Abstract
Potato is the fourth most consumed crop in the world. More than half of the crop is stored for three to nine months at cold temperatures (3–10 °C) for the fresh and seed market. One of the main causes of fresh potato waste in the retail supply chain is the processing of fungal and bacterial rots during storage. Dry rot is a fungal disease that mainly affects the potato crop during storage and is responsible for 1% of tuber losses in the UK. It is produced by Fusarium spp., such as Fusarium sambucinum and F. oxysporum, which can lead to the accumulation of mycotoxins in the potato tuber. Little is known about the impact of environmental factors on the accumulation of mycotoxins in potato tubers. Understanding the ecophysiology of these fungi is key to mitigating their occurrence under commercial storage conditions. Therefore, this work aimed to elucidate the effect of three different temperatures (5, 10, and 15 °C) and two different water activities (aw; 0.97, 0.99) on the ecophysiology and mycotoxin accumulation of F. sambucinum and F. oxysporum in a potato-based semi-synthetic medium. The mycotoxin accumulation was then studied in vivo, in potato tubers cultivated under organic farming conditions, stored for 40 days at 8.5 °C. Results showed that higher temperatures and aw enhanced fungal growth, lag time, and mycotoxin accumulation in vitro. Growth rate was 2 and 3.6 times higher when the temperature increased from 5 to 10 and 15 °C, respectively. Six different mycotoxins (T-2, HT-2, diacetoxyscirpenol, 15-acetoxyscirpenol, neosolaniol, and beauvericin) were detected in vitro and in vivo. T-2 was the most abundant mycotoxin detected in vitro, observing 106 ng of T-2/g media after 21 days of incubation at 10 °C and 0.99 aw. Due to the long period of time that potato tubers spend in storage, the fluctuations of environmental factors, such as temperature and relative humidity, could promote the development of fungal rot, as well as mycotoxin accumulation. This could result in important food and economic losses for the potato market and a threat to food safety.
Key Contribution:
This paper highlights the effect of storage temperature and relative humidity on the accumulation of mycotoxins produced by Fusarium spp. (dry rot agent) on potato tubers. High temperatures and relative humidity result in dry rot symptoms and accumulation of mycotoxins, increasing food waste and posing a food safety risk.
1. Introduction
Potato (Solanum tuberosum L.) is the fourth main food crop in the world after maize, rice, and wheat, and is widely consumed every year with a total production of 370 million tons in 2023 [1]. Potato susceptibility to fungal or bacterial diseases varies across potato cultivars. One of the main factors is the periderm thickness of the tubers [2]. During postharvest storage, more than half of the potato crop is stored for three to nine months at 4–10 °C for the fresh and processing markets and at temperatures below 4 °C for the seed market. During the storage period, the appearance of fungal or bacterial rots is a challenge that must be faced by the potato industry. The main fungal disease in Europe after black dot is dry rot, causing postharvest fungal decay and therefore consequently large economic losses [3]. Currently, 17 different species and five variants of Fusarium have been identified as responsible for dry rot in potato tubers, including F. avenaceum, F. solani var. coeruleum, F. sambucinum, and F. oxysporum, among others [3,4]. Fusarium spp. generally infect potato tubers through surface wounds or natural openings at pre- and postharvest stages, resulting in wrinkled brown skin and sunken tissue with a dry appearance [5].
Therefore, maintaining the optimal environmental conditions (temperature and relative humidity) during storage is essential for controlling the incidence of fungal or bacterial rots. Understanding the in vitro ecophysiology of Fusarium spp. responsible for these diseases will provide insight into how different environmental conditions affect the development of dry rot in potato tubers during storage. To determine the effect of different relative humidity on the development of Fusarium spp. in vitro, a range of water activities (aw) can be used. The aw is related to the water available in the matrix for fungal growth. It is considered one of the most important factors affecting fungal growth and their secondary metabolites production [6,7,8,9]. Therefore, this relationship will allow the simulation of a constant relative humidity in commercial storage facilities at a specific cold temperature.
Some of the Fusarium spp., such as F. sambucinum, F. sulphureum, F. coeruleum, F. graminearum, and F. oxysporum, responsible for dry rot in potato tubers can produce secondary metabolites known as mycotoxins. Fusarium spp. produce both trichothecenes and non-trichothecenes [3]. Trichothecenes are classified into four types (A, B, C, and D) based on their chemical structure, with types A and B being detected in rotten potato tubers [10]. T-2 toxin, HT-2 toxin, neosolaniol (NEO), diacetoxyscirpenol (DAS), and 15-acetoxyscirpenol (15-AS) are type A trichothecenes, while nivalenol, fusarenon X, deoxynivalenol (DON), 3-Acetyldeoxynivalenol (3-ADON), and 15-acetyldeoxynivalenol (15-ADON) are considered trichothecenes type B. Ingestion of trichothecenes can cause serious health issues in animals and humans, presenting immunosuppressive and mutagenic effects [10].
In previous studies, fusarenon X, 3-ADON, T-2, and DAS were detected in dry-rot-affected potato tubers infected with F. sulphureum, F. solani, and F. sambucinum at different storage temperatures (5 °C, 20 °C) in two potato cultivars [11,12]. F. graminearum was also identified as a producer of DON, nivalenol, 3-ADON, and 15-ADON in inoculated potato tubers [13].
The main non-trichothecenes are beauvericin, enniatin, fumonisin B1, fumonisin B2, fusarin C, zearalenone (ZEN), and fusaric acid; they all have been previously detected in potatoes infected with F. oxysporum [14].
Although previous studies have evaluated the effect of temperature and aw in some Fusarium spp., different temperatures were studied. In the present study, the effect of these environmental factors was evaluated in vitro and in vivo, not only on the fungal development, but also in their mycotoxin accumulation. There is limited research on the accumulation of mycotoxins in potato tubers stored under different environmental conditions. Therefore, elucidating the effect of storage temperature and relative humidity on the accumulation of mycotoxins will provide insight into the food safety of potato tubers. Besides, the time that potato tubers spend in storage, previous to their release to the market, could have an effect on the disease severity, and consequently on the mycotoxin accumulation.
The aims of this study were to (1) elucidate the effect of three different temperatures (5, 10, and 15 °C) and two different aw (0.97, 0.99) on the ecophysiology of F. sambucinum and F. oxysporum in a potato-based semi-synthetic media; (2) evaluate the effect of temperature and aw on the mycotoxin accumulation in vitro of both species; and (3) determine the mycotoxin accumulation in potato tubers under standard commercial storage conditions.
2. Results
2.1. Effect of Temperature and aw on Two Fusarium spp. Growth Parameters on Potato-Based Media
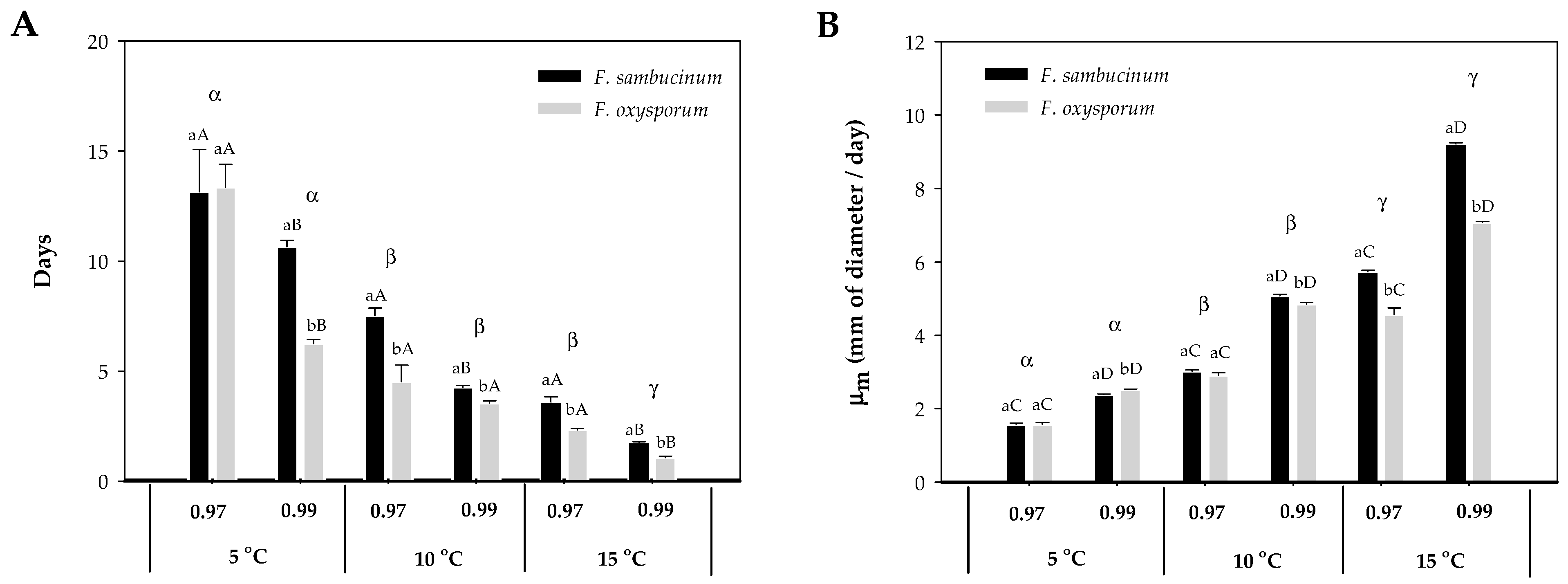
The increase in temperature from 5 to 15 °C resulted in a significant decrease in the lag time (λ) of both Fusarium spp. (F. sambucinum, F. oxysporum) (Figure 1A). At the lowest temperature, 5 °C, different aw did not affect the λ of either Fusarium spp. At 10 °C, aw only significantly affected F. sambucinum (p-value < 0.007), with a shorter λ at the highest aw (0.99). Similar results were obtained at the highest temperature, where significantly shorter λ were observed for both Fusarium spp. and aw (p-values < 0.004). The effect of temperature on λ of both Fusarium spp. differed between aw; at 0.97 aw, significant differences were only detected between 5 and 15 °C and 10 and 15 °C (p-values < 0.008). While at 0.99 aw, significant differences (p-values < 0.006) were detected between the three temperatures.
