An Emerging Way for Bacteria to Engage with Host Cells via Protein ADP-riboxanation
Abstract
:1. Introduction
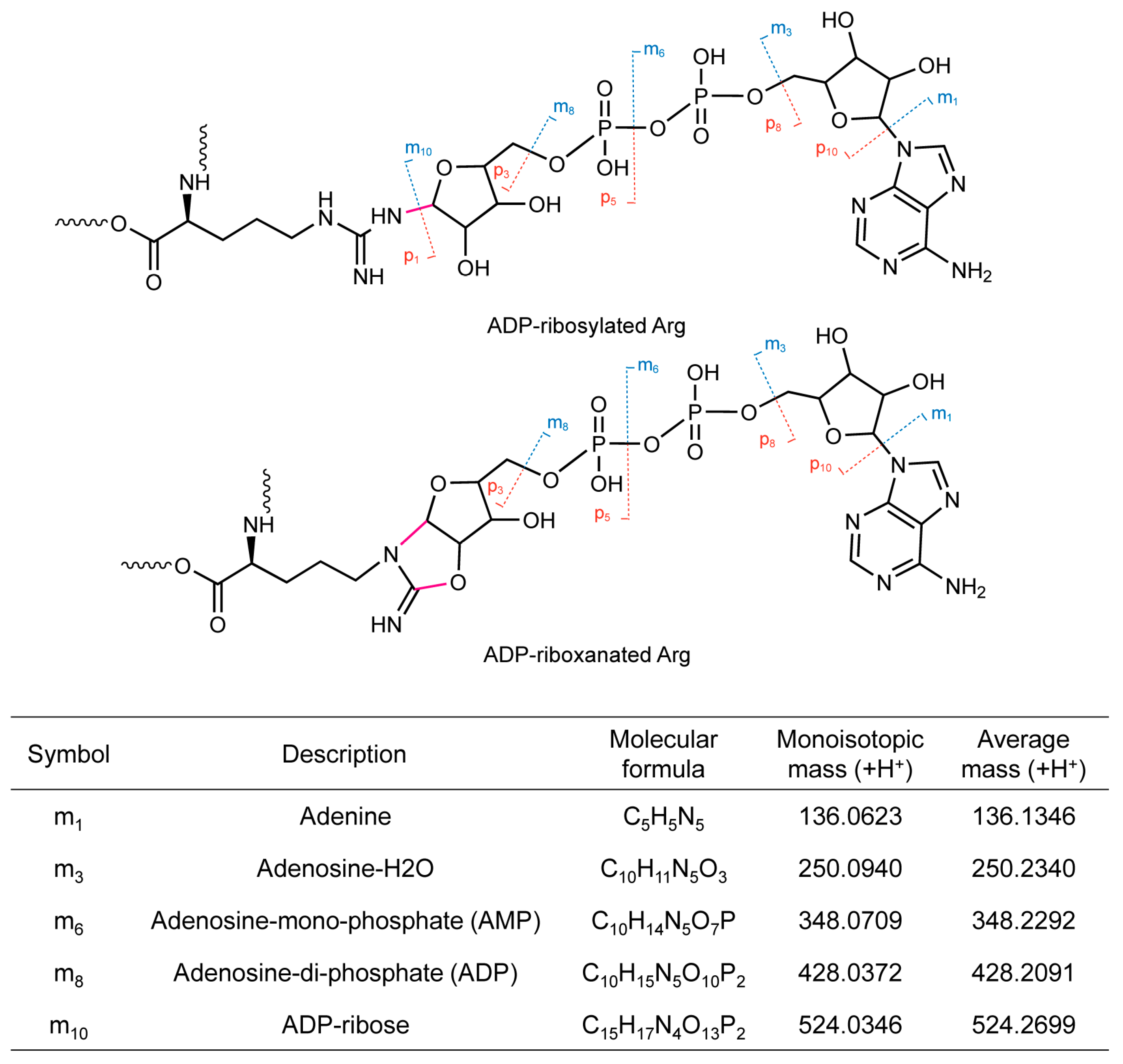
2. Protein ADP-ribosylation and ADP-riboxanation
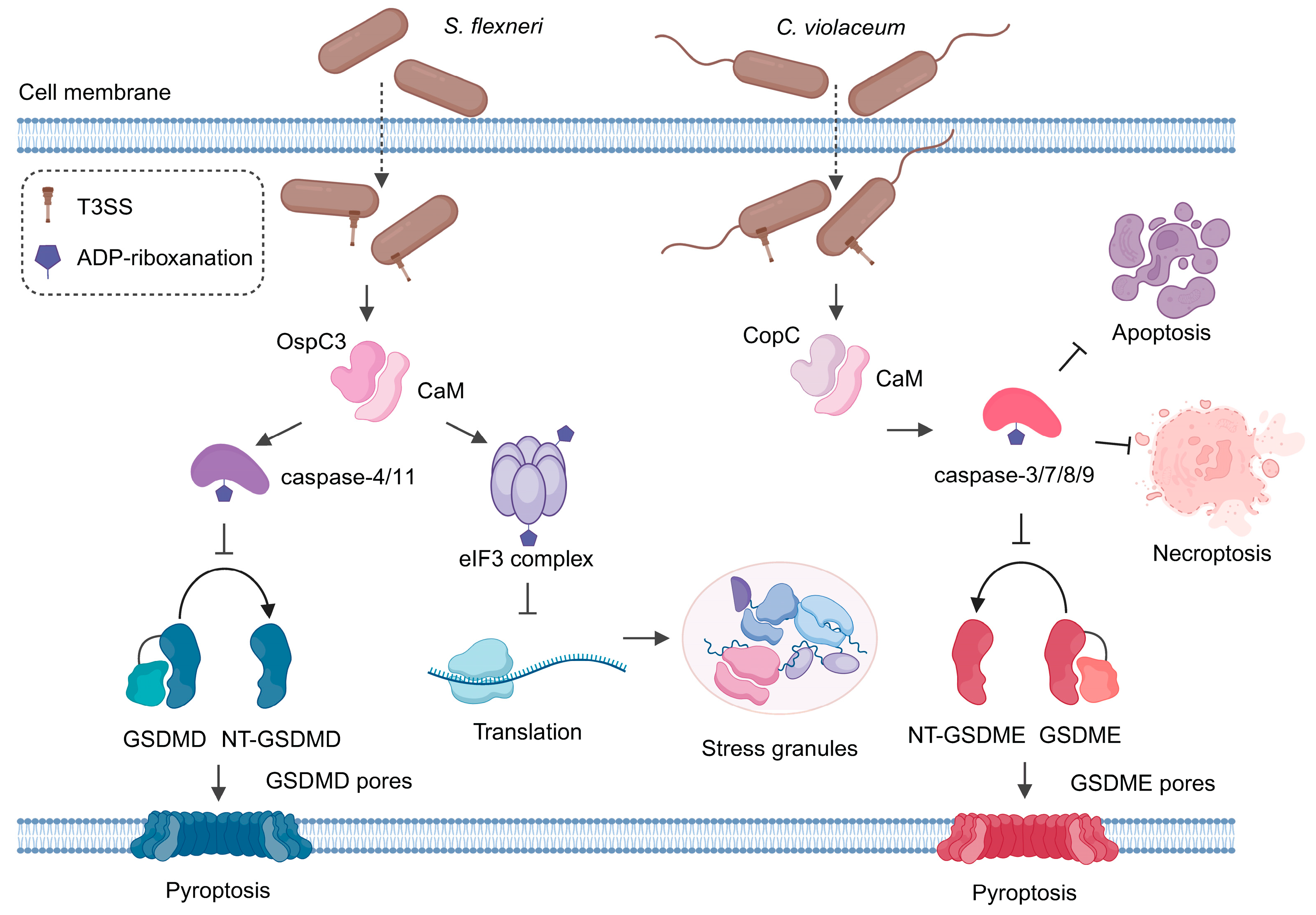
3. OspCs
4. CopC
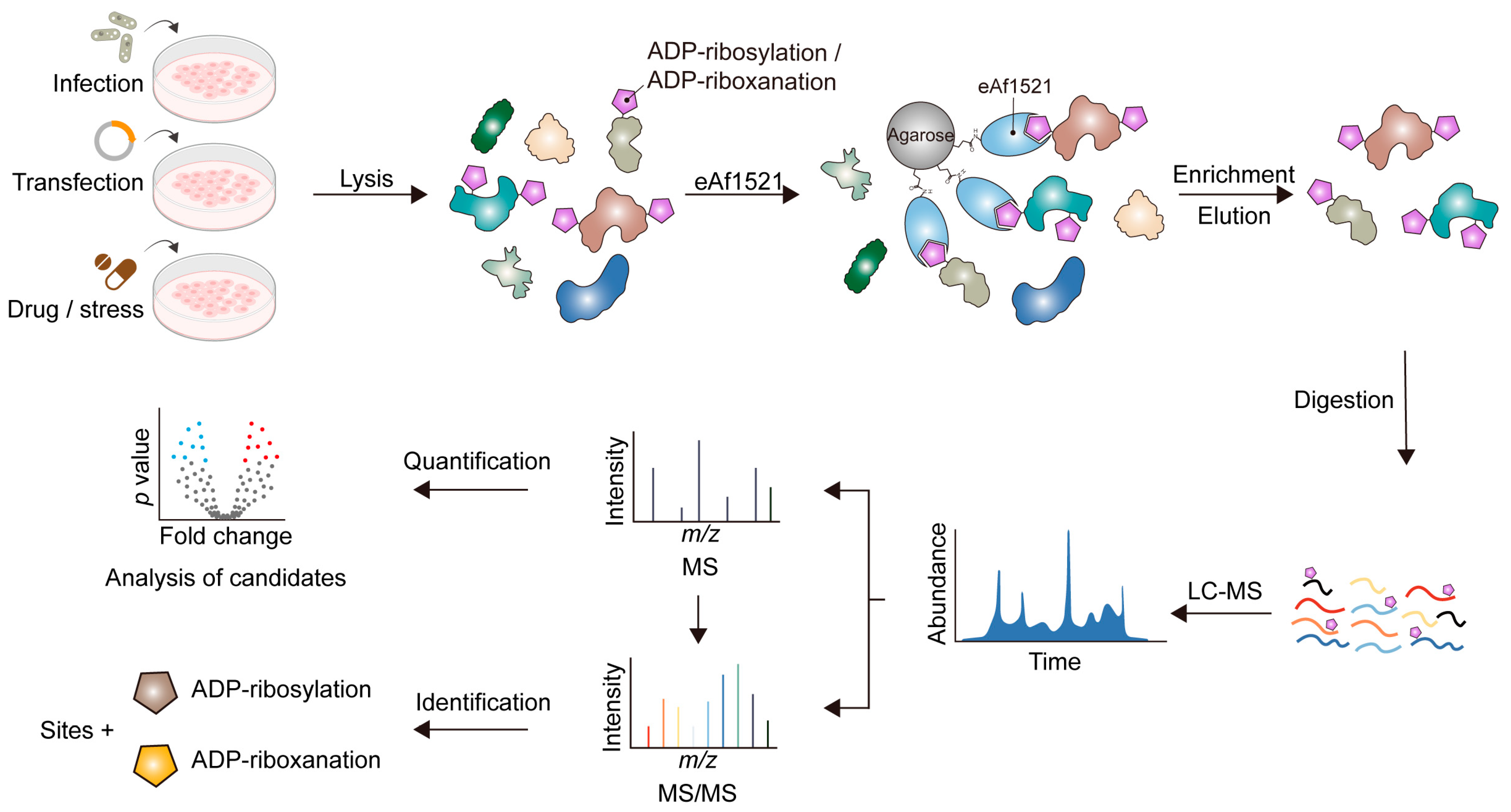
5. Broader Functions of ADP-riboxanation Revealed by Unbiased Proteomics
6. Limitation(s) of Proteomic Profiling During Target Identification
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salomon, D.; Orth, K. What Pathogens Have Taught Us about Posttranslational Modifications. Cell Host Microbe 2013, 14, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Luo, Z.-Q. Post-Translational Regulation of Ubiquitin Signaling. J. Cell Biol. 2019, 218, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.A.; Scheck, R.A. Bacterial Virulence Mediated by Orthogonal Post-Translational Modification. Nat. Chem. Biol. 2020, 16, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Stévenin, V.; Neefjes, J. Control of Host PTMs by Intracellular Bacteria: An Opportunity toward Novel Anti-Infective Agents. Cell Chem. Biol. 2022, 29, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.G.; Franklin, T.G.; Pruneda, J.N. Ubiquitin-Targeted Bacterial Effectors: Rule Breakers of the Ubiquitin System. EMBO J. 2023, 42, e114318. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Huang, C.; Wang, X.; Tan, J.; Cheng, S.; Wan, M.; Wang, Z.; Wang, S.; Luo, S.; Li, A.; et al. Threonine ADP-Ribosylation of Ubiquitin by a Bacterial Effector Family Blocks Host Ubiquitination. Mol. Cell 2020, 78, 641–652.e9. [Google Scholar] [CrossRef]
- Peng, T.; Tao, X.; Xia, Z.; Hu, S.; Xue, J.; Zhu, Q.; Pan, X.; Zhang, Q.; Li, S. Pathogen Hijacks Programmed Cell Death Signaling by Arginine ADPR-Deacylization of Caspases. Mol. Cell 2022, 82, 1806–1820.e8. