Abstract
Aflatoxin constitutes a significant concern for food and feed safety, posing detrimental health risks to both animals and humans. This study aimed to examine the prevalence and concentration of aflatoxins in maize feed, total mixed ration, and wheat bran collected from specialized dairy farms and local markets in three major urban centers in eastern Ethiopia. A total of 180 feed samples were collected from September 2021 to January 2022 in Chiro town, Dire Dawa city, and Harar city. These samples underwent thorough extraction and immunoaffinity clean-up before aflatoxin analysis using HPLC/FLD. The results revealed that AFB1, AFB2, AFG1, AFG2, and TAF contamination was detected in 72.2%, 66.1%, 71.1%, 68.7%, and 82.8% of the feed samples, respectively. The corresponding mean levels of each aflatoxin were 28.15 ± 3.50, 3.3 ± 0.40, 19.87 ± 1.87, 2.7 ± 0.32, and 54.01 ± 4.72 µg/kg, respectively. The occurrence and levels of aflatoxin varied across different study sites and feed types. Notably, feeds from Dire Dawa city exhibited significantly higher mean levels of AFB1 (43.98 ± 5.3 µg/kg), AFB2 (5.69 ± 0.6 µg/kg), AFG1 (32.25 ± 2.7 µg/kg), and AFG2 (5.01 ± 0.5 µg/kg) than feeds from other urban centers did. Additionally, a significantly higher occurrence of AFB1 (29.4%) and AFG1 (28.3%) was detected in feed from Dire Dawa city. Similarly, the total mixed ration (TMR) displayed significantly higher levels of AFB1 (50.67 ± 5.2 µg/kg), AFB2 (4.74 ± 0.6 µg/kg), AFG1 (32.87 ± 2.6 µg/kg), and AFG2 (3.86 ± 0.5 µg/kg) compared to the other feed types. Moreover, a significantly higher occurrence of AFB1 (30.7%) and AFG1 (28.7%) was detected in the TMR. Furthermore, a moderate correlation was observed between the count of aflatoxigenic Aspergillus species and the levels of TAF in the feed samples. Overall, this study underscores the widespread presence of aflatoxin contamination in dairy feeds in eastern Ethiopia, highlighting the urgent need for stringent monitoring and mitigation measures to ensure food and feed safety, as well as public health.
Keywords:
aflatoxins; feed and food safety; dairy farmers; feed retailers; maize feed; total mixed ration; wheat bran; HPLC/FLD; eastern Ethiopia Key Contribution:
Investigated the overall prevalence (82.8%) and mean level (54.01 ± 4.72 µg/kg) of aflatoxins in different feeds of dairy cows from specialized dairy farms and local markets in eastern Ethiopia and associated food safety and public health concerns.
1. Introduction
Aflatoxins are naturally occurring secondary metabolites, produced by Aspergillus species, primarily A. flavus and A. parasiticus [1]. Thus, A. flavus produces aflatoxin B1 (AFB1) and B2 (AFB2), while A. parasiticus secrets AFB1, AFB2, AFG1, and AFG2 [2,3]. Aspergillus fungi and subsequent aflatoxins are frequent contaminants of a wide variety of agricultural products, from the field to consumers’ plates [4]. Food and feed crops such as cereal grains, nuts, vegetables, fruits, and spices are frequently targeted by aflatoxins [5]. Thus, the structurally analogous aflatoxins, such as B1 (AFB1), B2 (AFB2), G1 (AFG1), and G2 (AFG2), are the major contaminants of animal feed and feed ingredients [2,3].
Exposure to aflatoxins poses a significant health risk to both humans and animals [6]. The International Agency for Research on Cancer (IARC) has classified aflatoxins as group 1 carcinogens [7,8]. The liver is the target organ, where chronic exposure of aflatoxin is associated with hepatocellular carcinoma (HCC) and other health complications [3,4]. On the other hand, acute aflatoxicosis can lead to liver disease and even death [4,9,10]. Consequently, investigations have demonstrated that thousands of human lives have been lost in numerous nations across the globe. Moreover, many millions of people in various countries are exposed to chronic aflatoxicosis, highlighting greater risks to feed and food safety and public health from aflatoxin [11,12].
Many countries have implemented stringent legal limits for aflatoxin levels in animal feeds and food products to minimize the risk of aflatoxins entering food chains. For instance, the European Commission has established a maximum tolerable limit of 20 µg/kg AFB1 in the feed for all animals and 5 µg/kg AFB1 in the feed for dairy cows [13]. The Ethiopian Standard Authority (ESA) set a maximum limit of 20 µg/kg for AFB1 and 40 µg/kg for total aflatoxin (TAF), whereas the Food and Drug Administration (FDA) set 20 µg/kg limit of TAF in dairy feed [14]. The implementation of strict regulations on aflatoxin levels in food and feed in developed countries has effectively reduced the risk of aflatoxin exposure [4]. However, in developing nations, the risk of aflatoxin exposure remains high due to insufficient regulations and implementation capacity.
Aflatoxin contamination in food and animal feeds is of particular concern in tropical and subtropical regions, where favorable climatic conditions promote the growth of aflatoxigenic Aspergillus fungi [15]. In addition, poor agricultural practices and crop damage prior to harvest significantly contribute to the increased risk of aflatoxin contamination in feed and feed ingredients [16]. Inadequate storage conditions such as air moisture, leakage of the floors/walls/roofs, and poor ventilation at dairy cattle farms and feed retailers also contribute to Aspergillus fungi and subsequent aflatoxin contamination in animal feed [17,18].
Contaminated feeds and feed ingredients are the main sources of aflatoxin exposure for animals. These contaminants then find their way into the food chain, primarily through milk and milk products, resulting in human contamination [19]. Aflatoxins frequently contaminate major crops such as maize, wheat, oilseeds, and others, which are the integral components of animal feeds [15]. Consequently, concentrate feeds such as wheat bran, oilseed cakes, maize-grain-based feed, and total mixed rations are the primary sources of aflatoxin contamination in animal and animal products [18,20,21].
Numerous investigations have demonstrated the prevalence of aflatoxin in dairy feeds. For instance, Nkosi et al. [22] reported that different dairy cattle feeds in Malawi had an average AFB1 concentration of 29.68 µg/kg and a contamination rate of 88.2% (n = 51), with 17.6% exceeding the 5 µg/kg standard set out by the EU. Similarly, AFB1 contamination was detected in 57% (n = 74) of concentrate feed samples in Kenya, with a mean level of 28.3 µg/kg, with 56% of the samples exceeding the standards set out by EU regulation [23]. In another study, a 48% contamination frequency and a mean level of 0.7 µg/kg AFB1, a 93% frequency and a mean level of 3.1 µg/kg AFB2, a 55% frequency and a mean level of 2.5 µg/kg AFG1, and a 100% frequency and a mean level of 41.3 µg/kg AFG2 were found in dairy feed from the Gauteng province of South Africa [24]. Additionally, Njobeh et al. [25] revealed that in South Africa, 14.7 µg/kg TAF and 52% (n = 25) prevalence were found in compound feed used for dairy cows, with 16% of samples exceeding the standards set out by EU regulation. Meanwhile, in Tanzania, a sunflower-based dairy feed had a prevalence of 72% (n = 18), with a higher mean level of 26 µg/kg for TAF [26].
In Ethiopia, like in most countries with tropical and subtropical climates, the presence of aflatoxin in dairy cattle feed poses significant concerns for food safety and public health [27,28]. Accordingly, numerous investigations have revealed substantial levels of aflatoxin contamination in dairy feed samples collected from various locations in Ethiopia. For instance, Gizachew et al. [29] reported a 100% contamination rate with a mean concentration of 362 ± 38 µg/kg in Noug cake, a 73% contamination rate with a mean concentration of 15 ± 6 µg/kg in wheat bran, and a 37% contamination rate with a mean concentration of 18 ± 11 µg/kg in maize grains for AFB1 in dairy feed collected from Addis Ababa, Ethiopia. Additionally, Rehrahie et al. [30] reported that 49.4% (n = 160) of dairy feed samples collected from various towns in central Ethiopia were contaminated with AFB1, with mean concentrations of 5.63 µg/kg, and 81.9% of the samples exceeded the FDA regulation limit of 20 µg/kg. Another study conducted by Yoseph et al. [31] reported a contamination rate of 31% (n = 33) with a mean concentration of 195.88 µg/kg, and 94% of the samples exceeded the FDA regulation limit for TAF in feed from Bishoftu town. The same authors reported mean concentrations of 17.93 µg/kg for AFB1, 91.37 µg/kg for AFB2, 13.79 µg/kg for AFG1, and 72.77 µg/kg for AFG2.
Furthermore, considering the rapid urbanization rate in Ethiopia and the significant role of specialized dairy farming in meeting milk demand in major urban centers [32,33], investigating the prevalence of aflatoxin in feed and milk is crucial. Therefore, this study focuses on concentrate feeds commonly used in specialized dairy farms and sold in local markets, such as wheat bran, maize feeds, and total mixed rations [34,35,36]. This study was conducted in three densely populated urban centers in eastern Ethiopia, which have many indoor dairy farms and feed retailers [37,38,39].
The objective of this study was to investigate the prevalence and concentration levels of aflatoxins in three types of feed collected from specialized dairy farms and local markets in Chiro town, Dire Dawa city, and Harar city in eastern Ethiopia. Additionally, this study aimed to evaluate the correlation between the counts of aflatoxigenic Aspergillus species and the levels of aflatoxins detected in the feed.