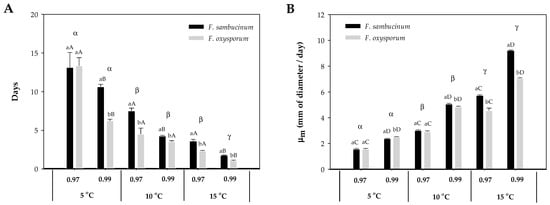
Figure 1.
Effect of temperature (5, 10, 15 °C) and aw (0.97, 0.99) on F. sambucinum and F. oxysporum growth. (A) Lag time (λ) and (B) growth rate (µm) of both Fusarium spp. on NPDA. Data show the means of six replicates ± standard deviation. a, b: Significant differences between Fusarium spp. at each specific aw and temperature. Different capital letters: Significant differences between aw at each temperature for each species. α, β, Ɣ: Significant differences between temperatures for each specific Fusarium spp. and aw (t-test, p-value < 0.05).
Differences in the λ were observed between both Fusarium spp.; significant differences were detected at the highest aw (0.99) at 5 °C, and at both aw at 10 and 15 °C (p-values < 0.007). F. sambucinum presented generally a higher lag time than F. oxysporum.
The effect of temperature and aw on both Fusarium species’ growth rate (µm) is presented in Figure 1B. The higher growth rate was achieved by F. sambucinum at 15 °C and 0.99 aw, with 9 mm of diameter per day. Overall, there was an increase in growth rate with temperature and aw. Significant differences (all p-values < 0.005) were detected for each of the Fusarium spp. between aw (0.97 and 0.99) at the three different temperatures (5, 10, and 15 °C) with a higher growth rate at the highest aw (0.99). Significant differences (p-values < 0.0001) in the growth rate of both Fusarium spp. were detected between the three different temperatures, with a higher growth rate at the highest temperature (15 °C).
When growth rate was compared between Fusarium spp., significant differences (p-values < 0.003) were only detected at the highest aw (0.99) at 5 and 10 °C, while at 15 °C, significant differences were detected at both aw (0.97, 0.99). At 5 °C × 0.99 aw, F. oxysporum presented a higher growth rate when compared with F. sambucinum, while at 10 °C × 0.99 aw and 15 °C at both aw, F. sambucinum grew faster.
2.2. Effect of Cultivar and Stage of Storage of Potato Tubers on the External Lesion Caused by F. sambucinum
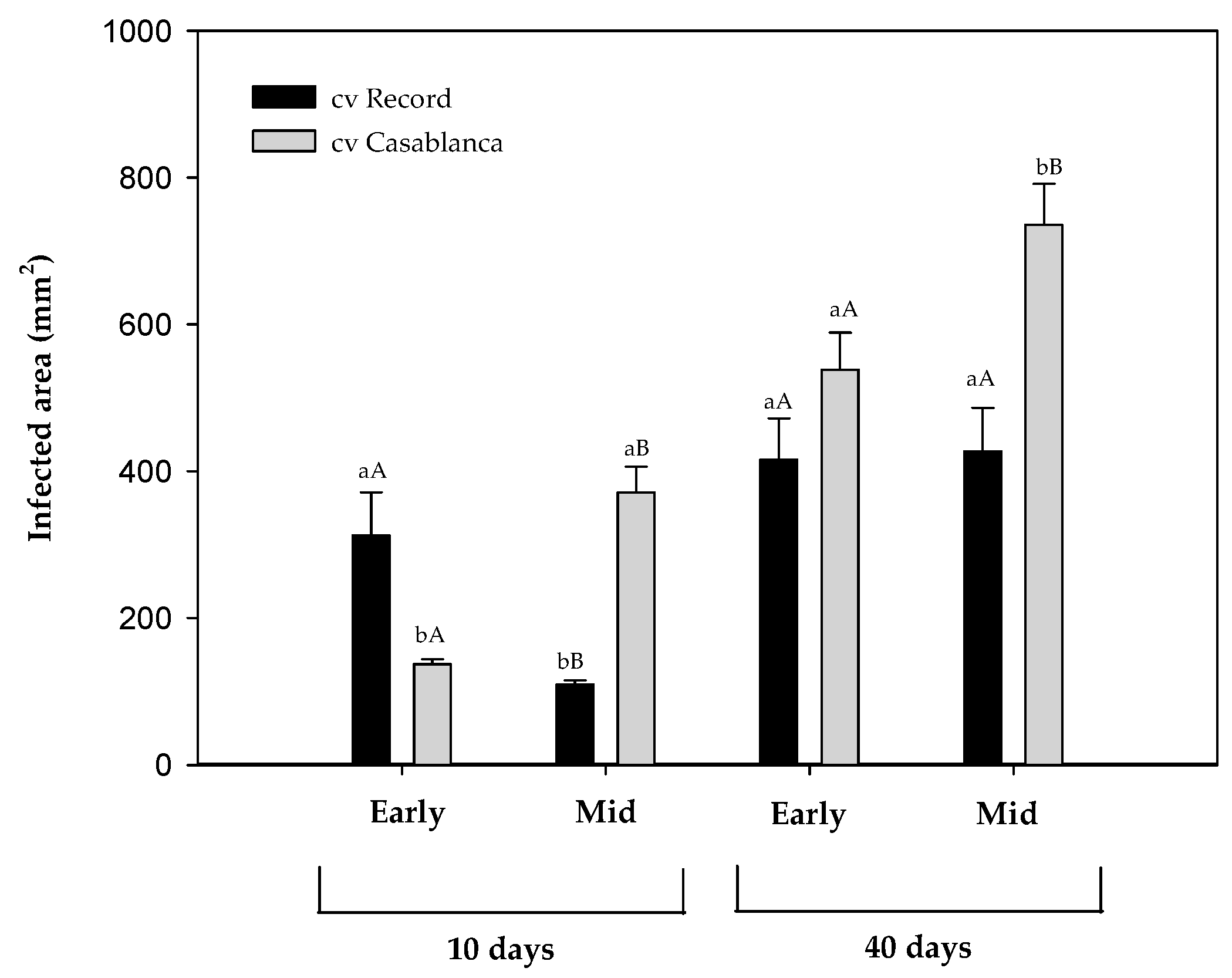
Two potato cultivars (cv. Record; cv. Casablanca), previously stored for 10 (early-stage) and 22 weeks (mid-stage) were inoculated with F. sambucinum and stored for 40 days at 8.5 °C. The external lesions were evaluated after 10 and 40 days of incubation at cold temperature and presented as an infected area (mm2) in Figure 2.
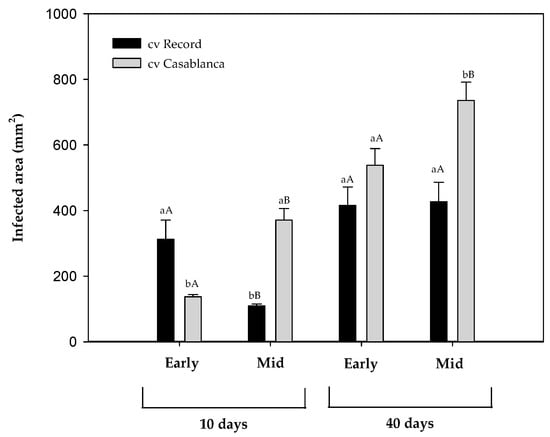
Figure 2.