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Hou, Y.; Li, Z.; Li, L.; Song, X.; Ding, J.; Shao, F.; Xu, Y. Calmodulin Binding Activates Chromobacterium CopC Effector to ADP-Riboxanate Host Apoptotic Caspases. mBio 2022, 13, e0069022. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, X.; Cui, J.; Xu, H.; Chen, J.; Gong, Y.-N.; Hu, L.; Zhou, Y.; Ge, J.; Lu, Q.; et al. Cysteine Methylation Disrupts Ubiquitin-Chain Sensing in NF-κB Activation. Nature 2011, 481, 204–208. [Google Scholar] [CrossRef]
- Zhang, Y.; Mühlen, S.; Oates, C.V.; Pearson, J.S.; Hartland, E.L. Identification of a Distinct Substrate-Binding Domain in the Bacterial Cysteine Methyltransferase Effectors NleE and OspZ. J. Biol. Chem. 2016, 291, 20149–20162. [Google Scholar] [CrossRef]
- Pearson, J.S.; Giogha, C.; Ong, S.Y.; Kennedy, C.L.; Kelly, M.; Robinson, K.S.; Lung, T.W.F.; Mansell, A.; Riedmaier, P.; Oates, C.V.L.; et al. A Type III Effector Antagonizes Death Receptor Signalling during Bacterial Gut Infection. Nature 2013, 501, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, L.; Yao, Q.; Li, L.; Dong, N.; Rong, J.; Gao, W.; Ding, X.; Sun, L.; Chen, X.; et al. Pathogen Blocks Host Death Receptor Signalling by Arginine GlcNAcylation of Death Domains. Nature 2013, 501, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.E.; Giogha, C.; Pollock, G.L.; Kennedy, C.L.; Webb, A.I.; Williamson, N.A.; Pearson, J.S.; Hartland, E.L. The Bacterial Arginine Glycosyltransferase Effector NleB Preferentially Modifies Fas-Associated Death Domain Protein (FADD). J. Biol. Chem. 2017, 292, 17337–17350. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yao, Q.; Li, S.; Ding, X.; Lu, Q.; Mao, H.; Liu, L.; Zheng, N.; Chen, S.; Shao, F. Glutamine Deamidation and Dysfunction of Ubiquitin/NEDD8 Induced by a Bacterial Effector Family. Science 2010, 329, 1215–1218. [Google Scholar] [CrossRef]
- Qiu, J.; Sheedlo, M.J.; Yu, K.; Tan, Y.; Nakayasu, E.S.; Das, C.; Liu, X.; Luo, Z.-Q. Ubiquitination Independent of E1 and E2 Enzymes by Bacterial Effectors. Nature 2016, 533, 120–124. [Google Scholar] [CrossRef]