2. Results
2.1. Frequency of Occurrence of Aflatoxins in Dairy Feed
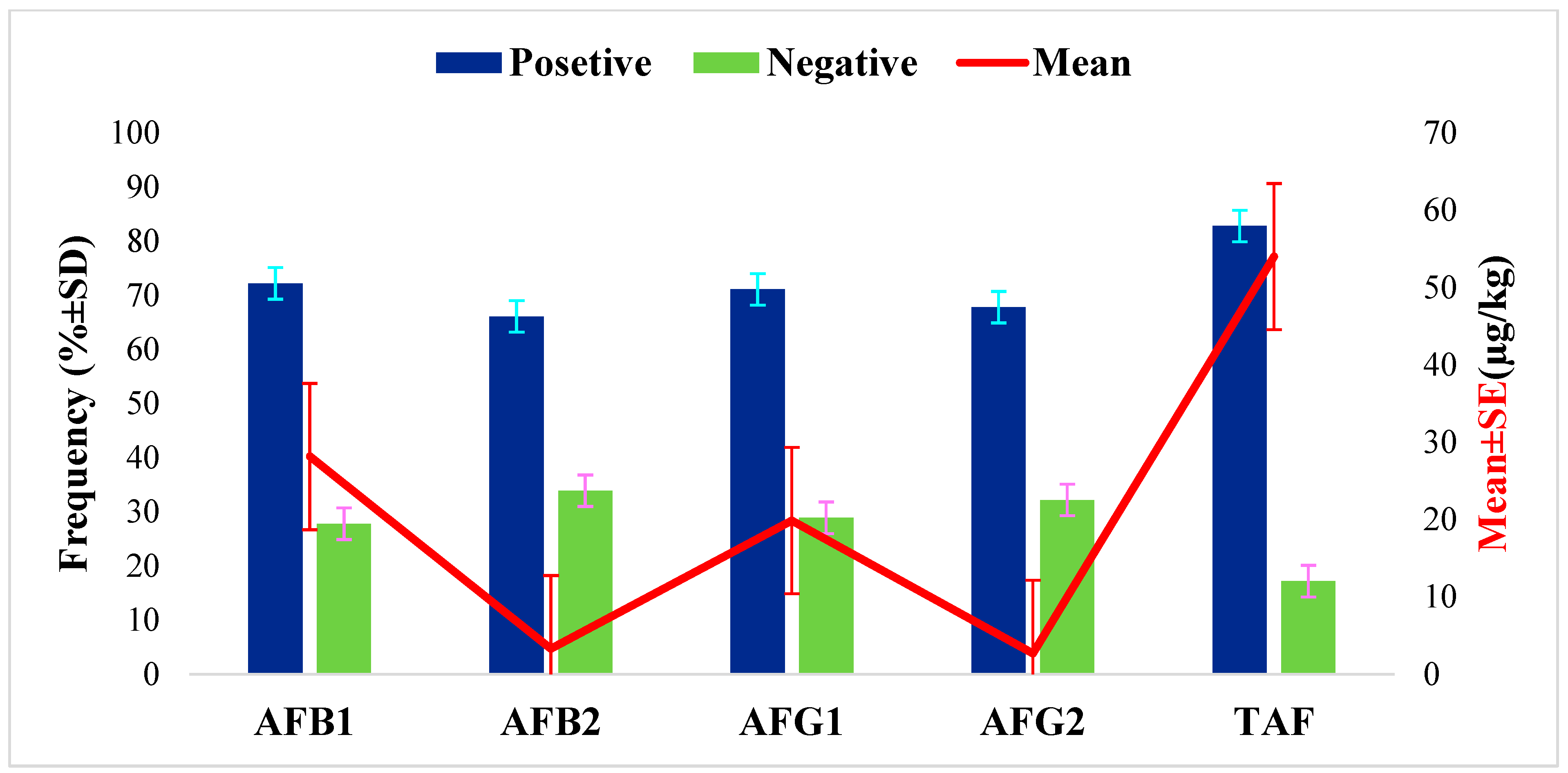
A total of 180 dairy cow feed samples were analyzed to quantify AFs using HPLC following validation of the measurement procedures as described in the methodology. The results revealed that 82.80% of samples (149/180) were contaminated with at least one AF, with an average concentration of 54.01 ± 4.72 µg/kg (Figure 1). The results also showed that all types of aflatoxin were highly prevalent, with 72.2%, 71.1%, 67.8% and 66.1% of feed samples being contaminated with AFB1, AFG1, AFB2, and AFG2, respectively, with mean concentrations of 28.15 ± 3.50, 19.87 ± 1.87, 3.30 ± 0.40, and 2.70 ± 0.32 µg/kg, respectively (Figure 1). However, in 17.2%, 27.8%, 33.9%, 28.9%, and 32.2% of the feed samples, TAF, AFB1, AFB2, AFG1, and AFG2, respectively, were not detectable.
Figure 1.
Overall frequency of occurrence and level of total aflatoxins in dairy feed (TAF = AFB1 + AFB2 + AFG1 + AFG2).
The analysis of variance showed that there was significant variation in the mean concentration of TAF among study sites (p < 0.01). Thus, a significantly higher (p < 0.05) mean concentration of TAF was detected in feed samples collected from Dire Dawa city (mean = 86.93 ± 8.2 µg/kg; range = ND–455.8 µg/kg) than in those from Harar city (mean = 41.50 ± 8.2 µg/kg; range = ND–229.2 µg/kg) and Chiro town (mean = 33.60 ± 8.2 µg/kg; range = 150.6 µg/kg). Similarly, the ANOVA result showed a highly significant variation in the mean concentration of TAF among the examined feed types, with TMR having a significantly (p < 0.01) higher mean level of TAF (mean = 92.20 ± 8.2 µg/kg; range = ND) compared to MF (mean = 43.80 ± 8.2 µg/kg; range = ND–175.9 µg/kg) and WB (mean = 26.0 ± 8.2 µg/kg; range = ND–245.5 µg/kg). However, no variation was observed among the sources of feed samples in the mean concentration of TAF (Table 1).

Table 1.
Frequency of occurrence and level of TAF in feed across study sites, feed sources, and feed types.
The results also revealed that the prevalence of feed samples contaminated with total aflatoxin did not vary (p > 0.05) across study sites, feed sources, and feed types (Table 1). However, a proportionally higher frequency of occurrence of TAF was found in the feed samples collected from Dire Dawa city (29.4%, n = 53) compared to the feed collected from Harar city (27.2% n = 49) and Chiro town (26.1%, n = 47). Likewise, a numerically higher frequency of occurrence of TAF was observed in TMR (30.0%, n = 54) than in MF (27.8%, n = 27.8) and WB (25.0%, n = 45). Moreover, a relatively similar frequency of occurrence of TAF was observed in samples from dairy farms (41.7%, n = 75) and local markets (41.1%, n = 74) (Table 1).
2.2. Frequency of Occurrence and Level of Aflatoxins in Feed across Study Sites, Feed Sources, and Feed Types
The analysis of variance showed that the mean concentration of AFB1 recovered from the dairy feeds using HPLC/FLD significantly varied among study sites and feed types (p < 0.001) (Table 2), as well as revealing an interaction between feed types and study sites (p < 0.05) (Table 2). However, variation in mean concentrations of AFB1 was not observed within feed sources across all study sites. Among the study sites, the highest mean concentration of AFB1 was recovered in the samples collected from Dire Dawa city (mean = 43.98 ± 5.3 µg/kg; range = LOD−303.5 µg/kg) compared to the other urban centers. On the other hand, among feed types, TMR had the highest significant (p < 0.05) mean concentration of AFB1 (mean = 50.67 ± 5.2 µg/kg; range = LOD−303.5 µg/kg) compared to the other feed types (Table 2). Moreover, the interaction in the mean concentration of AFB1 was observed to be significantly (p < 0.05) different among feed types and study sites. Thus, in Chiro town, the lowest significant (p < 0.05) mean concentration of AFB1 was observed in WB (6.6 ± 8.9 µg/kg) compared to the MF (29.8 ± 8.9 µg/kg) and TMR (18.7 ± 8.9 µg/kg), respectively. However, in Dire Dawa city and Harar city, the highest significant (p < 0.05) mean concentration of 94.9 ± 8.9 µg/kg and 38.4 ± 8.9 µg/kg for AFB1 was detected in TMR, respectively, compared to other feed types (Table 3).

Table 2.
Level of AFB1 and AFB2 in feeds across study sites, feed sources, and feed types.

Table 3.
Interaction between concentration of AFB1 and AFG1 and feed types and study sites.
The result of ANOVA also revealed that the mean concentration of AFB2 observed in this study varied significantly among the study sites (p < 0.001) and feed types (p < 0.05), while non-significant (p > 0.05) variation was observed between feed sources (Table 2). In comparison, the highest significant (p < 0.05) mean concentration of AFB2 was detected in feed samples collected from Dire Dawa city (mean = 5.69 ± 0.6 µg/kg; range = LOD–30.3 µg/kg) (Table 2). Among the feed types from both sources, the highest significant (p < 0.01) mean concentration of AFB2 was detected in TMR (mean = 4.74 ± 0.6 µg/kg, range = LOD–25.3 µg/kg) collected from all study sites (Table 2).
Furthermore, this study shows that the prevalence of AFB1 and AFB2 in feed samples varied across study sites, feed sources, and feed types (Table 2). Thus, the proportion of dairy feed contaminated with AFB1 (29.4%, n = 53/180) in feed samples from Dire Dawa city was significantly (p < 0.05) higher compared to the samples from Chiro town (22.3%, n = 40/180) and Harar city (20.7%, n = 37/180). Moreover, a numerically higher frequency of occurrence of AFB1 was observed in feed samples from dairy farms (37.2%, n = 67/180) than in the feed collected from markets (35.0%, n = 63/180). However, the frequency of occurrence of AFB2 did not significantly (p < 0.05) differ across the study sites, feed sources, and feed types. But the numerically highest occurrence of AFB1 was observed in feed samples from Dire Dawa city (25.0%, n = 45/180) and dairy farms (37.2%, n = 67/180), as well as in the TMR feed type (26.1%, n = 47/180) (Table 2).
In addition, the analysis of variance revealed that the mean level of AFG1 recovered from the examined feed samples significantly varied among the study sites and feed types (p < 0.01) (Table 4), as well as among feed types and the study sites interaction (p < 0.05) (Table 4). However, variation in the mean concentration of AFG1 was not observed within the feed sources across all sites. Regarding the study sites, the highest significant (p < 0.001) mean concentration of AFG1 was recovered in the feed samples collected from Dire Dawa city (mean = 32.25 ± 2.7 µg/kg; range = LOD–125.5 µg/kg) compared to the other urban centers (Table 4). Similarly, among feed types, TMR had a significantly (p < 0.01) higher mean concentration of AFG1 (mean = 32.87 ± 2.6; range = LOD−125.5) than the other feed types (Table 4). However, the variation in the mean concentration of AFB1 observed among feed types was not the same across the urban centers. As a result, in Dire Dawa city and Harar city, the TMR feed samples had a significantly (p < 0.001) higher mean concentration of 60.0 ± 4.6 µg/kg and 24.3 ± 4.6 µg/kg for AFG1, respectively, compared to the other feed types. However, in Chiro town, a non-significant (p > 0.05) mean concentration of AFG1 was observed between all feed type (Table 3).

Table 4.
Level of AFG1 and AFG2 in dairy feeds across study sites, feed sources, and feed types.
The result of ANOVA also revealed that the mean concentration of AFG2 observed in this study varied significantly among study sites (p < 0.01) and feed types (p < 0.01), while there was no significance (p > 0.05) observed between feed sources (Table 4). In comparison, a significantly higher mean concentration of AFG2 was detected in feed samples collected from Dire Dawa city (mean = 5.01 ± 0.5 µg/kg; range = LOD–21.5 µg/kg) compared to the feed samples from Chiro town (mean = 1.67 ± 0.5 µg/kg; range = LOD–9.4 µg/kg) and Harar city (mean = 1.37 ± 0.5 µg/kg; range = LOD–9.8 µg/kg) (Table 4). Similarly, among the examined feed types, the highest average concentration of AFG2 was detected in TMR feed samples (mean 3.86 ± 0.5 µg/kg; range = LOD–21.5 µg/kg) compared to the MF (mean = 2.60 ± 0.5 µg/kg; range = LOD–11.5 µg/kg) and WB (mean = 1.58 ± 0.5 µg/kg; range = LOD–21.5 µg/kg) collected from all study sites (Table 4).