Infected area (mm2) of Fusarium sambucinum on two different cultivars of potato tubers (cv. Record and cv. Casablanca) that were previously stored for 10 (Early) and 22 weeks (Mid) after 10 and 40 days at 8.5 °C. Data show the means of three replicates ± standard deviation. a, b: Significant differences between cultivars at each specific time and stage of storage. A, B: Significant differences between stages of the storage (early and mid) of potato tubers at each specific time and cultivar (Kruskal–Wallis, p-value < 0.05).
At the early storage stage, after 10 days of post-inoculation cold incubation, significant differences (p-value < 0.05) in the infected area were observed between cultivars, with cv. Record being more affected by F. sambucinum than cv. Casablanca. At the same stage, 40 days after inoculation, no significant differences were detected between cultivars.
At the mid-stage of storage, cv. Casablanca presented a significantly (p-value < 0.05) higher infected area than cv. Record at 10 and 40 days after inoculation. The stage of the storage of potato tubers reduced infection in cv. Record at an early the stage only but significantly increased the infection area of cv. Casablanca (p-value < 0.05) at both storage stages.
2.3. Effect of Temperature and aw on Mycotoxin Accumulation in vitro
2.3.1. Trichothecenes Accumulation
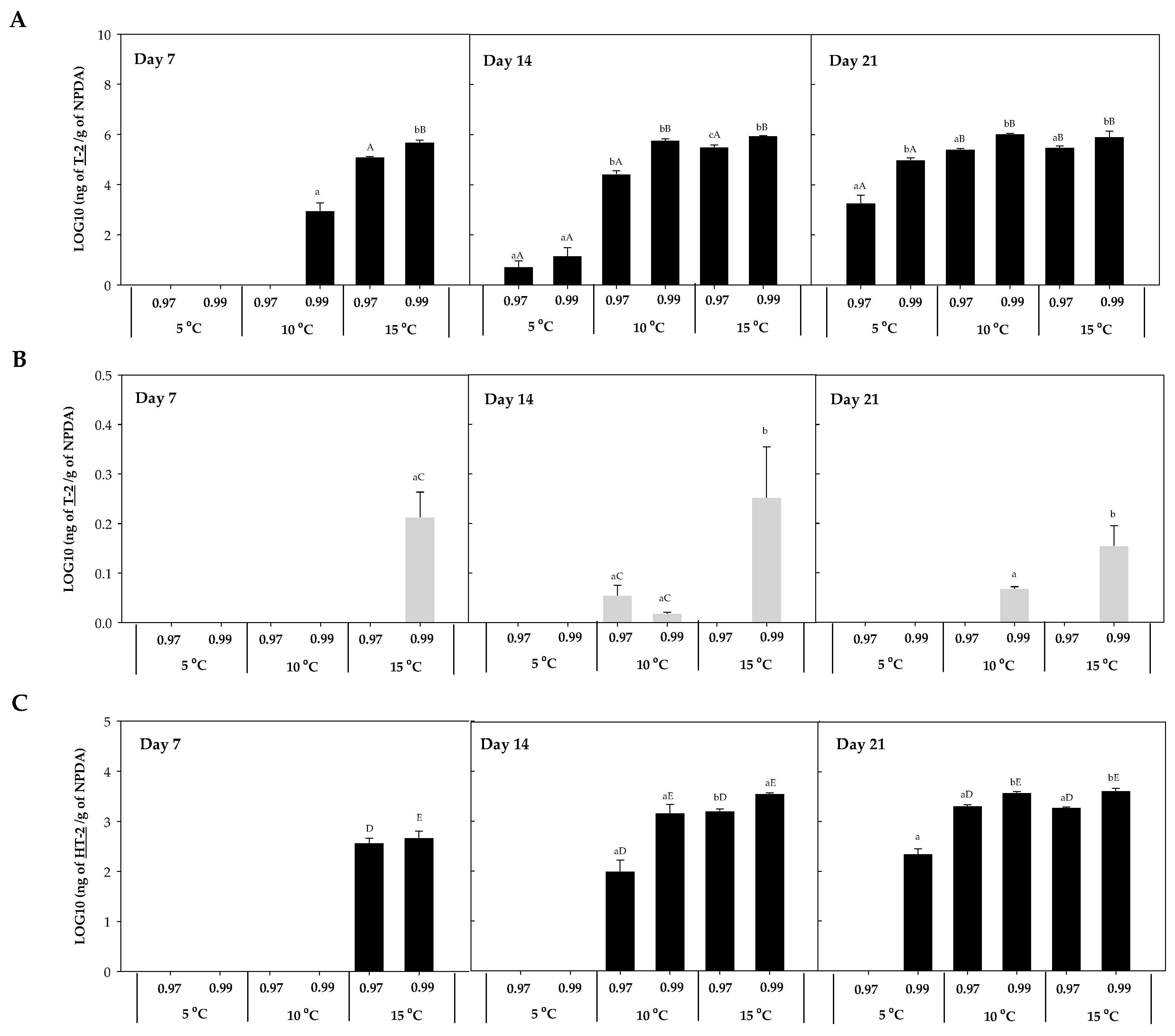
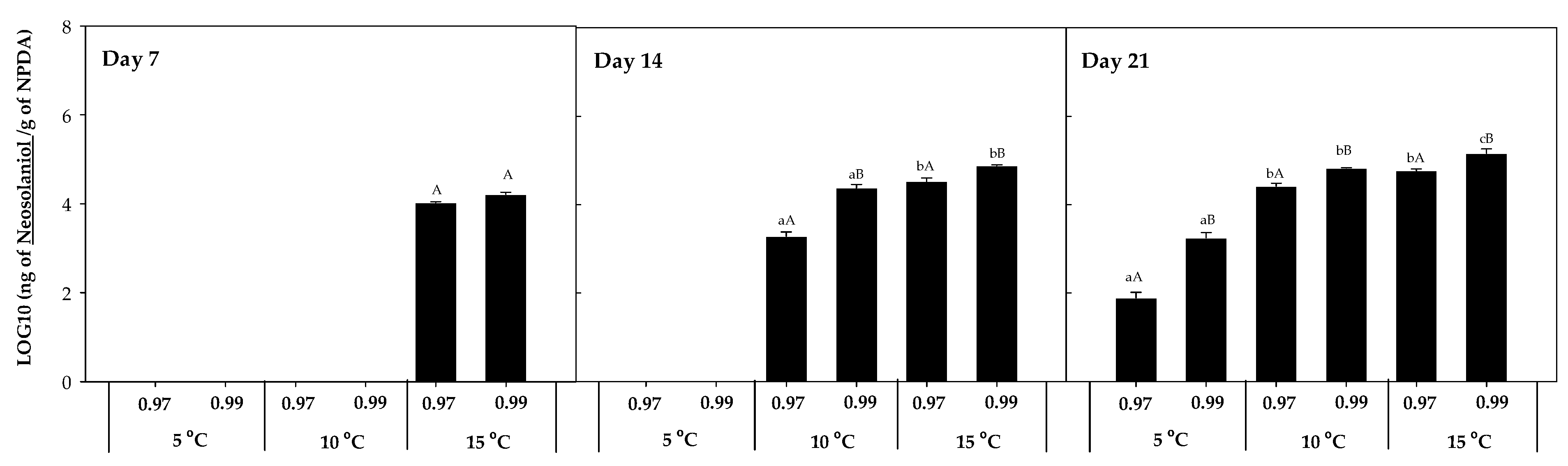
The mycotoxin accumulation on NPDA was studied after 7, 14, and 21 days. Five trichothecenes A, known to be produced by Fusarium spp., were detected in the present work (T-2, HT-2, DAS, 15-AS, and NEO). No trichothecenes B were detected in any of the samples analysed. T-2, HT-2, DAS, 15-AS, and NEO were detected in the presence of F. sambucinum, while in the presence of F. oxysporum only T-2 was detected. The accumulation of each of these five trichothecenes was studied at the three different temperatures (5, 10, 15 °C) and two different aw (0.97, 0.99). T-2 and HT-2 accumulation for F. sambucinum and T-2 accumulation for F. oxysporum are presented in Figure 3.