- Bhogaraju, S.; Kalayil, S.; Liu, Y.; Bonn, F.; Colby, T.; Matic, I.; Dikic, I. Phosphoribosylation of Ubiquitin Promotes Serine Ubiquitination and Impairs Conventional Ubiquitination. Cell 2016, 167, 1636–1649.e13. [Google Scholar] [CrossRef]
- Kotewicz, K.M.; Ramabhadran, V.; Sjoblom, N.; Vogel, J.P.; Haenssler, E.; Zhang, M.; Behringer, J.; Scheck, R.A.; Isberg, R.R. A Single Legionella Effector Catalyzes a Multistep Ubiquitination Pathway to Rearrange Tubular Endoplasmic Reticulum for Replication. Cell Host Microbe 2017, 21, 169–181. [Google Scholar] [CrossRef]
- Wang, T.; Song, X.; Tan, J.; Xian, W.; Zhou, X.; Yu, M.; Wang, X.; Xu, Y.; Wu, T.; Yuan, K.; et al. Legionella Effector LnaB Is a Phosphoryl-AMPylase That Impairs Phosphosignalling. Nature 2024, 631, 393–401. [Google Scholar] [CrossRef]
- Fu, J.; Li, S.; Guan, H.; Li, C.; Zhao, Y.-B.; Chen, T.-T.; Xian, W.; Zhang, Z.; Liu, Y.; Guan, Q.; et al. Legionella Maintains Host Cell Ubiquitin Homeostasis by Effectors with Unique Catalytic Mechanisms. Nat. Commun. 2024, 15, 5953. [Google Scholar] [CrossRef]
- Gan, N.; Nakayasu, E.S.; Hollenbeck, P.J.; Luo, Z.-Q. Legionella Pneumophila Inhibits Immune Signalling via MavC-Mediated Transglutaminase-Induced Ubiquitination of UBE2N. Nat. Microbiol. 2019, 4, 134–143. [Google Scholar] [CrossRef]
- Wan, M.; Wang, X.; Huang, C.; Xu, D.; Wang, Z.; Zhou, Y.; Zhu, Y. A Bacterial Effector Deubiquitinase Specifically Hydrolyses Linear Ubiquitin Chains to Inhibit Host Inflammatory Signalling. Nat. Microbiol. 2019, 4, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Choy, A.; Dancourt, J.; Mugo, B.; O’Connor, T.J.; Isberg, R.R.; Melia, T.J.; Roy, C.R. The Legionella Effector RavZ Inhibits Host Autophagy through Irreversible Atg8 Deconjugation. Science 2012, 338, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, P.; Cheng, S.; Lu, Q.; Nowak, K.; Hopp, A.