovács, S.; Győri, Z.; Pócsi, I.; Pusztahelyi, T. Biological Control and Mitigation of Aflatoxin Contamination in Commodities. Toxins 2021, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Sipos, P.; Peles, F.; Brassó, D.L.; Béri, B.; Pusztahelyi, T.; Győri, Z.; Pócsi, I. Physical and Chemical Methods for Reduction in Aflatoxin Content of Feed and Food. Toxins 2021, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Choi, W.S.; Park, E.S.; Bae, I.K.; Choi, S.D.; Paek, O.; Kim, S.H.; Chun, H.S.; Lee, S.E. Antifungal and Antiaflatoxigenic Methylenedioxy-containing Compounds and Piperine-like Synthetic Compounds. Toxins 2016, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.S.; Kim, L.; Chun, H.S.; Lee, S.E. 4-Hydroxy-7-methyl-3-phenylcoumarin Suppresses Aflatoxin Biosynthesis via Downregulation of aflK Expressing Versicolorin B Synthase in Aspergillus flavus. Molecules 2017, 22, 712. [Google Scholar] [CrossRef]
- Kim, H.M.; Kwon, H.; Kim, K.; Lee, S.E. Antifungal and Antiaflatoxigenic Activities of 1,8-cineole and t-cinnamaldehyde on Aspergillus flavus. Appl. Sci. 2018, 8, 1655. [Google Scholar] [CrossRef]
- Kim, G.; Lee, S.E. Antifungal and Antiaflatoxigenic Properties of Naphthoquinones toward Aspergillus flavus and their Mode of Iinhibitory Action on Aflatoxin Biosynthesis. Food Control 2021, 119, 107506. [Google Scholar] [CrossRef]
- Moon, Y.S.; Kim, H.M.; Chun, H.S.; Lee, S.E. Organic Acids Suppress Aflatoxin Production via Lowering Expression of Aflatoxin Biosynthesis-related Genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Hertiani, T.; Pratiwi, S.U.T.; Yuswanto, A.; Permanasari, P. Potency of Massoia Bark in Combating Immunosuppressed-related Infection. Pharmacogn. Mag. 2016, 12, S363–S370. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, H.Q.; Chi, Z.; Liu, G.L.; Chi, Z.M. Making of Massoia Lactone-loaded and Food-grade Nanoemulsions and their Bioactivities against a Pathogenic Yeast. J. Mar. Sci. Eng. 2022, 10, 339. [Google Scholar] [CrossRef]
- Barros, M.E.S.B.; Freitas, J.C.R.; Oliveira, J.M. Synthesis and Evaluation of (-)-Massoia lactone and Analogues as Potential Anticancer and Anti-inflammatory Agents. Eur. J. Med. Chem. 2014, 76, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Sang, Q.; Pan, Y.; Jiang, Z. HPLC Determination of Massoia Lactone in Fermented Cordyceps sinensis Mycelium Cs-4 and its Anticancer Activity in vitro. J. Food Biochem. 