The study results revealed that the prevalence of AFG1 and AFG2 in feed samples was observed at varying proportions across the study sites, feed sources, and feed types (Table 4). Thus, the proportion of dairy feed contaminated with AFG1 (28.3%, n = 51/180) in feed samples from Dire Dawa city was significantly (p < 0.05) higher compared to the proportion in feed samples from Chiro town (22.8%, n = 41/180) and Harar city (20.0%, n = 36/180). Moreover, the highest (p < 0.05) occurrence of AFG1 was observed in TMR (28.7%) compared to MF (21.9%) and WB (20.7%). However, a numerically higher occurrence of AFG1 was observed in feed samples from dairy farms (36.1%, n = 65/180) than in the feed collected from local markets (35.0%, n = 63/180). On the other hand, the frequency of occurrence of AFG2 did not significantly (p < 0.05) differ across study sites, feed sources, and feed types. However, a numerically higher frequency of occurrence of AFG2 was observed in feed samples from Dire Dawa city (25.6%, n = 46/180), dairy farms (35.6%, n = 64/180), and in the TMR feed type (23.3%, n = 42/180) (Table 4).
2.3. Level of Aflatoxins in Feeds beyond Different Regulatory Limits
Furthermore, the proportion of feed samples contaminated with TAF and AFB1 exceeding the limits set by the FDA/ESA is presented in Table 5. Overall, 33.3% and 38.9% of feed samples were found to exceed the 20.0 µg/kg regulation set by the FDA/ESA for AFB1 and the 40.0 µg/kg regulation set by the ESA for TAF, respectively. Moreover, a higher proportion of feed samples exceeding the ESA regulation for TAF was observed in the samples collected from Dire Dawa city (56.7%) compared to those from Chiro town (26.7%) and Harar city (33.3%). Similarly, a higher proportion of feed samples with an AFB1 content exceeding the FDA/ESA limit was found in the samples from Dire Dawa city (41.7%) compared to those from Chiro town (25.0%) and Harar city (33.3%).

Table 5.
Feed samples exceeding the FDA/ESA regulations limit for AFB1 and TAF.
On the other hand, a higher proportion of feed samples exceeding the ESA limit for TAF (43.3%), as well as feed samples exceeding the FDA/ESA regulation for AFB1 (42.2%), was found in samples from specialized dairy farms compared to those from local markets. Furthermore, among the analyzed feed types, a higher proportion of feed samples exceeding the ESA regulation limit for TAF (53.3%) and the FDA/ESA regulation limit for AFB1 (50.0%) was found in TMR. Meanwhile, 43.3% of MF and 20.0% of WB feed samples containing TAF exceeded the ESA regulation limit, while 33.3% of MF and 16.7% of WB feed samples containing AFB1 exceeded the FDA/ESA regulation limit.
2.4. Correlations between Aflatoxigenic Aspergillus Isolates and Aflatoxins Level
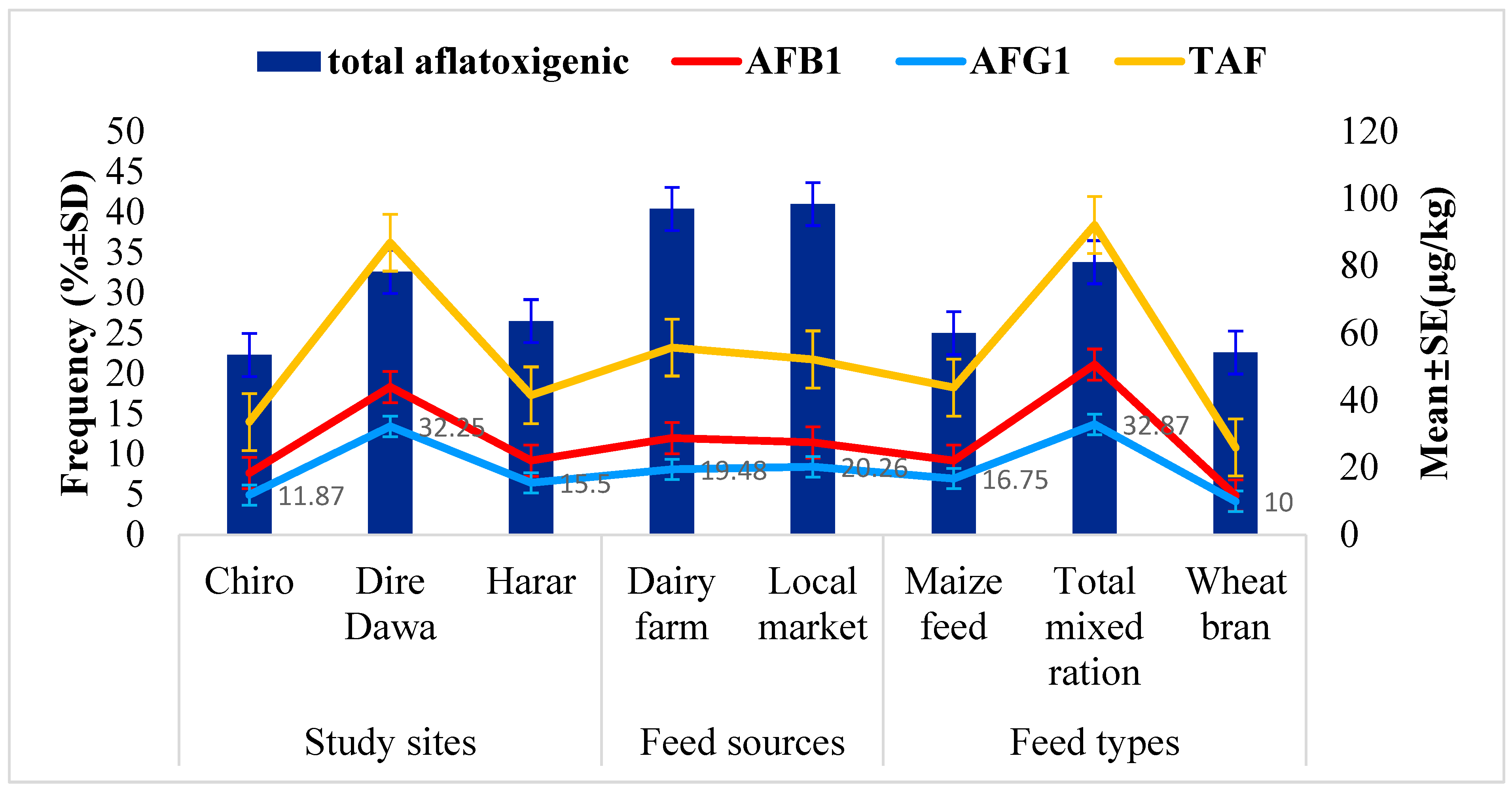
The relationship between the occurrence of aflatoxigenic Aspergillus species isolates and the TAF, AFB1 and AFG1 levels in feed samples across study sites, feed types, and feed sources is presented in Figure 2. Comparably, the highest occurrence of aflatoxigenic Aspergillus species isolates (32.6%) was found in feed samples from Dire Dawa city compared to those from the other urban centers. Similarly, the corresponding mean levels of 86.93 µg/kg for TAF, 43.98 µg/kg for AFB1 and 32.25 µg/kg AFG1 in the feed samples from Dire Dawa city were found to be higher than in those from the other study sites. The results revealed that the TMR feed type had the highest proportion of aflatoxigenic Aspergillus species (33.8%), as well as the highest mean levels of TAF (92.2 µg/kg), AFB1 (50.67 µg/kg), and AFG1 (32.87 µg/kg).
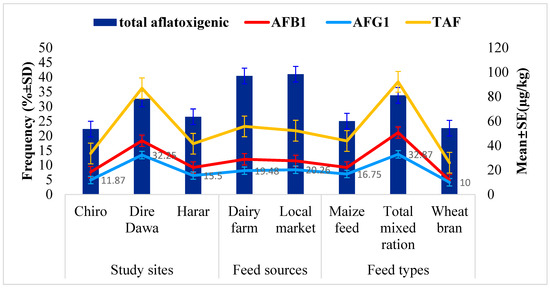
Figure 2.
Relationship between the contamination of aflatoxigenic Aspergillus species isolates and the level of TAF, AFB1, and AFG1.
On the other hand, comparable aflatoxigenic Aspergillus species contamination rates in the feed from dairy farms (40.4%) and local markets (41.0%) were observed. In line with this, comparable mean concentrations of TAF (55.7 µg/kg), AFB1 (28.81 µg/kg), and AFG1 (19.48 µg/kg) were observed in feed samples from dairy farms compared to the corresponding mean concentrations of 52.2 µg/kg, 27.48 µg/kg, and 20.26 µg/kg for the corresponding aflatoxins in feed from local markets.
Furthermore, Table 6 presents the correlation between the aflatoxigenic Aspergillus species isolates and the aflatoxin concentration levels in feed samples. Thus, the isolates of aflatoxigenic A. flavus showed a moderate correlation (p < 0.01) with AFB1 (r = 0.47) and AFB2 (r = 0.46). Similarly, the isolates of aflatoxigenic A. parasiticus showed a moderate correlation (p < 0.01) with AFB1 (r = 0.43), AFB2 (r = 0.40), AFG1 (r = 0.50), and AFG2 (r = 0.39). Overall, the correlation analysis revealed a moderately positive correlation between the counts of aflatoxigenic Aspergillus species and the concentrations of aflatoxins in the feeds of dairy cows. This confirms that the identified aflatoxigenic Aspergillus isolates were responsible for the contamination of aflatoxins in the examined dairy feeds. However, a correlation analysis of aflatoxigenic A. flavus revealed non-significant correlation (p > 0.05) with the concentration of AFG1 and AFG2, confirming the fact that not all aflatoxins were produced by A. flavus.

Table 6.
Correlation of aflatoxigenic A. flavus and A. parasiticus species with the levels of aflatoxins in feed samples.
3. Discussion
3.1. Occurrence and Levels of Aflatoxins in Feed
The persistent occurrence of aflatoxins in food and feed has detrimental effects on human and animal health. Aflatoxin contamination in feed for dairy cattle can then be transferred to humans via the consumption of products from animals that have been exposed to contaminated feed [19]. Therefore, it is essential to assess the prevalence of aflatoxins in dairy cow feed that is prone to aflatoxin contamination to understand the associated public health risks and thereby plan for their prevention and control. As a result, in this study, 180 samples of the three most susceptible feed types for dairy cows were collected from specialized dairy farms and local markets in three selected urban centers in eastern Ethiopia and examined for AFB1, AFB2, AFG1, and AFG2 contamination.
In the present study, among all the tested samples (N = 180), about 82.8% were contaminated with a mean level of 54.01 ± 4.62 µg/kg total aflatoxin (TAF) in dairy feeds, which is higher than the previous levels reported in Ethiopia by Mulugeta [40] and Yohannes et al. [41]. Contamination levels of 50.0%, with a mean concentration of 0.69 µg/kg in dairy feed collected from dairy farmers and feed traders around Addis Ababa, and 52.7%, with a mean concentration of 10.54 ± 3.82 µg/kg in dairy feed from the Gurage zone were reported in these studies [40,41]. However, a higher mean level of TAF in dairy feed (313.03 µg/kg) and Niger seed cake feed (385.45 µg/kg) collected from Addis Ababa, Ethiopia was reported [42]. Similarly, a study conducted in Bishoftu town in Ethiopia revealed a higher level of TAF (195.88 µg/kg) in poultry feed compared to our findings [31].