-K.; Li, L.; Shi, X.; Zhou, Z.; Gao, W.; et al. A Bacterial Effector Reveals the V-ATPase-ATG16L1 Axis That Initiates Xenophagy. Cell 2019, 178, 552–566.e20. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.; Kim, M.; Mimuro, H.; Suzuki, M.; Ogawa, M.; Oyama, A.; Ashida, H.; Kobayashi, T.; Koyama, T.; Nagai, S.; et al. The Shigella Flexneri Effector OspI Deamidates UBC13 to Dampen the Inflammatory Response. Nature 2012, 483, 623–626. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.F.; Liu, Z.; Chen, D.; Alto, N.M. Shigella Flexneri Suppresses NF-κB Activation by Inhibiting Linear Ubiquitin Chain Ligation. Nat. Microbiol. 2016, 1, 16084. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Fu, J.; Cheng, S.; Xu, Y.; Wang, Z.; Liu, X.; Shi, X.; Liu, Y.; Qi, X.; et al. Shigella Evades Pyroptosis by Arginine ADP-Riboxanation of Caspase-11. Nature 2021, 599, 290–295. [Google Scholar] [CrossRef]
- Zhang, Q.; Xian, W.; Li, Z.; Lu, Q.; Chen, X.; Ge, J.; Tang, Z.; Liu, B.; Chen, Z.; Gao, X.; et al. Shigella Induces Stress Granule Formation by ADP-Riboxanation of the eIF3 Complex. Cell Rep. 2024, 43, 113789. [Google Scholar] [CrossRef]
- Xian, W.; Fu, J.; Zhang, Q.; Li, C.; Zhao, Y.-B.; Tang, Z.; Yuan, Y.; Wang, Y.; Zhou, Y.; Brzoic, P.S.; et al. The Shigella Kinase Effector OspG Modulates Host Ubiquitin Signaling to Escape Septin-Cage Entrapment. Nat. Commun. 2024, 15, 3890. [Google Scholar] [CrossRef]
- Li, P.; Jiang, W.; Yu, Q.; Liu, W.; Zhou, P.; Li, J.; Xu, J.; Xu, B.; Wang, F.; Shao, F. Ubiquitination and Degradation of GBPs by a Shigella Effector to Suppress Host Defence. Nature 2017, 551, 378–383. [Google Scholar] [CrossRef]
- Wandel, M.P.; Pathe, C.; Werner, E.I.; Ellison, C.J.; Boyle, K.B.; von der Malsburg, A.; Rohde, J.; Randow, F. GBPs Inhibit Motility of Shigella Flexneri but Are Targeted for Degradation by the Bacterial Ubiquitin Ligase IpaH9.8. Cell Host Microbe 2017, 22, 507–518.e5. [Google Scholar] [CrossRef]