2020, 44, e13336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, Z.C.; Chi, Z.; Wang, Z.; Liu, G.L.; Li, X.F.; Hu, Z.; Chi, Z.M. Massoia Lactone Displays Strong Antifungal Property against Many Crop Pathogens and its Potential Application. Microb. Ecol. 2022, 84, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V. Essential oil: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, S.; Zhang, Q.; Liu, W.; Zhou, Y.; Yang, W. Screening Fungicides for Controlling Wheat Crown Rot Caused by Fusarium pseudograminearum across Hebei Province in China. Agriculture 2022, 12, 1643. [Google Scholar] [CrossRef]
- Rosam, K.; Monk, B.C.; Lackner, M. Sterol 14α-Demethylase Ligand-Binding Pocket-Mediated Acquired and Intrinsic Azole Resistance in Fungal Pathogens. J. Fungi 2021, 7, 1. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.; White, T.C. Hidden killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Goldman, G.H.; Santos, D.A. Fungicide Effects on Human Fungal Pathogens: Cross-resistance to Medical Drugs and Beyond. PLoS Pathog. 2022, 17, e1010073. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxins Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.W.; Vallieres, C.; Avery, S.V. Evolving Challenges and Strategies for Fungal Control in the Food Supply Chain. Fungal Biol. Rev. 2021, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Braïek, O.B.; Smaoui, S. Chemistry, Safety, and Challenges of the Use of Organic Acids and their Derivative Slats in Meat Preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar]
- Oser, B.L.; Weil, C.S.; Woods, L.A.; Bernard, B.K. Recent Progress in the Consideration of Flavoring Ingredients under the Food Additives Amendment. 14. GRAS substances. Food Technol. 1985, 39, 108–117. [Google Scholar]
- Lempart-Rapacewicz, A.; Kudlek, E.; Brukalo, K.; Rapacewicz, R.; Lempart, L.; Dudziak, M. The Threat of Food Additive Occurrence in the Environment- A Case Study on the Example of Swimming Pools. Foods 2023, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Canales-Martinez, M.; Rivera-Yañez, C.R.; Salas-Oropeza, J.; Lopez, H.R.; Jimenez-Estrada, M.; Rosas-Lopez, R.; Duran, D.A.; Flores, C.; Hernandez, L.B.; Rodriguez-Monroy, M.A. Antimicrobial Activitiy of Bursera Morelensis Ramirez Essential Oil. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 74–82. [Google Scholar] [CrossRef] [PubMed]
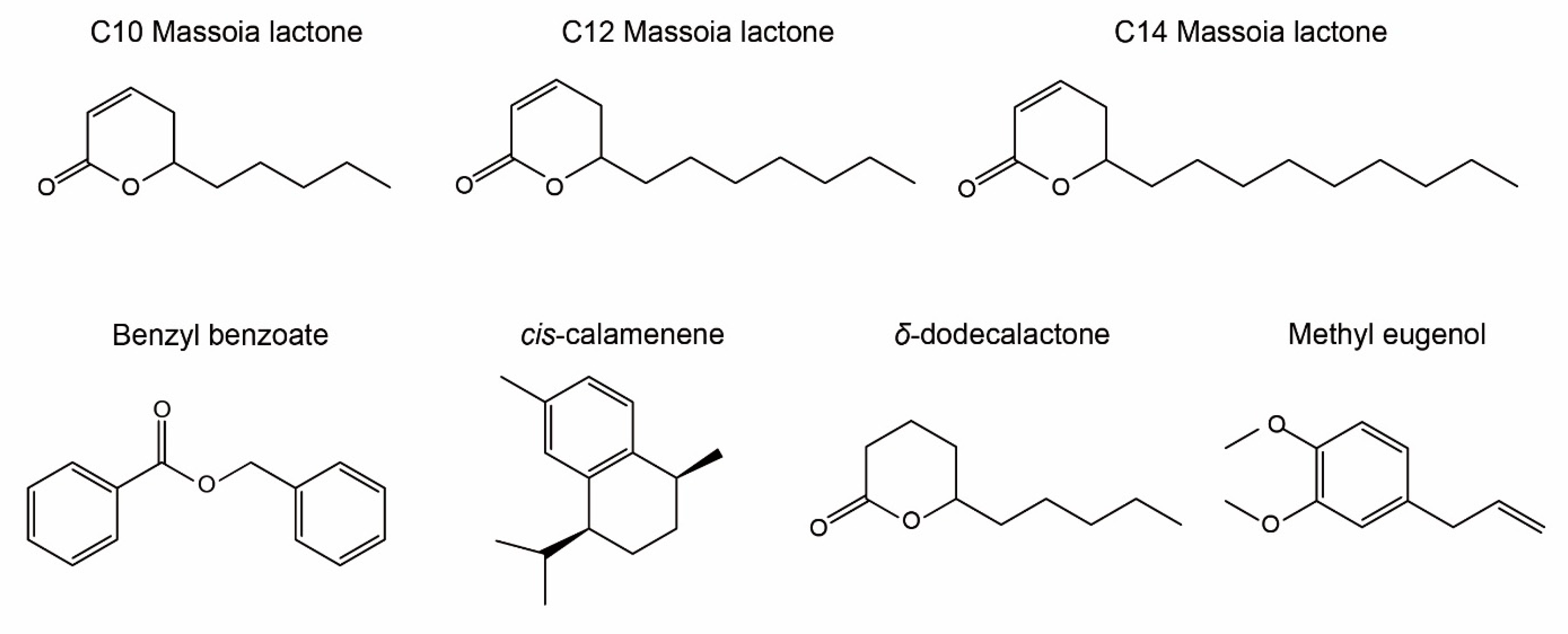
| No | Compound | MM | RI (5%) | RI (Wax) | Ref (5%) | Ref (Wax) | % |
|---|---|---|---|---|---|---|---|
| 1 | furfural | 96.084 | 832 | 1450 | 835 | 1468 | 0.2 |