Additionally, as shown in Table 2, the mean level of TAF in the feed samples varied among the different study sites, feed sources, and feed types. Consequently, the feed samples collected from Dire Dawa city showed a significantly higher mean concentration of 86.93 ± 8.2 µg/kg for TAF. In contrast, the mean concentration of 41.5 ± 8.2 µg/kg for TAF in the feed samples from Harar city was not statistically significant compared to the 33.6 ± 8.2 µg/kg mean concentration of TAF in the feed samples from Chiro town. In a study conducted by Yohannes et al. [41], a comparably lower mean of 10.54 ± 3.82 µg/kg for TAF in the feeds from Butajira town and 4.22 ± 8.2 µg/kg for TAF in feed from Emdibir town in the Gurage Zone, Ethiopia was detected. However, there was a higher mean of 313.03 µg/kg for TAF in dairy feed collected from feed factories in Addis Ababa, Ethiopia. Furthermore, poultry feed collected from different sites in Bishoftu town in Ethiopia had a higher mean TAF concentration (195.88 µg/kg) [31] compared with the present findings. These variations in aflatoxin levels may be attributed to various factors, including geographical location, climatic conditions, and feed storage practices [15,43,44]. In particular, poor storage practices among dairy farmers, such as inadequate air flow and ventilation, elevated air moisture and temperature, and prolonged storage period may have contributed to the proliferation of Aspergillus fungi and the production of aflatoxin.
Similarly, TMR had a significantly higher mean level of TAF (92.2 ± 8.2 µg/kg) compared to the other feed types. However, there was no significant difference between the average levels of TAF in maize feeds (43.8 ± 8.2 µg/kg) and those in wheat bran (26 ± 8.2 µg/kg). In a previous study, Genet et al. [42] reported a mean level of 385.45 µg/kg for TAF in Noug seed cake (Guizotia abyssinica), which was higher than the mean level of 26.92 µg/kg in maize and 3.7 µg/kg in wheat bran collected from Addis Ababa, Ethiopia. Furthermore, Mulugeta [40] reported a lower mean level of 0.06 µg/kg for TAF in wheat bran collected from different towns in central Ethiopia. The variations in aflatoxin levels in different types of feed could be due to the presence of susceptible feed ingredients such as Noug seed cake [6], which is a crucial ingredient in TMR. Consistent with this finding, Gizachew et al. [29] reported that Noug seed cake had the highest concentration of aflatoxin and noted it as a highly susceptible feed ingredient to aflatoxin contamination.
Our results also revealed high rates of contamination with AFB1, AFB2, AFG1, and AFG2 among the examined dairy feeds. Thus, this investigation demonstrated a common pattern of aflatoxin occurrence, with higher amounts of the highly potent AFB1 and AFG1 than AFB2 and AFG2. As a result, AFB1 was detected in 72.2% of samples, with a mean level of 28.15 ± 3.50 µg/kg, while AFG1 was detected in 71.1% of samples, with a mean level of 19.87 ± 1.87 µg/kg. In comparison, AFB2 had an occurrence rate of 66.1% with a mean level of 3.30 ± 0.40 µg/kg, whereas AFG2 had an occurrence rate of 67.8% with a mean level of 2.70 ± 0.32 µg/kg. Similar to our findings, higher levels of AFB1 (31.2 µg/kg) and G1 ((17.2 µg/kg) relative to AFB2 (3.27 µg/kg) and AFG2 (1.14 µg/kg) have been reported in dairy feed from the Guraghe Zone in Ethiopia [41]. Similarly, Genet et al. [42] reported higher levels of AFB1 (192.8 µg/kg) and AFG1 (104.35 µg/kg) compared to the levels of AFB2 (12.34 µg/kg) and AFG2 (3.55 µg/kg) in dairy feed, as well as higher level of AFB1 (288.34 µg/kg) and AFG1 (82.9 µg/kg) compared to AFB2 (10.91 µg/kg) and AFG2 (3.28 µg/kg) in maize feed from Addis Ababa, Ethiopia. However, Yoseph et al. [31] reported higher levels of AFB2 (91.37 µg/kg) and AFG2 (72.77 µg/kg) than the levels of AFB1 (17.93 µg/kg) and AFG1 (13.79 µg/kg) in poultry feed from Bishoftu town, Ethiopia.
These variations may be attributed to multiple factors that affect the growth of aflatoxin-producing fungi and their potential for aflatoxin production. AFB1 and AFB2 are known to be produced by A. flavus, whereas A. parasiticus produces all distinct types of aflatoxins B1, B2, G1, and G2 [45,46,47]. Moreover, another investigation demonstrated that the ratio of B and G aflatoxin is largely influenced by the conditions within the ecological niches where fungal species that produce aflatoxins grow [15]. Predominantly, temperature plays a greater role in AFB1 production, whereas water activity is vital for AFG1 biosynthesis [48]. Moreover, Medina et al. [49] noted that both water availability and temperature affect the expression of the structural gene (aflS) and regulatory gene (aflR), which determine the relative growth and aflatoxin production in both A. flavus and A. parasiticus. Similarly, a study by Abdel-Hadi et al. [50] revealed a good correlation between the early structural gene and aflatoxin production. In addition, Medina et al. [49] reported the interaction of conditions with relative gene expression and AFB1 production, involving temperature, water activity, and other factors.
3.2. Mean Concentration and Occurrence of Principal Aflatoxins in Feeds
The mean concentrations and frequency of occurrence of AFB1, AFB2, AFG1, and AFG2 in the feed samples were compared across the study sites, feed sources, and feed types (Table 2 and Table 4). Thus, significantly (p < 0.001) different mean concentrations of AFB1, AFB2, AFG1 and AFG2, as well as a significantly (p < 0.05) different frequency of occurrence of AFB1 and AFG1, were observed between the study sites. As a result, a significantly (p < 0.001) higher mean concentration of 43.98 ± 5.3 µg/kg for AFB1, with an occurrence rate of 29.4%, and a mean concentration of 32.25 ± 2.7 µg/kg for AFG1, with an occurrence rate of 28.3%, were found in feed samples collected from Dire Dawa city compared to the other urban centers. Similarly, the mean levels of AFB2 (5.69 ± 0.6 µg/kg) and AFG2 (5.01 ± 0.5 µg/kg) in the feed samples from Dire Dawa city were significantly (p < 0.01) higher compared to the other study sites. Since the feed types analyzed across the study sites were the same, the differences in climatic conditions, feed storage practices, and moisture content might be the cause of the variation in aflatoxin contamination in the feeds between the study sites.
In accordance with the current findings, Jalel et al. [18] reported a significant (p < 0.0001) mean concentration of AFB1 in dairy feed from Sululta town (23.37 ± 1.95 µg/kg) compared to Burayu town (21.99 ± 1.75 µg/kg) and Sebeta town (21.9 ± 1.9 µg/kg). Similarly, Gizachew et al. [29] reported significantly different levels of aflatoxin in feed between different study towns. Thus, the feed samples from Debre Zeit town had over three times higher levels of AFB1 compared to the feeds from Sendafa, Sululta, and Addis Ababa. Consistent with our findings, Jalel et al. [18] and Gizachew et al. [29] noted that environmental temperature, feed moisture content, and storage situation were the contributing factors to the variation in aflatoxins between the study areas. In contrast, Mulugeta, [40] reported that the levels of aflatoxins B1, B2, G1 and G2 in dairy feeds were not significantly different across three different towns (Sululta, Bishoftu, and Debre Berhan) in central Ethiopia.
Moreover, Rehrahie et al. [30] reported a lower mean level of AFB1 (5.63 µg/kg) in dairy feed from different towns in central Ethiopia compared to the present study, with a higher frequency of occurrence (49.4%). Compared with the present study, Changwa et al. [24] reported lower levels of AFB1 (0.7 µg/kg) and AFG1 (2.6 µg/kg) but higher levels of AFG2 (41.3 µg/kg) in dairy feeds from South Africa. However, the same authors reported a higher occurrence of AFB1 (48.0%), AFB2 (93.0%), AFG1 (55.0%) and AFG2 (100.0%) in dairy feeds compared with the present study. On the other hand, Genet et al. [42] reported higher mean concentrations of AFB1 (192.80 µg/kg), AFB2 (12.34 µg/kg), and AFG1 (104.35 µg/kg) in dairy feed collected from Addis Ababa compared to the present study.
In line with this finding, several studies have revealed that the variation in the level of aflatoxins across different study areas may be attributed to geographical locations, climatic conditions, and feed storage practices or a combination of these factors under which the Aspergillus fungi that produce aflatoxin grow [15,51,52]. A. flavus is widely known to produce AFB1 and AFB2, whereas A. parasiticus produces all distinct types of aflatoxin, including B1, B2, G1, and G2 [45,46,47]. According to Matumba et al. [48], temperature plays a greater role in AFB1 production, whereas water activity/moisture content contributes to the biosynthesis of AFG1.
The study results revealed that significantly different (p < 0.01) mean concentrations of AFB1, AFB2, AFG1 and AFG2, as well as a significantly (p < 0.05) different frequency of occurrence of AFB1 and AFG1, were observed among the examined feed types (Table 2 and Table 4). Compared with the other feed types, the TMR feed exhibited a significantly higher mean concentration of 50.67 ± 5.2 µg/kg (LOD–303.5 µg/kg) and a 30.7% frequency of occurrence for AFB1, as well as a 32.87 ± 2.6 µg/kg (LOD–125.5 µg/kg) mean concentration and 28.7% frequency of occurrence for AFG1. Similarly, the average levels of 4.74 ± 0.6 µg/kg (LOD–25.3 µg/kg) for AFB2 and 3.86 ± 0.5 µg/kg (LOD–25.3 µg/kg) for AFG2 in TMR were significantly higher than those in the other feed types, although their frequency of occurrence did not significantly differ between feed types.
In line with this finding, Makau et al. [53] reported a highly significant (p < 0.001) variation in the mean concentration of AFB1 in different feed types, with a higher mean level detected in the mixed concentrate feed (147.86 µg/kg) than in the other feed types. Similarly, Mulugeta et al. [40] reported various mean concentrations of AFB1 (0.34 µg/kg), AFB2 (0.22 µg/kg), AFG1 (0.03 µg/kg), AFG2 (0.33 µg/kg), and TAF (0.61 µg/kg) in Noug seed cake compared with the corresponding mean levels of 0.021 µg/kg, 0.018 µg/kg, LOD µg/kg, 0.018 µg/kg, and 0.06 µg/kg in wheat bran collected around Addis Ababa, Ethiopia.