- Ji, C.; Du, S.; Li, P.; Zhu, Q.; Yang, X.; Long, C.; Yu, J.; Shao, F.; Xiao, J. Structural Mechanism for Guanylate-Binding Proteins (GBPs) Targeting by the Shigella E3 Ligase IpaH9.8. PLoS Pathog. 2019, 15, e1007876. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, M.L.; Li, Y.; Kinch, L.N.; Grishin, N.V.; Ball, H.L.; Orth, K. AMPylation of Rho GTPases by Vibrio VopS Disrupts Effector Binding and Downstream Signaling. Science 2009, 323, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and Mortality Due to Shigella and Enterotoxigenic Escherichia Coli Diarrhoea: The Global Burden of Disease Study 1990-2016. Lancet Infect Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Moss, J.; Vaughan, M. Mechanism of Action of Choleragen. Evidence for ADP-Ribosyltransferase Activity with Arginine as an Acceptor. J. Biol. Chem. 1977, 252, 2455–2457. [Google Scholar] [CrossRef]
- Lüscher, B.; Ahel, I.; Altmeyer, M.; Ashworth, A.; Bai, P.; Chang, P.; Cohen, M.; Corda, D.; Dantzer, F.; Daugherty, M.D.; et al. ADP-Ribosyltransferases, an Update on Function and Nomenclature. FEBS J. 2022, 289, 7399–7410. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ogawa, M.; Sanada, T.; Mimuro, H.; Kim, M.; Ashida, H.; Akakura, R.; Yoshida, M.; Kawalec, M.; Reichhart, J.-M.; et al. The Shigella OspC3 Effector Inhibits Caspase-4, Antagonizes Inflammatory Cell Death, and Promotes Epithelial Infection. Cell Host Microbe 2013, 13, 570–583. [Google Scholar] [CrossRef]
- Li, H.; Zhou, F.; Zhang, L. ADP-Riboxanation: A New Pyroptosis Evasion Strategy. J. Mol. Cell Biol. 2022, 14, mjab077. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, H.; Li, Z.; Feng, N.; Meng, F.; Xu, Y.; Li, L.; Shao, F.; Ding, J. Structural Mechanisms of Calmodulin Activation of Shigella Effector OspC3 to ADP-Riboxanate Caspase-4/11 and Block Pyroptosis. Nat. Struct. Mol. Biol. 2023, 30, 261–272. [Google Scholar] [CrossRef]