| 2 | (E)-1,3-nonadiene | 124.223 | 926 | 1257 | 924 | 0.8 | |
| 3 | 5-methyl-2-furancarboxaldehyde | 110.111 | 962 | 1557 | 963 | 1555 | 0.2 |
| 4 | 2-methoxyphenol | 124.137 | 1086 | 1839 | 1090 | 1846 | 0.1 |
| 5 | linalool | 154.249 | 1099 | 1538 | 1098 | 1537 | 0.2 |
| 6 | 1,4-undecadiene | 152.277 | 1126 | 1257 | 0.7 | ||
| 7 | trans-3-nonen-2-one | 140.223 | 1138 | 1523 | 1137 | 1500 | 0.1 |
| 8 | 1,3,5-undecatriene | 150.261 | 1185 | 1402 | 1187 | 0.1 | |
| 9 | ylangene | 204.351 | 1378 | 1480 | 1373 | 1485 | 0.1 |
| 10 | copaene | 204.351 | 1382 | 1488 | 1384 | 1488 | 0.2 |
| 11 | 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane | 204.351 | 1388 | 1585 | 0.1 | ||
| 12 | methyl eugenol | 178.228 | 1399 | 1995 | 1401 | 2020 | 0.3 |
| 13 | β-bergamotene | 204.351 | 1442 | 1581 | 1436 | 1586 | 0.1 |
| 14 | γ-muurolene | 204.351 | 1453 | 1682 | 0.2 | ||
| 15 | alloaromadendrene | 204.351 | 1472 | 1636 | 1467 | 1639 | 0.3 |
| 16 | C10 massoia lactone | 168.233 | 1485 | 2210 | 1483 | 45.2 | |
| 17 | δ-decalactone | 170.249 | 1495 | 2168 | 1494 | 2160 | 1.2 |
| 18 | β-bisabolene | 204.351 | 1512 | 1721 | 1512 | 0.3 | |
| 19 | δ-Cadinene | 204.351 | 1526 | 1751 | 1528 | 0.2 | |
| 20 | cis-calamenene | 202.335 | 1530 | 1824 | 1532 | 1839 | 3.2 |
| 21 | C12 massoia lactone | 196.286 | 1697 | 2442 | 36.7 | ||
| 22 | δ-dodecalactone | 198.302 | 1709 | 2399 | 1711 | 1.9 | |
| 23 | benzyl benzoate | 212.244 | 1776 | 2606 | 1770 | 2566 | 3.8 |
| 24 | benzyl salicylate | 228.243 | 1880 | 2762 | 1870 | 2737 | 0.3 |
| 25 | C14 massoia lactone | 224.339 | 1907 | 2673 | 1910 | 1.4 | |
| Total | 98.9 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Park, S.J.; Kim, K.; Kim, T.-O.; Lee, S.-E. Antifungal and Antiaflatoxigenic Activities of Massoia Essential Oil and C10 Massoia Lactone against Aflatoxin-Producing Aspergillus flavus. Toxins 2023, 15, 571. https://doi.org/10.3390/toxins15090571
Lee Y, Park SJ, Kim K, Kim T-O, Lee S-E. Antifungal and Antiaflatoxigenic Activities of Massoia Essential Oil and C10 Massoia Lactone against Aflatoxin-Producing Aspergillus flavus. Toxins. 2023; 15(9):571. https://doi.org/10.3390/toxins15090571
Chicago/Turabian StyleLee, Yubin, Soo Jean Park, Kyeongnam Kim, Tae-Oh Kim, and Sung-Eun Lee. 2023. "Antifungal and Antiaflatoxigenic Activities of Massoia Essential Oil and C10 Massoia Lactone against Aflatoxin-Producing Aspergillus flavus" Toxins 15, no. 9: 571. https://doi.org/10.3390/toxins15090571