Moreover, research has revealed varied levels of aflatoxins in different feed types of dairy cows. Thus, compared with the present findings, lower mean levels of AFB1 (31.2 µg/kg) and AFG1 (17.1 µg/kg) were detected in the mixed feed of dairy cattle [41]. The same authors also reported comparable mean levels of AFB2 (3.27 µg/kg) and AFG2 (1.14 µg/kg) in mixed feed of dairy cows. Similarly, Genet et al. [42] reported lower concentrations of AFB1 (12.71 µg/kg), AFB2 (1.32 µg/kg), AFG1 (11.54 µg/kg), and AFG2 (1.35 µg/kg) in maize feed collected from Addis Ababa. In contrast, higher average levels of AFB1 (40.56 ± 9.58 µg/kg) were detected in maize bran from Malawi [22], whereas a comparable mean level of AFB1 (18 ± 11.0 µg/kg) was detected in maize grain collected from dairy farms from greater Addis Ababa milk shed, Ethiopia [29].
Additionally, in wheat bran from around Addis Ababa in Ethiopia, the mean concentrations of AFB1 (0.021 µg/kg), AFB2 (0.018 µg/kg), AFG1 (0.187 µg/kg), and AFG2 (0.0 µg/kg) were lower than in our findings [40]. Similarly, wheat bran collected from Addis Ababa feed factories had lower mean concentrations of AFB1 (2.29 µg/kg) and AFG1 (1.5 µg/kg) [42]. However, Gizachew et al. [29] reported a higher mean concentration of 15 ± 6 µg/kg for AFB1 in wheat bran collected from dairy farms in Addis Ababa city and the surrounding areas. Compared with the presented study, Elzupir et al. [54] reported higher mean concentrations of AFB1 (21.84 ± 0.7 µg/kg), AFB2 (5.03 ± 0.52 µg/kg), AFG1 (23.49 ± 0.75 µg/kg), and AFG2 (38.41 ± 0.41 µg/kg) in wheat bran from Khartoum, Sudan.
In addition to environmental factors, the type of substrate, nutrient composition, and moisture content of feed may play critical roles in the variation in the content of aflatoxin-producing fungi as well as aflatoxin levels between the examined feed types. In line with this finding, Kos et al. [55] noted that the degree of colonization by Aspergillus fungi in a given food or feedstuff depends on numerous factors, including the composition of the substrate, the availability of nutrients, moisture content, among others. Moreover, Daou et al. [56] noted that fungi may grow quickly on a substrate that contains high levels of carbohydrates and is rich in carbon and nitrogen. Thus, this may hold true in our cases, where the TMR (13.0%) has a greater level of crude fiber than the MF (2.2%) and WB (8.2%) do [57].
Furthermore, a significant (p < 0.001) interaction effect on the mean concentrations of AFB1 and AFG1 was observed among different feed types and study sites (Table 3). Thus, in Dire Dawa city and Harar city, the significantly (p < 0.001) highest mean concentrations of 94.908 ± 8.9 µg/kg and 38.4 ± 8.9 µg/kg for AFB1, respectively, were detected in TMR feed, whereas in Chiro town, the significantly (p < 0.001) lowest mean concentration of AFB1 was detected in the WB (6.6 ± 8.9 µg/kg). Similarly, the TMR from Dire Dawa city (60.0 ± 4.6 µg/kg) and Harar city (24.3 ± 4.6 µg/kg) had significantly (p < 0.001) higher mean concentrations of AFG1 than the other feed types. The significant variation in mean concentrations of AB1 and AFG1 in TMR feed samples from Dire Dawa city and Harar city may be attributed to a combined effect of relatively higher temperatures in these urban centers, as well as high carbohydrate (13.0%) and moisture content (11.8%) in TMR feed [58], which promote Aspergillus fungal growth and aflatoxin production. In line with this finding, Matumba et al. [48] noted that higher environmental temperature plays a greater role in AFB1 production, whereas moisture content contributes more for the biosynthesis of AFG1 in Aspergillus fungi.
3.3. Level of Aflatoxins in Feeds beyond Different Standard Regulations
The proportions of feed samples contaminated with AFB1 and TAF at levels exceeding the regulatory limits set by the FDA/ESA were determined across the study sites, feed sources, and feed types (Table 5). As a result, 33.3% (60/180) of the feed samples contaminated with AFB1 displayed levels exceeding the FDA/ESA limit of 20.0 µg/kg for dairy cattle feed. However, 38.9% (70/180) of the feed samples contaminated with TAF displayed levels exceeding the ESA limit of 40.0 µg/kg. A study conducted by Rehrahie et al. [30] revealed that 81.9% of feed samples exceeded the AFB1 level set out by the FDA/ESA regulation limit in dairy feed, which was higher than the proportion reported in the present study. Similarly, studies conducted on dairy feed collected from Nakuru County, Kenya [53] and Qazvin, Iran [59] revealed that 52.0% and 50.0% of feed samples containing AFB1 displayed levels exceeding the FDA regulatory limit, respectively. However, Jalel et al. [18] reported that 26.7% of feed samples contained AFB1 at concentrations exceeding 20.0 µg/kg.
On the other hand, a relatively higher percentage (62.5%) of feed samples with TAF above the FDA limit in dairy feeds has been reported [24]. The discrepancy in the proportion of feed samples with aflatoxins exceeding different regulatory limits may be attributed to geographical location, climatic conditions, or other factors favorable for aflatoxin production by Aspergillus fungus [15].
3.4. Correlation of Aflatoxigenic Aspergillus Isolates and Aflatoxins Level
Figure 2 presents the correlation of total aflatoxigenic Aspergillus isolates and the levels of TAF, AFB1, and AFG1 detected in the examined dairy feed samples. The highest occurrence of total aflatoxigenic Aspergillus species isolates (32.6%) was found in the feed samples from Dire Dawa city, with corresponding mean levels of 43.98 µg/kg for AFB1, 32.25 µg/kg for AFG1, and 86.93 µg/kg for TAF, which were also higher in the feed samples from Dire Dawa city. Similarly, the highest proportion of total aflatoxigenic Aspergillus species isolates (33.8%) was found in the TMR feed samples, with corresponding mean levels of 92.2 µg/kg for TAF, 32.87 µg/kg for AFG1, and 50.37 µg/kg for AFB1.
Although data on the relationship between aflatoxigenic Aspergillus species and aflatoxin levels are difficult to find, Krnjaja et al. [60] reported a comparable aflatoxigenic Aspergillus species occurrence rate of 85.71%, with a corresponding mean level of 4.47 µg/kg (1.79–16.01 µg/kg) in chicken feed, as well as a 100.0% occurrence rate with corresponding mean levels of 4.56 µg/kg (1.34–18.29 µg/kg) in layer feed for AFB1. Similarly, the highest occurrence of aflatoxigenic A. flavus in concentrate feed with a mean of 11.5 ± 8.0 µg/kg (2.6–24.8 µg/kg) for AFB1 was found compared to the other feed types of dairy cattle [61].
Moreover, Table 6 displays the Pearson correlation between the counts of aflatoxigenic A. flavus and A. parasiticus and the levels of aflatoxins. A highly significant (p < 0.01) moderate correlation was observed between AFB1 and AFB2 and aflatoxigenic A. flavus. A similar correlation was observed between aflatoxigenic A. parasiticus counts and the levels of AFB1, AFB2, AFG1, and AFG2 in dairy feed. Similarly, Kim et al. [60] reported a moderately positive correlation (r = 0.41) between the total fungal counts and the levels of AFB1 in poultry feed. However, a strong positive correlation (r = 0.76) was reported between the isolates of A. flavus in feed and the level of AFM1 in milk [11].
4. Conclusions
The investigation of aflatoxin prevalence in feed samples revealed significant occurrences and mean concentrations across study sites, feed sources, and feed types. Among the examined samples, an 82.8% prevalence with a mean level of 54.01 ± 4.62 µg/kg for TAF was found. Moreover, a higher occurrence of 72.2% with a mean level of 28.15 ± 3.50 µg/kg for AFB1 and an occurrence of 71.1% with a mean level of 19.87 ± 1.87 µg/kg for AFG1 were detected. Furthermore, the feed samples collected from Dire Dawa city exhibited a significantly higher prevalence and mean concentrations of highly potent AFB1 and AFG1 compared to the feed from Chiro town and Harar city. This could be attributed to suitable climatic factors for fungal colonization and aflatoxin production in feed samples from Dire Dawa city. Similarly, compared to MF and WB feed samples, the TMR feed samples showed a significantly higher prevalence and mean concentrations of the highly potent AFB1 and AFG1. This could be attributed to the nutrient composition and relatively favorable moisture content of TMR for Aspergillus fungal contamination and aflatoxin production.
In addition, a considerable proportion of the feed samples exceeded the FDA/ESA regulatory limits for AFB1 and TAF, particularly among the TMR feed samples. Moreover, the proportions of feed samples from specialized dairy farms that surpassed the FDA/ESA regulatory limits were higher than those from local markets. Moreover, there was a moderately positive correlation between aflatoxigenic Aspergillus species counts and the levels of TAF, indicating the aflatoxin production potential of Aspergillus species in feeds from eastern Ethiopia. This study revealed the widespread prevalence of aflatoxin contamination in dairy feed from the study areas. Thus, there is an urgent need for rigorous monitoring of aflatoxins in feed and implementation of mitigation measures to curb the associated feed and food safety risks. Additionally, it is crucial to investigate aflatoxin contamination levels in milk to better understand the potential risks associated with aflatoxin exposure. Overall, these findings indicate significant feed and food safety concerns related to aflatoxins, found in specific locations in this study, that require attention from policy-makers, researchers, administrators, and other concerned bodies.