- Ashida, H.; Sasakawa, C.; Suzuki, T. A Unique Bacterial Tactic to Circumvent the Cell Death Crosstalk Induced by Blockade of Caspase-8. EMBO J. 2020, 39, e104469. [Google Scholar] [CrossRef]
- Alphonse, N.; Wanford, J.J.; Voak, A.A.; Gay, J.; Venkhaya, S.; Burroughs, O.; Mathew, S.; Lee, T.; Evans, S.L.; Zhao, W.; et al. A Family of Conserved Bacterial Virulence Factors Dampens Interferon Responses by Blocking Calcium Signaling. Cell 2022, 185, 2354–2369.e17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Peng, T.; Tao, X.; Tian, M.; Li, Y.; Wang, Z.; Ma, S.; Hu, S.; Pan, X.; Xue, J.; et al. Structural Insights into Caspase ADPR Deacylization Catalyzed by a Bacterial Effector and Host Calmodulin. Mol. Cell 2022, 82, 4712–4726.e7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cheng, S.; Zeng, H.; Zhou, P.; Ma, Y.; Li, L.; Liu, X.; Shao, F.; Ding, J. ARF GTPases Activate Salmonella Effector SopF to ADP-Ribosylate Host V-ATPase and Inhibit Endomembrane Damage-Induced Autophagy. Nat. Struct. Mol. Biol. 2022, 29, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Rosenthal, F.; Karlberg, T.; Bütepage, M.; Thorsell, A.-G.; Dreier, B.; Grossmann, J.; Sobek, J.; Imhof, R.; Lüscher, B.; et al. Engineering Af1521 Improves ADP-Ribose Binding and Identification of ADP-Ribosylated Proteins. Nat. Commun. 2020, 11, 5199. [Google Scholar] [CrossRef] [PubMed]
- Belyi, Y.; Tabakova, I.; Stahl, M.; Aktories, K. Lgt: A Family of Cytotoxic Glucosyltransferases Produced by Legionella Pneumophila. J. Bacteriol. 2008, 190, 3026–3035. [Google Scholar] [CrossRef]
- Belyi, Y.; Niggeweg, R.; Opitz, B.; Vogelsgesang, M.; Hippenstiel, S.; Wilm, M.; Aktories, K. Legionella Pneumophila Glucosyltransferase Inhibits Host Elongation Factor 1A. Proc. Natl. Acad. Sci. USA 2006, 103, 16953–16958. [Google Scholar] [CrossRef]