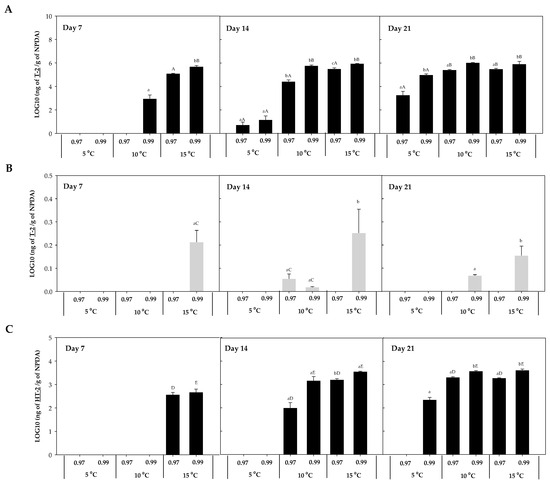
Figure 3.
T-2 accumulation in presence of F. sambucinum (A) and F. oxysporum (B) and HT-2 accumulation in presence of F. sambucinum (C) after 7, 14, and 21 days of incubation at different environmental conditions (temperature and water activity). Data show the means of six replicates ± standard deviation. a–c: Significant differences between temperatures at each specific aw. Different capital letters:: Significant differences between aw at each specific temperature (t-test, p-values < 0.05).
T-2 concentration in the presence of F. sambucinum ranged from not detected to 106 ng/g in NPDA (Figure 3A), with significantly higher (p-values < 0.05) concentrations at 0.99 aw compared to 0.97 aw. Temperature significantly (p-values < 0.05) affected the accumulation of T-2 in F. sambucinum. After 7 days of incubation, its presence was only observed at the highest temperature, while after 14 days it was also detected at 10 °C. Besides, at the end of the incubation period it was higher at 10 °C and detected at the lowest temperature (5 °C) as well.
The accumulation of T-2 in the presence of F. oxysporum (Figure 3B) followed a similar tendency, although the levels detected were 106 times lower than the ones found in F. sambucinum and some values were below the Limit of Detection (LOD). In presence of F. oxysporum, T-2 concentrations ranged from not detected to 60 ng/g in NPDA. T-2 was the only mycotoxin detected in presence of F. oxysporum over all the different combinations of temperature and aw tested.
The accumulation of HT-2 in F. sambucinum cultures (Figure 3C) followed a similar tendency to T-2, although its concentration was 103 time lower than T-2. After 7 days of incubation, 400.73 ± 30.42 and 547.87 ± 23.21 ng of HT-2/g NPDA were detected at 15 °C and 0.97 and 0.99 aw, respectively. While after 14 days of incubation, HT-2 detection was also observed at 10 °C, and was significantly higher (p-values < 0.05) at 15 °C (compared to 10 °C). At the end of the incubation period, HT-2 was also detected at 5 °C (225.25 ± 55.51 ng HT-2/g NPDA). Significant differences (p-values < 0.05) were only detected at 0.99 aw between 5 and 10 °C, while no differences were observed after 21 days of incubation between 10 and 15 °C. Overall, the aw of the media significantly affected the HT-2 accumulation; at the highest aw, the concentration was higher.
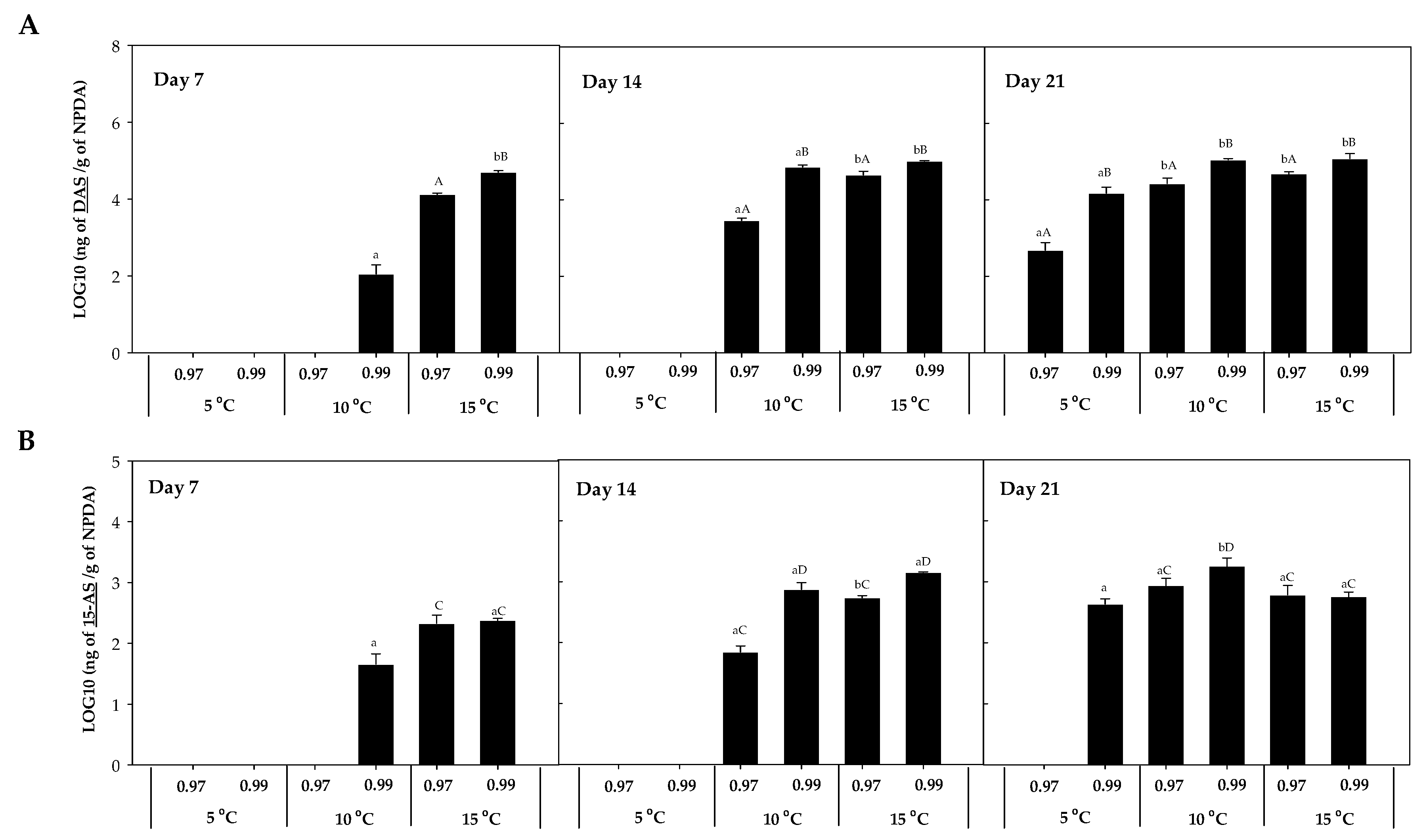
The accumulation of DAS was 103 times higher than 15-AS, observing the highest concentration at 10 °C × 0.99 aw (1807.2 ± 55.51 ng HT-2/g NPDA; Figure 4). After 7 days of incubation, their presence was detected at 15 °C, except for 15-AS which was also detected at 10 °C × 0.99 aw (Figure 4B). After 14 days, their detection was also predominant at 10 and 15 °C, and after 21 days, their presence was detected in almost all the conditions, with the lowest concentration detected at 5 °C × 0.97 aw. Significant differences (p-values < 0.05) were detected between temperatures (5, 10, and 15 °C) for these two mycotoxins (DAS and 15-AS). The concentration of DAS after 14 and 21 days of incubation was significantly higher at the highest water activity (0.99) compared to 0.97 aw (p-values < 0.05) (Figure 4A).
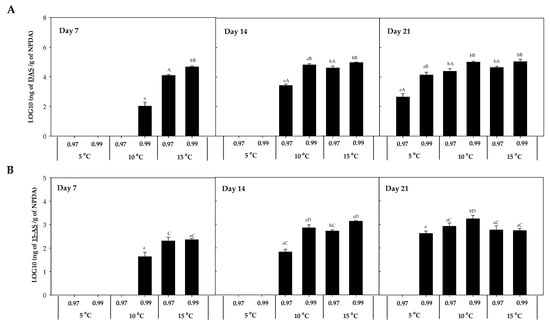
Figure 4.
Diacetoxyscirpenol (DAS) (A) and 15-acetoxyscirpenol (15-AS) (B) accumulation in the presence of F. sambucinum after 7, 14, and 21 days of incubation in different environmental conditions (temperature and water activities). Data show means of six replicates ± standard deviation. a, b: Significant differences between temperatures at each specific aw. Different capital letters:: Significant differences between aw at each specific temperature (t-test, p-values < 0.05).
NEO was also detected in presence of F. sambucinum (Figure 5), and its accumulation was mainly detected at 10 and 15 °C, independently of the time of incubation. Its presence at 5 °C was only detected at the highest aw after 21 days of incubation. The highest concentration of NEO was detected after 21 days of incubation at the highest temperature and 0.99 aw (1.45·105 ± 2.31·103 ng NEO/g NPDA). Significantly higher (p-values < 0.05) accumulation was detected with higher temperatures and water activities.