5. Materials and Methods
5.1. Description of the Study Area
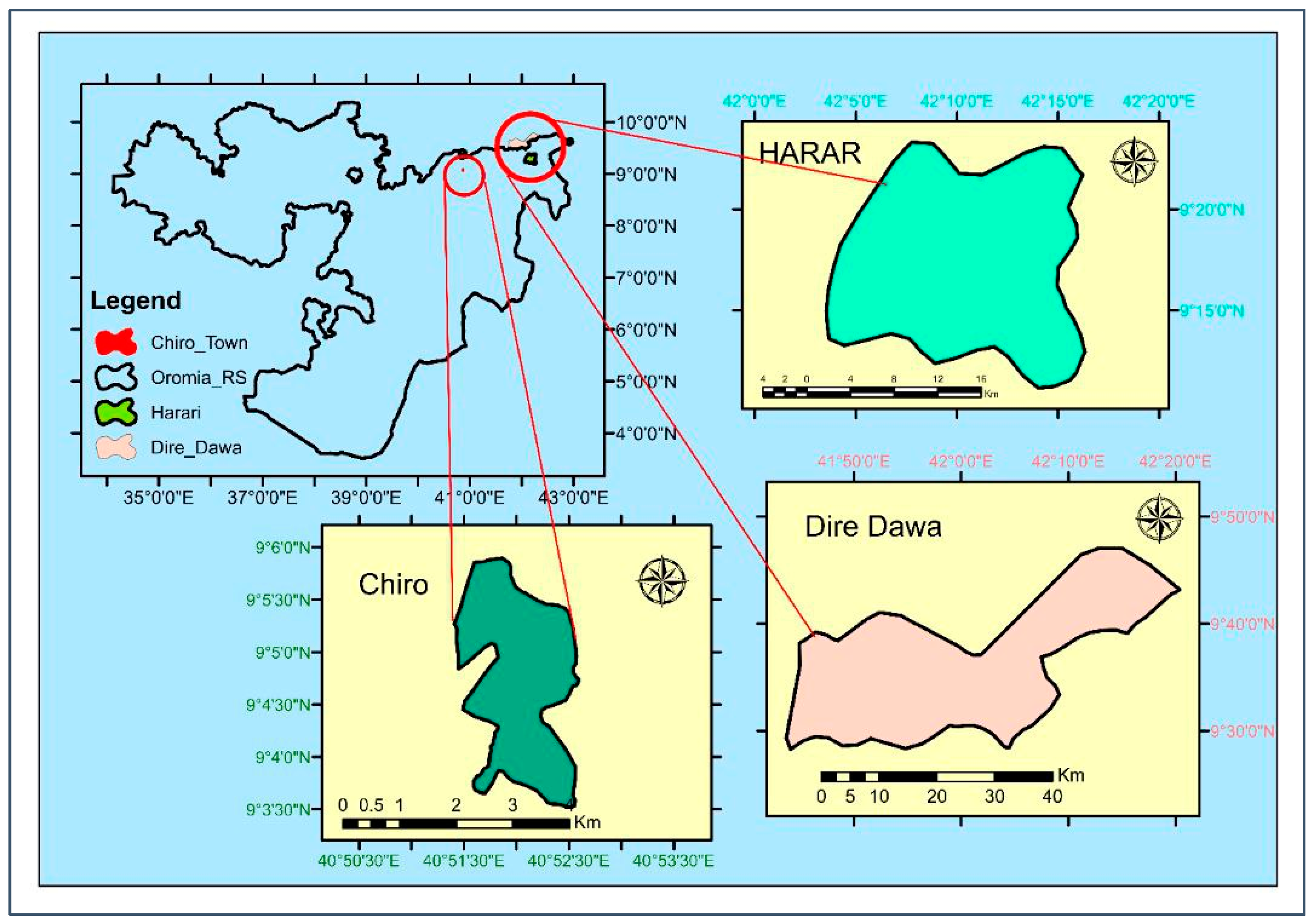
Considering their potential for dairy production and their role as the main market centers for milk distribution for the surrounding districts, the three major urban centers of eastern Ethiopia, Dire Dawa city, Chiro town, and Harar city (Figure 3), were specifically selected for this study [38,62,63]. These selected urban centers are situated in different agroecological areas according to elevation. Dire Dawa city is situated in a lowland agroecology with an elevation of 1170 m above sea level and an average annual temperature of 25.2 °C (17.4–33.0 °C), whereas Chiro town has a semiarid agroclimate and is situated at 1757 m above sea level, with an average annual temperature of 20.6 °C (13.3–27.8 °C) [64,65,66]. On the other hand, the majority of Harar city’s land is situated in a midland agroecological location, at 1900–2200 m above sea level, where a few areas are situated in a highland agroecology [67]. Harar city has an annual average temperature of 20.5 °C, ranging from 14.1 to 26.8 °C [66].
Figure 3.
Map showing the location of the study areas: Chiro town (dark green), Dire Dawa city (rose), and Harar city (light green).
5.2. Feed Sample Collection
For this study, 180 concentrate feed samples, comprising wheat bran (WB), total mixed ration (TMR), and maize feed (MF), were gathered from two primary feed sources: specialized dairy farms and local markets. Between September 2021 and January 2022, feed samples were gathered from the selected urban centers (i.e., Chiro town, Harar, city and Dire Dawa city). Ten samples of each feed type—MF (10), TMR (10), and WB (10)—were gathered from the specialized dairy farms in each of the identified urban centers. Similarly, ten samples from local markets in each urban center were collected for each of the three feed types: MF (10), TMR (10), and WB (10). As a result, 180 feed samples (10 × 3 × 2 × 3 = 180) were taken and analyzed for aflatoxin contamination.
The sample size was determined using an 86% (0.86) expected prevalence [68] and a 5% level of precision, following the method described by Daniel and Cross [69]. Prior to the collection of feed samples, detailed consultations were held with agricultural administrators, livestock experts, and extension workers in the target urban centers to identify potential kebeles (the smallest administrative unit) for specialized dairy farming and the major feeds. Thus, the livestock development offices were provided a list of specialized dairy farms, from which the sampled dairy farms (which were feeding their cows from MF, TMR, or WB) were randomly selected for feed sample collection. In collaboration with livestock extension workers, local feed retailers or feed shops (selling MF, TMR, or WB) were subsequently selected by visiting the identified marketing centers using systematic random sampling. The feed samples were bought from the identified feed retailers/shops.
Thus, to form an aggregated portion of the sample, a small amount of feed was taken by sampling spears from many locations in different containers/sacks where the lactating cows were being fed. After the samples were well mixed, a 0.5 kg feed sample was collected from the aggregated portion and placed into a labeled sampling bag. The feed samples were subsequently brought to the Dairy Sciences Laboratory of Haramaya University and kept under cool conditions until further analysis was performed. Aflatoxin analysis of the feed samples was performed at the Animal Products, Veterinary Drug and Feeds Quality Assessment Center (APVDFAC) in Addis Ababa.
5.3. Feed Sample Extraction
Feed samples were extracted following the procedures outlined in the AOAC official method with slight modifications [70]. Briefly, the feed samples were ground to a particle size of 0.01 mm using the Chincan mill (FW100, Taichung, China). The feed samples were thoroughly mixed to achieve homogeneity. Then, 20 g of each feed sample was weighed into a 500 mL graduated plastic cylinder containing 2 g of sodium chloride and 80 mL of a methanol–water (0.8:0.2 v/v) solution and shaken using a wrist-action shaker (Wrist-Action Shaker: Model-75, Burrell Company, Sheffield, England) for 30 min. Then, the solutions were filtered into 250 mL glass beakers through a Whatman No. 1 paper.
Next, 20 mL of n-hexane was added to each filtrate, and the solutions were transferred to Falcon test tubes and centrifuged at 2500 rpm for 10 min using the Hermle Labortechnik GmbH, Wehingen, Germany. Following centrifugation, the solutions were added to standing glass beakers for phase separation, and the defatted desiccant was then drained from the supernatant fatty layer of each solution. Subsequently, 7 mL of each defatted solution was mixed with 43 mL of phosphate-buffered saline (PBS) solution and filtered through a 0.22 µm micropore syringe filter. The filtered sample solutions were then subjected to sample clean-up.
5.4. IAC Feed Sample Clean-Up
Sample clean-up was performed using the solution obtained from the sample extraction process. Thus, AflaClean immunoaffinity column (IAC) kits (AflaClean LCTech GmbH, Wehingen, Germany) were used to clean up the sample solution for aflatoxins, including AFB1, AFB2, AFG1, and AFG2 in feeds. The instructions enclosed with the IAC kits were followed for sample clean-up. Thus, 50 mL of each defatted sample solutions was eluted through IAC kit columns at a rate of 1–2 drops per second using a vacuum pressure pump (Supelco Visiprep™, Darmastadt, Germany). The columns were washed with 10 mL of distilled water at the same flow rate. Subsequently, the bound aflatoxins were eluted with 3 mL of HPLC grade acetonitrile. The eluate was collected in vials and injected into the HPLC for aflatoxins analysis.
5.5. HPLC Instrumentation
An Agilent 1260 system (Agilent Technologies, Santa Clara, CA, USA) with a high-performance liquid chromatography (HPLC) instrument coupled with a fluorescence detector (FLD), Chemstation Software (Agilent Technologies), a binary pump, a vacuum degasser, an autosampler, and an Agilent column (Eclipse XDB-C18, 1.8 µm, 4.6 × 50 mm) was used to analyze aflatoxin in the feed samples. The mobile phase was composed of an isocratic mixture of water/acetonitrile/methanol (65:20/15, v/v) at a flow rate of 1.4 mL/min. With the column temperature adjusted to 40 °C, the injection volume was set to 20 µL. Fluorescence wavelengths at 365 nm excitation and 435 nm emission were used to detect AFB1, AFB2, AFG1, and AFG2 in the feed samples.
5.6. Method Validation
Method validation was carried out in compliance with the standards set forth in the AOAC [70]. Consequently, method validation was conducted on the basis of linearity, the limit of detection (LOD), the limit of quantification (LOQ), the percentage of recovery (%R), and the percentage of relative standard deviation (%RSD) (Table 7).

Table 7.
Method performance of aflatoxin analysis in feed samples.
The linearity of the method was determined by preparing calibration curves for each aflatoxin using a standard solution (Sigma-Aldrich® Darmastadt, Germany) at six concentration levels (2–32 µg/kg). The peak areas of aflatoxins against the concentration level of standards were plotted, and linear regression and coefficients of determination (R2) were computed. Thus, the coefficients of determination (R2) of 0.9997, 0.9998, 0.9999, and 0.9981 for AFB1, AFB2, AFG1, and AFG2, respectively, demonstrated linearity.
In addition, the limit of detection (LOD) and limit of quantification (LOQ) were used to demonstrate the method’s sensitivity. Thus, the minimum concentration of an analyte that can be detected was set to be the LOD, whereas the lowest amount of an analyte in a sample that can be quantitatively determined with suitable precision was termed the LOQ. The LOD was calculated as the lowest concentration of the analyte giving a signal response 3 times greater than the average of the baseline noise obtained from ten blank samples (S/N 3:1), and the LOQ was determined as the analyte signal response 10 times greater than the average of the baseline noise obtained from ten blank samples (S/N 10:1). As a result, the limits of detection for AFB1, AFB2, AFG1, and AFG2 were 0.54, 0.43, 0.35, and 1.39 µg/kg, respectively. Similarly, the limits of quantification for AFB1, AFB2, AFG1, and AFG2 were determined to be 1.65, 1.30, 1.07, and 4.20 µg/kg, respectively.
Furthermore, the accuracy and precision of the method were evaluated by spiking samples at six known aflatoxin concentrations in triplicate (Table 7). The method’s repeatability was measured using the percent of recovery (%R), whereas precision was assessed using the percent of relative standard deviation (%RSD), as described in the following equations:
Accordingly, the %R ranged from 72.0 to 97.28%, 75.9 to 90.10%, 73.2 to 114.79%, and 70.45 to 100.24% for AFB1, AFB2, AFG1, and AFG2, respectively. This demonstrates that the analytical method was accurate, as the percentages of recovery were within the range of 70–120% as specified in the AOAC [70]. Similarly, ranges of 0.11% to 0.85% of the percentage relative standard deviation of 0.21%, 0.15%, 0.11%, and 0.57% for AFB1, AFB2, AFG1, and AFG2, respectively, were found. The %RSD of this investigation demonstrated that the method is precise as the values were less than 5%.