- Subramanian, A.; Wang, L.; Moss, T.; Voorhies, M.; Sangwan, S.; Stevenson, E.; Pulido, E.H.; Kwok, S.; Chalkley, R.J.; Li, K.H.; et al. A Legionella Toxin Exhibits tRNA Mimicry and Glycosyl Transferase Activity to Target the Translation Machinery and Trigger a Ribotoxic Stress Response. Nat. Cell Biol. 2023, 25, 1600–1615. [Google Scholar] [CrossRef]
- Joseph, A.M.; Shames, S.R. Affecting the Effectors: Regulation of Legionella Pneumophila Effector Function by Metaeffectors. Pathogens 2021, 10, 108. [Google Scholar] [CrossRef]
- Pollock, G.L.; Grishin, A.M.; Giogha, C.; Gan, J.; Oates, C.V.; McMillan, P.J.; Gaeta, I.; Tyska, M.J.; Pearson, J.S.; Scott, N.E.; et al. Targeting of Microvillus Protein Eps8 by the NleH Effector Kinases from Enteropathogenic E. Coli. Proc. Natl. Acad. Sci. USA 2022, 119, e2204332119. [Google Scholar] [CrossRef]
| Bacteria | Effectors | Host Targets | PTMs | Functions | References |
|---|---|---|---|---|---|
| Chromobacterium violaceum | CteC | Ubiquitin | ADP-ribosylation | Blocks ubiquitin signaling | [6] |
| Chromobacterium violaceum | CopC | Caspase-3/7/8/9 | ADP-riboxanation | Blocks cell death | [7,8] |
| Enteropathogenic E. coli | NleE | TAB2, TAB3 | Cysteine methylation | Inhibits host NF-κB signaling | [9,10] |
| Enteropathogenic E. coli | NleB | FAS-associated death domain protein (FADD), TNFR1-associated death domain protein (TRADD) | GlcNAcylation | Inhibits death receptor-induced apoptosis | [11,12,13] |
| Enteropathogenic E. coli | Cif | Ubiquitin-like modifier NEDD8 | Deamidation | Arrests cell cycle | [14] |
| Legionella pneumophila | SidEs | Rab1, Rtn4 | Ubiquitination independent of E1/2 | Interferes with vesicle trafficking and tubular ER | [15,16,17] |
| Legionella pneumophila | LnaB | Phosphoribosyl ubiquitin, Src | Phosphoryl-AMPylation | Activates NF-κB signaling, impairs phosphosignaling | [18,19] |
| Legionella pneumophila | MavC (Lpg2147) | Ubiquitin, UBE2N | Deamidation, transglutaminase-induced ubiquitination | Inhibits host NF-κB signaling | [20] |