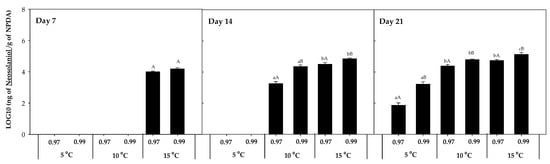
Figure 5.
Neosolaniol (NEO) accumulation in the presence of F. sambucinum after 7, 14, and 21 days of incubation in different environmental conditions (temperature and water activities). Data show means of six replicates ± standard deviation. a–c: Significant differences between temperatures at each specific aw. A, B: Significant differences between aw at each specific temperature (t-test, p-values < 0.05).
2.3.2. Other Mycotoxins Accumulation
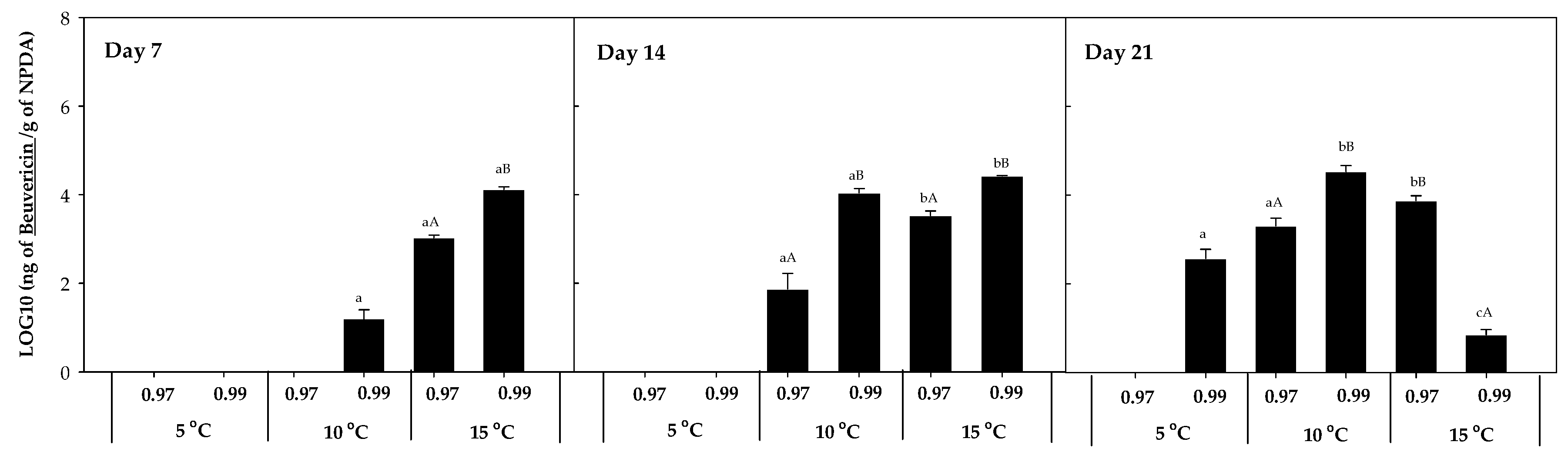
The accumulation of other mycotoxins, such as beauvericin, fumonisin B1 and B2, fusaric acid, and ZEN, was also evaluated. However, beauvericin was the only mycotoxin detected in the presence of F. sambucinum, with no additional mycotoxins detected in the presence of F. oxysporum. The accumulation of beauvericin (Figure 6) was significantly affected by the aw of the media. After 7 days of incubation, it was mainly detected at 15 °C × 0.99 aw (1.35 × 104 ± 2.00 × 102 ng beauvericin/g NPDA), while after 14 days of incubation it was detected at 10 and 15 °C at the highest aw (0.99). After 21 days, its detection was significantly higher (p-values < 0.04) at 10 °C × 0.99 aw and was detected at 5 °C but not at 15 °C × 0.99 aw.
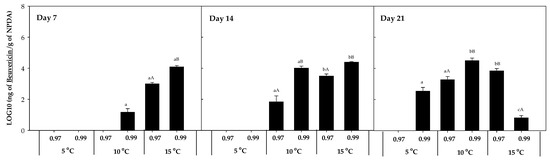
Figure 6.
Beauvericin accumulation in presence of F. sambucinum after 7, 14, and 21 days of incubation in different environmental conditions (temperature and water activities). Data show means of six replicates ± standard deviation. a–c: Significant differences between temperatures at each specific aw. A, B: Significant differences between aw at each specific temperature (t-test, p-values < 0.05).
2.4. Effect of Cultivar and Stage of the Storage on Mycotoxin Accumulation on Potato Tubers Inoculated with Fusarium sambucinum
The mycotoxin accumulation on potato tubers inoculated with F. sambucinum was studied on two different cultivars (cv. Record, cv. Casablanca) after 10 and 40 days of incubation at 8.5 °C in potato tubers that had been stored for 10 (early-stage) and 22 weeks (mid-stage) prior to inoculation. Six mycotoxins (T-2, HT-2, DAS, 15-AS, NEO, and beauvericin), known to be produced by Fusarium spp., were detected in both cultivars. As it can be observed in Table 1, the highest accumulation of mycotoxins was detected in potato tubers from a mid-stage. No significant differences (p-values < 0.05) were detected between cultivars after 10 days of incubation at the early stage of storage, except for NEO, where higher accumulation was observed in cv. Record. Although, in general a slightly higher accumulation of mycotoxins was observed in cv. Record compared to cv. Casablanca, the infected area produced by F. sambucinum after 10 days of incubation was higher in cv. Record than in Casablanca. The mycotoxin that was detected at the highest concentration was T-2, followed by HT-2, DAS, and 15-AS. Besides, when the effect of the stage of storage of the potato tubers was analysed based on the mycotoxin accumulation after 10 days of incubation, a significant increase in the mycotoxin content was observed for the cv. Casablanca compared to Record. Casablanca potato tubers that were stored for a longer period resulted in a significantly higher content of mycotoxins (p-values < 0.05), while this effect was not observed for cv. Record. The mycotoxin content of non-inoculated potato tubers (control) was also evaluated; as expected, no mycotoxins were detected and none of them presented any visible fungal development.

Table 1.
Mycotoxin accumulation (ng of toxin/g of potato tuber) in cv. Record and cv. Casablanca potato tubers stored for a short (early-stage) and a long period of time (mid-stage), under cold storage conditions (8.5 °C), 10 and 40 days after inoculation with F. sambucinum. Data show means of three replicates ± standard deviation. a, b: Significant differences between cultivars were detected A, B: Significant differences between storage stages (early and mid) were detected (t-test, p-value < 0.05).
3. Discussion
This study has compared the effect of two-way interacting environmental conditions (temperature, aw) on F. sambucinum and F. oxysporum growth and temporal mycotoxin accumulation in vitro. It has been previously observed that in commercial potato stores, spatial differences are often observed in temperature and relative humidity conditions [15,16]. Therefore, unveiling how the different environmental conditions can affect fungal development and mycotoxin accumulation of Fusarium spp. is essential for the potato industry. Providing farmers with good practices to avoid or reduce the development of dry rot is beneficial for storage management and can contribute to the reduction of potato losses, while maintaining high food safety standards.
In this study, both Fusarium spp. have shown a reduction in lag time and an increase in growth rate because of increased temperature and aw. An increase in temperature modified the overall lag time values from 13.1 to 3.6 days. The growth rate of F. sambucinum at 15 °C was 3.6 times higher than at 5 °C, and double that at 10 °C. F. sambucinum showed a lower lag time and higher growth rate compared to F. oxysporum under the same conditions. In vivo studies on the development of Fusarium spp., responsible for dry rot in potato tubers, suggested that changes in temperature from 5 °C to 20–25 °C were directly affecting the severity of damage in potato tubers infected with Fusarium spp. (F. coeruleum, F. sambucinum, F. avenaceum), responsible for dry rot [17,18,19,20,21,22]. An increase in the severity of dry rot, produced by F. sambucinum, has also been observed under high relative humidity conditions [19]. Although these results were observed in vivo, they were in accordance with our results. Therefore, an increase in the humidity conditions allowed a quicker development of Fusarium spp.