5.7. Identification of Aflatoxigenic Aspergillus Species
To screen for aflatoxigenic Aspergillus species, 1 g of a ground feed sample was added to a sterilized test tube along with 9 mL of distilled water. The mixture was then vortexed for 5 min [71]. Next, the serial dilution technique was employed, with dilutions of 10−1, 10−2, 10−3, and 10−4 [72]. From each dilution, 1 mL of the solution was dispensed into a 90 mm Petri dish containing Aspergillus flavus and Parasiticus Agar (AFPA) to isolate and identify Aspergillus species from the feed samples. The AFPA medium was prepared from 20 g/L yeast extract, 10 g/L bacteriological peptone, 0.5 g/L ferric ammonium citrate, and 15 g/L agar [3].
Furthermore, the technique described by Ahmed et al. [73] and Abd El-Aziz et al. [74] was employed to assess the aflatoxigenic potential of Aspergillus species colonies by coconut agar medium (CAM) using UV fluorescence UVITEC, Cambridge, UK at a wavelength of 365 nm. To obtain pure cultures and screen for their aflatoxigenic potential colonies of A. flavus and A. parasiticus species grown on AFPA were sub-cultured onto CAM and incubated at 26 ℃ in the dark for 5–7 days. Accordingly, the colonies that produced aflatoxin exhibited blue-green fluorescence, whereas the colonies that did not produce aflatoxin did not exhibit such fluorescence.
5.8. Statistical Analysis
The data collected were checked and entered into Microsoft Excel 2016 (MS Excel®) and then exported to SAS v9.4 software (SAS Institute, Cary, NC, USA) for analysis. The occurrence of aflatoxins in dairy feed samples from different study sites, feed sources, and feed types was analyzed and presented in graphs and frequency tables. For the mean concentration of aflatoxin in the feed samples, analysis of variance (ANOVA) was performed to compare their differences across the study sites, feed sources, and feed types. Meanwhile, Duncan’s test was used to determine the difference in the mean concentration (µg/kg) of each aflatoxin in the feed samples. Furthermore, aflatoxigenic Aspergillus species were correlated with aflatoxin levels in feed.
Author Contributions
A.T.: Conceptualization, methodology, data curation, formal analysis, investigation, writing—original draft preparation, and writing—review and editing. M.Y.K.: supervision, validation, and writing—review and editing. Y.Y.M.: supervision, validation, and writing—review and editing. A.M.: supervision, validation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
This study was made possible with the support of many esteemed individuals, community members, institutions, and others. Therefore, the authors would like to acknowledge all parties who contributed to the completion of this research work. Specifically, the authors are grateful and express their gratitude to the FDRE Ministry of Education, Haramaya University, and Bule Hora University for their esteemed support of this research work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashiq, S. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Compr. Rev. Food Sci. Food Saf. 2014, 1, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Bouti, K.; Verheecke-Vaessen, C.; Mokrane, S.; Meklat, A.; Djemouai, N.; Sabaou, N.; Mathieu, F.; Riba, A. Polyphasic characterization of Aspergillus section Flavi isolated from animal feeds in Algeria. J. Food Saf. Wiley 2019, 40, e12743. [Google Scholar] [CrossRef]
- Variane, A.C.F.; Dos, S.C.F.; Castro, F.F.d.; Barbosa-Tessmann, I.P.; Santos, G.T.d.; Pozza, M.S.d.S. The occurrence of aflatoxigenic Aspergillus spp. in dairy cattle feed in Southern Brazil. Braz. J. Microbiol. 2018, 49, 919–928. [Google Scholar] [CrossRef]
- Klingelhofer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.K.; Brüggmann, D.; Groneberg, D.A.; Klingelhöfer, D.; Zhu, Y.; Braun, M.; Bendels, M.H.K.; et al. Aflatoxin-Publication analysis of a global health threat. Food Control 2018, 89, 280–290. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Bervis, N.; Lorán, S.; Juan, T.; Carramiñana, J.J.; Herrera, A.; Ariño, A.; Herrera, M. Field Monitoring of Aflatoxins in Feed and Milk of High-Yielding Dairy Cows under Two Feeding Systems. Toxins 2021, 13, 201. [Google Scholar] [CrossRef]
- IARC (Internation Agency for Research on Cancer). Aflatoxins. IARC Monogr. 2012, 100F, 225–248. [Google Scholar]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Mary, A. Aflatoxin Exposure and its Association with stunting among Young Children in Butajira District, South-Central Ethiopia. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2018. [Google Scholar]
- Sarma, U.P.; Bhetaria, P.J.; Devi, P.; Varma, A. Aflatoxins: Implications on Health. Indian J. Clin. Biochem. 2017, 32, 124–133. [Google Scholar] [CrossRef]
- Claudious, G.; Sheperd, M.; Charles, M.T.; Jambwa, P.; Marumure, J. Isolation of Aspergillus flavus from Dairy Cattle Feed and Assessment of Aflatoxin M1 In Milk from Small Dairy Farms Around Harare, Zimbabwe. Adv. Microbiol. Res. 2019, 3, 009. [Google Scholar] [CrossRef]
- Unnevehr, L.; Grace, D. Tackling Aflatoxins: An Overview of Challenges and Solutions; International Food Policy Research Institute: Washington, DC, USA, 2013. [Google Scholar]
- EU. Commission Directive 2003/100/EC of 31 October 2003 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed. Off. J. Eur. Union 2002, 285, 33–37. [Google Scholar]
- FDA (Food and Drug Authority). FDA Compliance Policy Guide CPG Sec. 527.400 “Whole Milk, Lowfat Milk, Skim Milk—Aflatoxin M1”. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1 (accessed on 29 February 2024).
- Awuchi, C.; Nyakundi Ondari, E.; Josiah Eseoghene, I.; Twinomuhwezi, H.; Otuosorochi Amagwula, I.; Morya, S. Fungal Growth and Mycotoxins Production: Types, Toxicities, Control Strategies, and Detoxification. In Fungal Reproduction and Growth; Sultan, S., Singh, G.K.S., Eds.; IntechOpen Limited: London, UK, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Nishimwe, K.; Bowers, E.; Ayabagabo, J.D.D.; Habimana, R.; Mutiga, S.; Maier, D. Assessment of aflatoxin and fumonisin contamination and associated risk factors in feed and feed ingredients in Rwanda. Toxins 2019, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, O.Z.; Indabo, S.S.; Ahmed, H.O.; Aliyu, A.; Muhammad, H.U.; Sakariyahu, S.K.; Aliyu, R. Surveillance of aflatoxin levels in maize (Zea mays L.) grains sold in some major markets of Kaduna State, Nigeria. Environ. Technol. Sci. J. 2024, 15, 14–22. [Google Scholar] [CrossRef]
- Jalel, F.; Berhan, T.; Ulfina, G.; Kefena, E. Feed Quality, Prevalence of Aflatoxin Contamination in Dairy Feed and Raw Milk in Oromia Special Zone Surrounding Finfinne, Ethiopia. Asian J. Dairy. Food Res. 2021, 41, 8–14. [Google Scholar] [CrossRef]
- Natalia, A.-M.; Jose, H.-P.; Ana, G.-C.; Laura, G.-G. Aflatoxins in animal feeds: A straightforward and cost-effective analytical method. Food Control 2015, 54, 74–78. [Google Scholar] [CrossRef]
- Vickers, M.; Pick, S. Mycotoxin Contamination in Animal Feed and Forages; Agriculture & Horticulture Development Board (AHDB): Warwickshire, UK, 2016. [Google Scholar]
- Dawit, G.; Szonyi, B.; Azage, T.; Hanson, J.; Grace, D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control 2016, 59, 773–779. [Google Scholar]
- Nkosi, M.M.; Safalaoh, A.C.L.; Mtegha, C. Prevalence and contamination levels of aflatoxins in dairy cattle feeds from milk bulking groups in Malawi. Afr. J. Rural. Dev. 2018, 3, 883–894. [Google Scholar]
- Kang’ethe, E.K.; Gatwiri, M.; Sirma, A.J.; Ouko, E.O.; Mburugu-Musoti, C.K.; Kitala, P.M.; Nduhiu, G.J.; Nderitu, J.G.; Mungatu, J.K.; Hietaniemi, V.; et al. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Qual. Saf. 2017, 1, 131–137. [Google Scholar] [CrossRef]
- Changwa, R.; Abia, W.; Msagati, T.; Nyoni, H.; Ndleve, K.; Njobeh, P. Multi-mycotoxin occurrence in dairy cattle feeds from the gauteng province of South Africa: A pilot study using UHPLC-QTOF-MS/MS. Toxins 2018, 10, 294. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the middle East and Africa. Food Addit. Contam. Part B 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Firew, T.M.; Birhan, A.A.; Kassahun, T.; Nie, C.; Wang, G.; Liu, Y. Mycotoxins in Ethiopia: A Review on Prevalence, Economic and Health Impacts. Toxins 2020, 12, 648. [Google Scholar] [CrossRef]
- Anja, A. Review on the Impact of Aflatoxine in Dairy Industry: Occurrence and Control the Case of Ethiopia. Food Sci. Qual. Manag. 2016, 50, 56–64. Available online: https://www.iiste.org/Journals/index.php/FSQM/article/view/29915 (accessed on 12 September 2024).
- Dawit, G.; Szonyi, B.; Azage, T.; Hanson, J.; Grace, D. Feed Storage Practices and Aflatoxin Contamination of Dairy Feeds in the Greater Addis Ababa Milk Shed, Ethiopia; ILRI: International Livestock Research Institute (ILRI): Nairobi, Kenya, 2015. [Google Scholar]
- Rehrahie, M.; Getnet, A.; Fassil, A. Determination of aflatoxin in dairy feeds and milk in some selected areas of Ethiopia. Food Environ. Saf. 2018, XVII, 286–299. Available online: http://fens.usv.ro/index.php/FENS/article/view/597 (accessed on 9 November 2023).
- Yoseph, C.M.; Tadesse, S.K.; Fanos, T.W. Burden of Aflatoxin in Poultry Feeds in Selected Chicken Rearing Villages of Bishoftu-Ethiopia. Res. Sq. 2016, 2, 1. [Google Scholar] [CrossRef]
- Melaku, M. Urban and Peri-Urban Dairy Cattle Production in Ethiopia: A Review. Online J. Anim. Feed. Res. 2019, 9, 173–177. [Google Scholar]
- CSA (Central Statistical Authority). Federal Democratic Republic of Ethiopia Central Statistical Authority. Population Projections for Ethiopia 2007–2037. Cent. Stat. Agency 2013, 188, 1. Available online: http://www.csa.gov.et/census-report/population-projections/category/368-population-projection-2007-2037 (accessed on 13 March 2024).