| Legionella pneumophila | RavD | Linear ubiquitin chains | Deubiquitination | Inhibits linear ubiquitin chain-mediated signaling (e.g., NF-κB signaling) | [21] |
| Legionella pneumophila | RavZ | Atg8 | Hydrolysis | Blocks autophagy | [22] |
| Salmonella Typhimurium | SopF | ATP6V0C | ADP-ribosylation | Blocks xenophagy | [23] |
| Shigella flexneri | OspI | UBC13 | Deamidation | Inhibits host NF-κB signaling | [24] |
| Shigella flexneri | IpaH1.4, IpaH2.5 | HOIL-1-interacting protein (HOIP) | Ubiquitination | Inhibits host NF-κB signaling | [25] |
| Shigella flexneri | OspCs | Caspase-4/11, eIF3 | ADP-riboxanation | Blocks pyroptosis and protein translation, induces stress granules | [26,27] |
| Shigella flexneri | OspG | Cullin-associated NEDD8-dissociated protein 1 (CAND1) | Phosphorylation | Blocks septin cage assembly | [28] |
| Shigella flexneri | IpaH9.8 | Guanylate-binding proteins (GBPs) | Ubiquitination | Inhibits GBP-mediated immunity | [29,30,31] |
| Vibrio parahaemolyticus | VopS | Rho, Rac, Cdc42 | AMPylation | Inhibits actin assembly | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, W.; Tang, Z.; Zhang, Q.; Wang, Y.; Liu, X. An Emerging Way for Bacteria to Engage with Host Cells via Protein ADP-riboxanation. Toxins 2024, 16, 467. https://doi.org/10.3390/toxins16110467
Xian W, Tang Z, Zhang Q, Wang Y, Liu X. An Emerging Way for Bacteria to Engage with Host Cells via Protein ADP-riboxanation. Toxins. 2024; 16(11):467. https://doi.org/10.3390/toxins16110467
Chicago/Turabian StyleXian, Wei, Zhiheng Tang, Qinxin Zhang, Ying Wang, and Xiaoyun Liu. 2024. "An Emerging Way for Bacteria to Engage with Host Cells via Protein ADP-riboxanation" Toxins 16, no. 11: 467. https://doi.org/10.3390/toxins16110467
APA StyleXian, W., Tang, Z., Zhang, Q., Wang, Y., & Liu, X. (2024). An Emerging Way for Bacteria to Engage with Host Cells via Protein ADP-riboxanation. Toxins, 16(11), 467. https://doi.org/10.3390/toxins16110467