The effect of temperature on the growth of Fusarium spp. in vitro has been studied several times on different media, such as PDA. Daami-Remadi et al. (2006) studied the effect of temperature on both pathogens, different isolates, in vivo and in vitro. In their in vitro experiment, both Fusarium spp. were inoculated in non-modified PDA (0.995 aw), and the diameter of colonies was observed six days after incubation at the same temperatures as the present study (5, 10, 15 °C) [23]. The main differences between these results and ours were for F. oxysporum. They did not observe any growth at 5 °C and the colony diameter was lower at 10 and 15 °C, by 10 and 15 mm, respectively. Also, they observed larger colonies for F. sambucinum compared to F. oxysporum; the opposite of what was observed in the present study. Those differences might be due to the different formulation of the media. In our study, a 30%-containing mashed potato media to be more representative of in vivo potato tubers. Therefore, the findings achieved in this work suggest that higher temperatures in storage will directly increase the growth of F. sambucinum and F. oxysporum.
The effect of temperature and aw on F. sambucinum, isolated from rice, in rice extract agar (REA) was previously studied by Ferre et al. (2007). The growth rate of F. sambucinum at 15 °C at 0.95, 0.98, and 0.995 aw increased along with the aw tested [24]. These data were in accordance with the present study. They observed values between 1 and 3 mm/day at 0.95 and 0.995 aw, respectively; values that were almost 1/6 of our results, achieving a growth rate at 15 °C × 0.99 aw of 9 mm/day. This difference might be due to the media and the specific strain of F. sambucinum. These results are in accordance with the present findings regarding the effect of the relative humidity on the growth of F. sambucinum. The increase in the relative humidity of potato storage rooms will benefit the growth of F. sambucinum.
This is the first time where the effect of storage stage of potato tubers has been evaluated on F. sambucinum development in two different potato cultivars. A higher lesion severity was observed in cv. Casablanca compared to cv. Record. This severity difference could be because Record is a maincrop cultivar, while Casablanca is an earlier cultivar. This is supported by previous studies where a higher susceptibility to dry rot was observed in earlier cultivars compared to maincrop due to their physiological characteristics [17,21]. This might be caused by the effect of storage time on lesion severity of dry rot. Casablanca, an earlier cultivar, showed higher disease severity with the increase in storage time. This could be due to the physiological changes that the cultivar was experiencing during storage. Although the effect of long-term storage in potato tubers has been previously evaluated in soft rot, a bacterial potato disease [25], there are no studies focused on the effect of dry rot. More recently, the Agriculture and Horticulture Development Board has confirmed that Casablanca is susceptible to both F. coeruleum and F. sulphureum [26], and our results confirm a higher degree of severity of damage to F. sambucinum in cv. Casablanca compared to cv. Record. Our research is the first highlighting studying disease severity of F. sambucinum in cv. Record.
Different mycotoxins were detected in vitro in the presence of both Fusarium spp. The trichothecenes detected in this study were T-2, HT-2, DAS, NEO, and 15-AS, while the only non-trichothecene detected was beauvericin. Elucidating the accumulation of those mycotoxins by F. sambucinum and F. oxysporum, both responsible for dry rot in potato tubers, at the different environmental conditions studied, can provide interesting information regarding the food safety of potato tubers infected with dry rot. In the presence of F. sambucinum, a larger number of mycotoxins were detected: T-2, HT-2, DAS, NEO, 15-AS, and beauvericin. While in presence of F. oxysporum, only T-2 was detected. This is in accordance with what has already been reported regarding the mycotoxins produced by both Fusarium spp. In presence of F. oxysporum, T-2 and beauvericin were detected on different matrixes, while in presence of F. sambucinum, T-2, HT-2, NEO, DAS, 15-AS, and beauvericin were detected [27].
In this study, the accumulation of most mycotoxins was directly related to the growth of both Fusarium spp. Indeed, an increased growth of the fungi led to a higher accumulation of toxins. Therefore, their production was directly affected by the temperature and aw. The accumulation of mycotoxin was also evaluated in vivo. T-2, followed by HT-2, DAS, and 15-AS were detected at the highest concentration with a predominance for an increase in mycotoxins presence with time. The same effect as the one observed for the disease severity was observed in cv. Casablanca; those tubers that were stored for a longer time presented a higher accumulation of mycotoxins. Previous research has been carried out on the detection of mycotoxins in potato tubers [10,11,12,13,14,28]. The influence of cultivar and storage temperature on the accumulation of mycotoxins in potato tubers was studied on three Fusarium spp. (F. sambucinum, F. solani, F. sulphureum). They evaluated the accumulation of mycotoxins after 21 and 60 days when potato tubers were stored at 20 and 5 °C, respectively. Although their accumulation was higher at 20 °C, after 60 days of storage at 5 °C, Fusarenon X, 3-AcDON, DAS, and T-2 were detected at low concentrations [10]. Their results were in accordance with the results observed here, where a higher concentration of trichothecenes was detected at higher temperatures. However, our study is the first studying the effect of storage time of potato tubers on the mycotoxin accumulation [10,11,12,13,14,28].
The effect of temperature is notable as mycotoxins were already detected at low temperatures (5, 10 °C). This is the first study where mycotoxins, such as T-2, HT-2, DAS, 15-AS, and NEO have been detected in the presence of Fusarium spp. responsible for dry rot under cold temperature conditions (below 10 °C). Twenty-one days were needed for both Fusarium spp., mainly F. sambucinum, to start producing mycotoxins at the lowest temperature studied (5 °C). Therefore, considering that potato tubers can be stored for up to 10 months, if the environmental conditions are optimal for the development of Fusarium spp., the accumulation of mycotoxins can take place in the potato tuber. Previous studies have already detected the accumulation of beauvericin in the presence of F. sambucinum in MEA and potato tubers, when they were stored at 25 °C. However, DAS was only detected in vitro, while its detection was lower than the LOD in vivo [29]. This could be due to the differential severity that different potato cultivars present to pathogen infection. Since July 2024, there are EU maximum limits for the sum of T-2 and HT-2 in different commodities, such as cereals. As an example, in maize, the maximum level for direct human consumption is 50 µg/kg [30]. Although there is no current limit for potatoes, a concentration of 3 × 104 and 104 of HT-2 and T-2 ng per g of potato was observed in this study. Considering those values and comparing them with the EU recommendation in maize, they would be exceeding the limit if a regulation is to be imposed. Considering that potato is the fourth most consumed crop in the world, a high concentration of mycotoxins could be present once potato tubers are sold.
These findings were achieved under controlled laboratory conditions, which included surface-sterilisation and space between potato tubers. In a commercial potato storage facility, tubers are stored in bulk in 1-ton boxes, in close contact, where infection between tubers will spread quicker and remain not visible until a significant portion of the box is already infected. This suggests that, not only should there be a regular control of the storage facility, focusing on the sensible areas to changes in temperature, but also new storage systems that allow the early detection of rotten tubers.
Maintaining food safety is our first objective. Therefore, the fluctuation in temperature and relative humidity of potato storage facilities could result in an increase in dry rot in stores, higher severity of the damage appearing in fewer days, and higher accumulation of mycotoxins. Good practices in storage should be reviewed, such as including regular checks for the presence of fungal rots in those areas of the storage where the environmental conditions are more susceptible to change (lower and upper zones). Especially considering that some cultivars, in particular cv. Casablanca, could change their susceptibility to suffer severe damages by dry rot depending on the storage time of potatoes. Those potatoes stored for a longer time, prior to be sold, will present higher severity damages of dry rot as less time will be required for the fungi to infect and higher concentrations of mycotoxins. Furthermore, considering that once the potato tuber arrives to the consumer, the storage temperature will increase and so will the Fusarium spp. development and, consequently, the presence of mycotoxins. This will result in consumer exposure to mycotoxins and a large amount of food loss and waste in households, as well as a potential risk to the sustainability of the potato supply chain.
4. Conclusions
In this paper, we present evidence that supports the need for data-driven decisions to manage potato storage conditions. Indeed, any increases in temperature and relative humidity in potato storage facilities would result in a higher development of dry rot, and higher accumulation of mycotoxins (T-2, HT-2, DAS, 15-AS, NEO, and beauvericin). Moreover, this work combines in vitro and in vivo studies showcasing the potential of these pathogens not only to develop under different environmental conditions, but also their potential importance in terms of food safety due to their ability to produce mycotoxins, even at cold temperatures. This is the first study elucidating the accumulation of mycotoxins in the presence of Fusarium spp. responsible for dry rot under cold temperatures (5 and 10 °C). These results showed levels of trichothecenes, such as T-2, that are overpassing the new maximum limit in the European Union enforced since July 2024 [30]. Therefore, good practices for potato storage facilities would require an optimal and regular control of potato rots, focusing on storage settings where the environmental conditions are more susceptible to change, such as potato tubers from the lower layer of the bulk where water is accumulated, and higher RH conditions are faced.