- Asheber, T.; Baudronb, F.; Wegary, D. Comparative Performance of Five Maize Varieties as Livestock Feed in the Rift Valley of Ethiopia. Acad. Res. J. Agric. Sci. Res. 2017, 5, 366–379. [Google Scholar] [CrossRef]
- Dejene, T.; Lemma, F.; Fekede, F.; Kitaw, G.; Wondatir, Z. Effect of Total Mixed Ration on Dry Matter Intake, Milk Yield and Composition of Early Lactating Jersey Cows. J. Biol. Agric. Healthc. 2019, 7, 19–24. Available online: https://www.researchgate.net/publication/330448605 (accessed on 12 April 2024).
- Zeleke, M. Ethiopia’s Livestock Systems: Overview and Areas of Inquiry; Feed the Future Innovation Lab for Livestock Systems: Gainesville, FL, USA, 2021; Available online: https://www.taylorfrancis.com/books/9781003319665/chapters/10.4324/9781003319665-11 (accessed on 29 February 2024).
- Abdurehman, A.; Yusuf, Y. Milk Production Performance, Challenges and Opportunities of Dairy Cattle Production in West Hararghe, Oromiya Regional State. Open J. Anim. Sci. 2020, 10, 219–235. [Google Scholar] [CrossRef]
- Brandsma, W.; Mengistu, D.; Kassa, B.; Yohannes, M.; van der Lee, J. The Major Ethiopian Milksheds; Wageningen, Livestock Research Report 735; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Mohammed, Y.; De-Waal, H.O. Herd management, milk production and reproduction of urban dairy farms in the Harar milk shed. Ethiop. J. Anim. Prod. 2009, 9, 57–75. Available online: https://www.africabib.org/rec.php?RID=Q00048917 (accessed on 21 December 2023).
- Mulugeta, F. Study on Level of Aflatoxin in Dairy Cattle Feeds and Assess Knowledge, Attitude, and Practice of Feed Producers, Dairy Farmers, and Feed Traders around Addis Ababa. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2017. Available online: https://projectng.com/topic/fo7183/ (accessed on 25 January 2024).
- Yohannes, B.; Wondossen, A.; Anteneh, G. Analysis to Ascertain the Determination for Aflatoxin Contamination of Milk and Feeds from Gurage Zone, Ethiopia. Int. J. Agric. Res. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Genet, M.; Tilahun, B.; Henok, A.; Ashagrie, Z. Level of aflatoxins in dairy feeds, poultry feeds, and feed ingredients produced by feed factories in Addis Ababa, Ethiopia. Mycotoxin Res. 2024, 8, 1–10. [Google Scholar] [CrossRef]
- Rajarajan, P.; Sylvia, K.; Periasamy, M.P.; Subramanian, M. Detection of aflatoxin producing Aspergillus flavus from animal feed in Karnataka, India. Environ. Anal. Health Toxicol. 2021, 36, e2021017. [Google Scholar] [CrossRef]
- Fitalew, T.; Biruk, D.; Alebachew, A.; Habtamu, D.; Zelalem, M.; Argaw, A.; Chalachew, Y. Aflatoxin contamination of animal feeds and its predictors among dairy farms in Northwest Ethiopia: One Health approach implications. Front. Vet. Sci. 2023, 10, 1123573. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Khan, M.Z.; Khan, A.; Hameed, M.R.; Khatoon, A.; Abadin, Z.u.; Hassan, Z.U. Study of fungi and their toxigenic potential isolated from wheat and wheat bran. Toxin Rev. 2017, 36, 80–88. [Google Scholar] [CrossRef]
- Sharma, R.K.; Parisi, S. Aflatoxins in Indian Food Products. In Toxins and Contaminants in Indian Food Products; Sharma, R.K., Parisi, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar]
- Fallah, A.A.; Pirali-Kheirabadi, E.; Rahnama, M.; Saei-Dehkordi, S.S.; Pirali-Kheirabadi, K. Mycoflora, aflatoxigenic strains of Aspergillus section Flavi and aflatoxins in fish feed. Qual. Assur. Saf. Crops Foods 2014, 6, 419–424. [Google Scholar] [CrossRef]
- Matumba, L.; Sulyok, M.; Njoroge, S.M.C.; Ediage, E.N.; Poucke, C.; Saeger, S.D.; Krska, R. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnuts from Malawi. Mycotoxin Res. 2015, 31, 57–62. [Google Scholar] [CrossRef]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 2012, 9, 757–767. [Google Scholar] [CrossRef]
- Sissinto, A.Y.C.; Mintognisse, F.J.P.; Mawuton, A.H.U. Geographic Distribution of Aspergillus Section Flavi Subspecies Isolated from Crops, Foods, and Feedstuffs in Benin. Adv. Microbiol. 2023, 13, 361–372. [Google Scholar] [CrossRef]
- Ráduly, Z.; Szabó, L.; Madar, A.; Pócsi, I.; Csernoch, L. Toxicological and Medical Aspects of Aspergillus-Derived Mycotoxins Entering the Feed and Food Chain. Front. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef]
- Makau, C.M.; Matofari, J.W.; Muliro, P.S.; Bebe, B.O. Aflatoxin B1 and Deoxynivalenol contamination of dairy feeds and presence of Aflatoxin M1 contamination in milk from smallholder dairy systems in Nakuru, Kenya. Int. J. Food Contam. 2016, 3, 6. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Younis, Y.M.H.; Fadul, M.H.; Elhussein, A.M. Determination of aflatoxins in animal feed in Khartoum State, Sudan. J. Anim. Vet. Adv. 2009, 8, 1000–1003. [Google Scholar] [CrossRef]
- Kos, J.; Ani’c, M.; Radi’c, B.; Zadravec, M.; Hajnal, E.J.; Pleadin, J. Climate Change-A Global Threat Resulting in Increasing Mycotoxin Occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Effect of Temperature and Relative Humidity on Growth of Aspergillus and Penicillium spp. and Biocontrol Activity of Pseudomonas protegens AS15 against Aflatoxigenic Aspergillus flavus in Stored Rice Grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef]
- Kim, D.H.; Choi, S.H.; Park, S.K.; Lee, S.S.; Choi, C.W. Effect of corn grain particle size on ruminal fermentation and blood metabolites of Holstein steers fed total mixed ration. Asian-Australas. J. Anim. Sci. 2018, 31, 80–85. [Google Scholar] [CrossRef]
- Shad, Z.; Ghavami, M.; Atungulu, G.G. Occurrence of Aflatoxin in Dairy Cow Feed Ingredients and Total Mixed Ration. Dict. Genom. Transcr. Proteomics. 2015, 35, 679–686. [Google Scholar] [CrossRef]
- Krnjaja, V.; Petrovic, T.; Stankovic, S.; Lukic, M.; Skrbic, Z.; Mandic, V.; Bijelic, Z. Mycobiota and aflatoxin B1 in poultry feeds. Biotechnol. Anim. Husb. 2019, 35, 61–69. [Google Scholar] [CrossRef]
- Omeiza, G.K.; Kabir, J.; Kwaga, J.K.P.; Kwanashie, C.N.; Mwanza, M.; Ngoma, L. A risk assessment study of the occurrence and distribution of aflatoxigenic Aspergillus flavus and aflatoxin B1 in dairy cattle feeds in a central northern state, Nigeria. Toxicol. Rep. 2018, 5, 846–856. [Google Scholar] [CrossRef]
- Ketema, M.; Aman, M.; Seifu, E.; Getachew, T.; Hawaz, E.; Hailu, Y. The dairy value chain and factors affecting choice of milk channels in Harar and Dire Dawa areas, Eastern Ethiopia. Rev. Agric. Appl. Econ. 2016, 19, 10–18. [Google Scholar] [CrossRef]
- Sisay, L.; Dagne, T.; Gashaw, G.; Getu, A. Assessment of Cow’s Milk Hygienic Practices Under Small Scale Farmers in West Hararghe Zone, Oromia National Regional State, Ethiopia. Adv. Life Sci. Technol. 2018, 68, 46–55. Available online: https://www.researchgate.net/publication/376617166 (accessed on 22 December 2023).
- Abibeker, S.A.; Mume, A.A.; Mariye, M.; Desalegn, D.G.; Furgasa, W. Causes of Water Pollution in Chiro River Eastern Oromia, Ethiopia. Int. J. Sci. Res. Publ. 2023, 13, 111–119. [Google Scholar] [CrossRef]
- Arabali, M.; Amare, E.G. A Cross Sectional Study on Prevalence of Cephalopina titillator Infection in Camel (Camelus dromedaries) in Dire Dawa Administrative Region, Ethiopia. Adv. Biol. Res. 2015, 9, 225–229. [Google Scholar] [CrossRef]
- NMA (National Meteorological Agency). Eastern Ethiopian Meteorological Report of 2022; National Meteorological Service Agency of Federal Democratic Republic of Ethiopia: Addis Ababa, Ethiopia, 2022. [Google Scholar]
- Alemayehu, B.; Kibret, K.; Solomon, A.; Dagnachew, L. Analysis of Crop Production Constraints Through Participatory Rural Appraisal in Harari Region, Eastern Ethiopia; Implications for Research and Development. J. Agric. Crops 2019, 5, 209–217. [Google Scholar]
- Tahira, I.; Sultana, N.; Munir, A.; Hasan, S.M.; Hanif, N.Q. Occurrence of Aflatoxin M1 in raw and processed milk consumed in Pakistan. Pak. J. Pharm. Sci. 2019, 32, 1097–1101. [Google Scholar]
- Daniel, W.W.; Cross, C.L. Biostatistics: A Foundation for Analysis in the Health Sciences, 10th ed.; Daniel, W.W., Cross, C.L., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Method of Analysis; Association of Official Analytical Chemistry: Washington, DC, USA, 2002. [Google Scholar]
- Alsalabi, F.A.; Hassan, Z.U.; Al-Thani, R.F.; Jaoua, S. Molecular identification and biocontrol of ochratoxigenic fungi and ochratoxin A in animal feed marketed in the state of Qatar. Heliyon 2023, 9, e12835. [Google Scholar] [CrossRef]
- Álvarez-Días, F.; Torres-Parga, B.; Valdivia-Flores, A.G.; Quezada-Tristán, T.; Alejos-De La Fuente, J.I.; Sosa-Ramírez, J.; Rangel-Muñoz, E.J. Aspergillus flavus and Total Aflatoxins Occurrence in Dairy Feed and Aflatoxin M1 in Bovine Milk in Aguascalientes, Mexico. Toxins 2022, 14, 292. [Google Scholar] [CrossRef]
- Ahmed, M.Z.; Alqahtani, A.S.; Nasr, F.A.; Rehman, M.T.; Abdullah Alsufyani, S.; AlAjmi, M.F.; Alhuzani, M.R. Detection and isolation of aflatoxin producing Aspergillus sp. in chewing and smokeless tobacco by microbial and molecular methods. Saudi J. Biol. Sci. 2023, 103704. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.; Shehata, S.M.; Hisham, S.M.; Alobathani, A.A. Molecular profile of aflatoxigenic and non-aflatoxigenic isolates of Aspergillus flavus isolated from stored maize. Saudi J. Biol. Sci. 2021, 28, 1383–1391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).