Furthermore, it has been demonstrated that Casablanca potato tubers with a longer storage time present severe dry rot symptoms, as well as a higher accumulation of mycotoxins. The potato industry should consider the fact that potato tubers might change their disease severity once infected based on their storage time. Therefore, elucidating the time that each potato cultivar can be stored prior to changing their disease severity could help improve the final quality of potato tubers once they arrive to the consumer. It is especially important considering the long periods of storage and that once the potatoes are taken to retail and to households, where conditions of temperature and water availability are not controlled, these fungal species can develop, produce mycotoxins (e.g., T-2 and HT-2), and be exposed to the consumers. It is important to notice that these molecules can most likely diffuse within the potato tissue. Hence, to avoid any potential food safety problems, the recommendation until more research is developed should be to dispose of any contaminated tubers to avoid the risk of consuming Fusarium toxins.
5. Materials and Methods
5.1. Fungal Pathogens
Fusarium sambucinum, responsible for dry rot on potato tubers, was supplied from the Plant Breeding and Acclimatization Institute (IHAR, Błonie, Poland). While F. oxysporum was isolated in Bedfordshire, UK from rotten potato tubers cv. Markies in October 2018 and the identification was molecularly confirmed based on Gil-Serna et al. (2016) technique at Cranfield University (UK) [31]. Both fungal strains were subcultured in Potato Dextrose Agar (PDA, Sigma-Aldrich, Dorset, UK) for 7 days at 25 °C in the dark. A glycerol: water (70:30, v/v) stock solution of the previous culture was prepared separately and stored at -20 °C until its use.
5.2. Inoculation and Incubation of F. sambucinum and F. oxysporum In Vitro
Both Fusarium spp. were studied in vitro in a natural potato-based media at three different temperatures (5, 10, and 15 °C) and at two different water activities (0.97 and 0.99 aw). The two different water activities simulated high and low relative humidity conditions, 97% and 99%, respectively.
A semi-synthetic growth medium, Natural Potato Dextrose Agar (NPDA), was formulated to simulate the natural crop commodity. NPDA was prepared with 2% of glucose (Fisher Scientific, Waltham, MA, USA), 1.5% of agar (Sigma-Aldrich, Dorset, UK), and 30% of mashed potato in 1 L of potato infusion. Potato infusion was prepared with 200 g of peeled potatoes (cv. Maris Piper) boiled in 1 L of dH2O for 20 min and filtered through a single layer of cheesecloth (Fisher Scientific, USA). After filtration, the glucose and agar were added, as well as the mashed potato from the resulted boiled potatoes. The aw of the resulting solution was then modified with glycerol, based on a previously conducted aw regression line. The aw of the NPDA was measured with a AQUALAB 4TE aw meter (Decagon Instruments; Pullman, Washington, DC, USA). The NPDA media was dispensed in Petri dishes (Ø 9 cm) (ThermoFisher Scientific, Waltham, MA, USA).
An aliquot of the glycerol stock of each of the fungal strains was inoculated on PDA and incubated for 7 days at 25 °C, prior to the experiment. A spore suspension of 108 spores/mL was prepared using a Helber Haemocytometer and 1 µL was plated in the centre of the solid media. NPDA plates were incubated at three temperatures (5, 10, and 15 °C) and two different aw conditions (0.97, 0.99) for 30 days. To maintain the aw of the media, cultures from each pair of conditions (T° × aw) were stored inside 12 L airtight boxes containing a solution of glycerol: water adapted to the specific aw. Three replicates (12 L airtight boxes) were used per temperature and aw with NPDA non-inoculated plates and inoculated with F. sambucinum and F. oxysporum.
The mycotoxin content was studied in vitro at three different sampling points (7, 14, and 21 days). Three replicates were included and samples were kept at −20 °C until mycotoxin analysis was carried out.
5.3. Inoculation and Incubation of F. sambucinum on Potato Tubers
A parallel experiment was carried out in vivo, where F. sambucinum was inoculated in surface-sterilised potato tubers and incubated at 8.5 °C for 40 days. Two cultivars of potato tubers, cultivated under organic farming conditions, were selected based on their susceptibility to dry rot (cv. Casablanca, cv. Record). Both cultivars were stored for two different periods of time under cold conditions, prior to the experiment. Half were stored for 10 weeks (early stage of storage), and the other half were stored for 22 weeks (mid-stage of storage) at 4 °C.
Potato tubers were washed by soaking them in tap water for five minutes followed by ten minutes in distilled water, removing the excess of soil with a scrubber. Potato tubers were submerged for 15 min in 0.5% NaClO. Afterwards, tubers were washed twice with sterile distilled water and immersed in sterile distilled water for two to three hours before their inoculation. Tubers were wounded (5 mm in diameter) in the centre and inoculated with 200 µL of the spore suspension. Three replicates of twelve potato tubers were stored in 12 L airtight boxes and incubated at 8.5 °C under 98% of relative humidity. Non-inoculated potato tubers were also included as a control with the water/glycerol solution to maintain the relative humidity.
The mycotoxin content of potato tubers was analysed after 10 and 40 days of incubation at 8.5 °C. Three replicates were included per combination of conditions and samples were kept at −20 °C until the analysis was carried out.
5.4. Diametric Growth Rates of F. sambucinum and F. oxysporum on Potato-Based Media
Fungal growth was assessed daily after the inoculation from six different plates of each of the different conditions (temperature × aw). Colony diameters were measured in two perpendicular directions to each other. Two different growth parameters were calculated, growth rate (µm, mm of diameter per day) and lag time (λ, days), using Microsoft Excel (version 2203). The regression line slope was considered as the growth rate, while the lag time was estimated as the interception between the regression line and the x-axis [32].
5.5. External Lesion Assessment of Potato Tubers Infected with F. sambucinum
The external lesion was assessed after 10 and 40 days of storage at 8.5 °C. Five potato tubers from each treatment were considered. Assuming the potato lesion to be round, the area of the rots (A) was calculated based on the area of a circle, where r was half of the average of the measured diameters of the rot. The area of non-inoculated tubers (control) was subtracted from the area of each rot on each specific day measured.
5.6. Mycotoxin Analysis
The mycotoxin content was studied in vitro and in vivo. The mycotoxin extraction from the semi-synthetic media consisted of three plugs of NPDA of 5 mm in diameter, submerged in Liquid Nitrogen (N2) and homogenised with 450–600 µm of glass beads using the Precellys 24 Tissue homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France). For the potato samples, 100 mg of rotten tissue were agitated in a Tissue Homogenizer at 5500 rpm for 20 s followed by a 5 s interval and another 20 s of agitation. Then, a volume of extraction buffer (acetonitrile: water: formic acid, 79:20.9:0.1, v/v/v) was added and they were again homogenised. The volume of the extraction buffer was 4 times the weight of each of the samples. Samples were then left for incubation for 90 min at 25 °C at 300 rpm on a rotary shaker in the dark. Afterwards, the extracts were centrifuged for 10 min at 22,600× g, the supernatant (200 µL), transferred to HPLC vials containing 250 µL microinserts (Fisher Scientific, USA), and kept at −20 °C until their analysis.
Ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) (Sciex Technologies, Warrington, UK) was used for the mycotoxin detection. Chromatographic separation was achieved on a reverse-phase ACE 3-C18 column (2.1 × 100 mm, 3 µm particle size; Hichrom, Reading, UK) equipped with a C18 security guard cartridge (4 × 3 mm, Gemini, Agilent Technologies, Santa Clara, CA, USA) kept at 40 °C, as described in Gil-Serna et al. (2022) [33]. The method recovery was validated in potato tissue and NPDA media including the targeted mycotoxins (Supplementary Material). Fifteen mycotoxins were included with standards for the sample analysis. Five trichothecenes A were included: T-2, HT-2, DAS, and 15-AS; five trichothecenes B: DON, 3-ADON, 15-ADON, nivalenol, and fusarenon X; and, five non-trichothecenes mycotoxins: beauvericin, fumonisin B1 and B2, fusaric acid, and ZEN. Multiquant 3.03 software (AB Sciex, Foster City, CA, USA) was used for the analysis of the results.
5.7. Statistical Analysis
Statistical analysis was performed using JMP Pro 14 (SAS Institute, INC., Cary, NC, USA). Data sets were tested for normality and homoscedasticity using the Shapiro–Wilk and Levene’s tests, respectively. Those datasets that succeeded in the previous test were analysed using parametric test, ANOVA. A post hoc analysis (Tukey–Kramer HSD method) was carried out when significant differences were detected (p-value < 0.05). For those datasets that the normality and homoscedasticity test failed, a non-parametric test (Kruskal–Wallis) was performed with a post hoc analysis (Wilcoxon test) when significant differences were detected (p-value < 0.05).
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins16100414/s1, Figure S1: Effect of temperature (5, 10, 15°C) and aw (0.97, 0.99) on F. sambucinum and F. oxysporum growth on colony morphology of F. sambucinum and F. oxysporum after 10 days of storage on potato-based media (NPDA). Table S1: Mycotoxin, retention time, qualifier (Q1) and quantifier (Q3) and recovery information about the matrix effect (LOD and LOQ).
Author Contributions
A.M., S.K. and L.A.T. were responsible for the project resources. A.M., S.K., C.V.-V. and M.G.-P. conceived and designed the experiments. M.G.-P. and C.V.-V. carried out the experiments and performed data collection and analyses. The original draft was written by M.G.-P., which was then edited and reviewed by all authors. Project administration was conducted by A.M., and project supervision was carried out by A.M., S.K. and L.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Interreg North-West Europe program (project number: NWE 363) and BSRC research grant Oats for the future between the Applied Mycology Group at Cranfield University, UK (BB/P001432/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting this study are openly available from CORD, at this link: https://doi.org/10.57996/cran.ceres-2594.
Acknowledgments
Jadwiga Sliwka from the Plant Breeding and Acclimatization Institute (IHAR, Poland) for suppling the F. sambucinum strain.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 September 2023).
- Lulai, E.C. Tuber periderm and disease resistance. In Compedium of Potato Diseases, 2nd ed.; Stevensor, W.R., Loria, R., Franc, G.D., Weigartner, D.P., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2001; pp. 3–6. [Google Scholar]
- Xue, H.; Liu, Q.; Yang, Z. Pathogenicity, mycotoxin production, and control of potato dry rot caused by Fusarium Spp.: A review. J. Fungi 2023, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Estrada, R.; Gudmestad, N.C.; Rivera, V.V.; Secor, G.A. Fusarium graminearum as a dry rot pathogen of potato in the USA: Prevalence, comparison of host isolate aggressiveness and factors affecting aetiology. Plant Pathol. 2010, 59, 1114–1120. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Dagnas, S.P.; Membré, J.M. Predicting and preventing mold spoilage of food products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef]
- Medina, A.; Rodríguez, A.; Sultan, Y.; Magan, N. Climate change factors and Aspergillus flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015, 8, 171–179. [Google Scholar] [CrossRef]
- Medina, A.; Gilbert, M.K.; Mack, B.M.; OBrian, G.R.; Rodríguez, A.; Bhatnagar, D.; Payne, G.; Magan, N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int. J. Food Microbiol. 2017, 256, 36–44. [Google Scholar] [CrossRef]
- Xue, H.L.; Bi, Y.; Tang, Y.M.; Zhao, Y.; Wang, Y. Effect of cultivars, Fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food. Chem. 2014, 151, 236–242. [Google Scholar] [CrossRef]
- Xue, H.; Bi, Y.; Wei, J.; Tang, Y.; Zhao, Y.; Wang, Y. New method for the simultaneous analysis of types A and B trichothecenes by Ultrahigh-Performance Liquid Chromatography coupled with tandem mass spectrometry in potato tubers inoculated with Fusarium sulphureum. J. Agric. Food Chem. 2013, 61, 9333–9338. [Google Scholar] [CrossRef]
- Ellner, F.M. Mycotoxins in potato tubers infected by Fusarium sambucinum. Mycotoxin Res. 2002, 18, 57–61. [Google Scholar] [CrossRef]
- Delgado, J.A.; Schwarz, P.B.; Gillespie, J.; Rivera-Varas, V.V.; Secor, G.A. Trichothecene mycotoxins associated with potato dry rot caused by Fusarium graminearum. Phytopathology 2010, 100, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Song, H.H.; Lee, H.S.; Jeong, J.H.; Park, H.S.; Lee, C. Diversity in beauvericin and enniatins H, I, and MK1688 by Fusarium oxysporum isolated from potato. Int. J. Food Microbiol. 2008, 122, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Cunnington, A.; Pringle, R. Store Managers Guide 2012; Potato Council & Agriculture and Horticulture Development Board: Coventry, UK, 2012; Available online: https://media.ahdb.org.uk/media/Default/Imported%20Publication%20Docs/Potato%20store%20managers%20guide.pdf (accessed on 5 December 2022).
- Cargill, B.F. The Potato Storage: Design, Construction, Handling and Environmental Control, 1st ed.; Michigan State University: East Lansing, MI, USA, 1976; pp. 259–279. [Google Scholar]
- Ayers, G.W.; Robinson, D.B. An inoculation technique for the study of dry rot of potatoes. Am. Potato J. 1954, 31, 278–281. [Google Scholar] [CrossRef]
- McKee, R.K.; Boyd, A.E.W. Dry-rot disease of the potato: IX. The effect of diphenyl vapour on dry-rot infection of potato tubers. Ann. Appl. Biol. 1962, 50, 351–357. [Google Scholar] [CrossRef]
- Gachango, E.; Hanson, L.E.; Rojas, A.; Hao, J.J.; Kirk, W.W. Fusarium spp. causing dry rot of seed potato tubers in Michigan and their sensitivity to fungicides. Plant Dis. 2012, 96, 1767–1774. [Google Scholar] [CrossRef]
- Lui, L.H.; Kushalappa, A.C. Response surface models to predict potato tuber infection by Fusarium sambucinum from duration of wetness and temperature, and dry rot lesion expansion from storage time and temperature. Int. J. Food Microbiol. 2002, 76, 19–25. [Google Scholar] [CrossRef]
- Sangalang, A.E.; Backhouse, D.; Burgess, L.W. Survival and growth in culture of four Fusarium species in relation to occurrence in soils from hot climatic regions. Mycol. Res. 1995, 99, 529–533. [Google Scholar] [CrossRef]
- Mckee, R.K. Dry-rot disease of the potato. VIII. A study of the pathogenicity of Fusarium caeruleum (Lib.) Sacc. and Fusarium avenaceum (Fr.) Sacc. Ann. Appl. Biol. 1954, 41, 417–434. [Google Scholar] [CrossRef]
- Daami-Remadi, M.; Jabnoun-Khiareddine, H.; Ayed, F.; El Mahjoub, M. Effect of temperature on aggressivity of Tunisian Fusarium species causing potato (Solanum tuberosum L.) tuber dry rot. J. Agron. 2006, 5, 350–355. [Google Scholar] [CrossRef][Green Version]
- Ferre, F.S.; Caselles, J.R.; Siurana, M.P.S. Interacciones competitivas entre Fusarium sambucinum fuckel y Phoma glomerata (corda) Wollenweber & Hochapfel en condiciones in vitro. Rev. Iberoam. Micol. 2007, 24, 29–33. [Google Scholar] [CrossRef]
- Potato Variety Database. Available online: https://potatoes.agricrops.org/varieties/view/Casablanca (accessed on 25 July 2024).
- Chung, Y.S.; Goeser, N.J.; Cai, X.; Jansky, S. The effect of long term storage on bacterial soft rot resistance in potato. Am. J. Pot. Res. 2013, 90, 351–356. [Google Scholar] [CrossRef]
- Bojanowski, A.; Avis, T.J.; Pelletier, S.; Tweddell, R.J. Management of potato dry rot. Postharvest Biol. Technol. 2013, 84, 99–109. [Google Scholar] [CrossRef]
- El-Banna, A.A.; Scott, P.M.; Lau, P.-Y.; Sakuma, T.; Platt, H.W.; Campbell, V. Formation of trichothecenes by Fusarium solani var. coeruleum and Fusarium sambucinum in potatoes. Appl. Environ. Microbiol. 1984, 47, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Steglińska, A.; Sulyok, M.; Janas, R.; Grzesik, M.; Liszkowska, W.; Kręgiel, D.; Gutarowska, B. Metabolite formation by fungal pathogens of potatoes (Solanum tuberosum) in the presence of bioprotective agents. Int. J. Environ. Res. Public Health 2023, 20, 5221. [Google Scholar] [CrossRef] [PubMed]
- 2013/165/EU: Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products Text with EEA Relevance. 2013, pp. 12–15. Available online: https://eur-lex.europa.eu/eli/reco/2013/165/oj (accessed on 25 July 2024).
- Gil-Serna, J.; Gálvez, L.; París, M.; Palmero, D. Fusarium proliferatum from rainwater and rooted garlic show genetic and pathogenicity differences. Eur. J. Plant Pathol. 2016, 146, 199–206. [Google Scholar] [CrossRef]
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marín, S. Predicting mycotoxins in foods: A review. Food Microbiol. 2009, 26, 757–769. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Patiño, B.; Verheecke-Vaessen, C.; Vázquez, C.; Medina, Á. Searching for the Fusarium spp. which are responsible for trichothecene contamination in oats using metataxonomy to compare the distribution of toxigenic species in fields from Spain and the UK. Toxins 2022, 14, 592. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).