Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor
Abstract
1. Introduction
2. Results
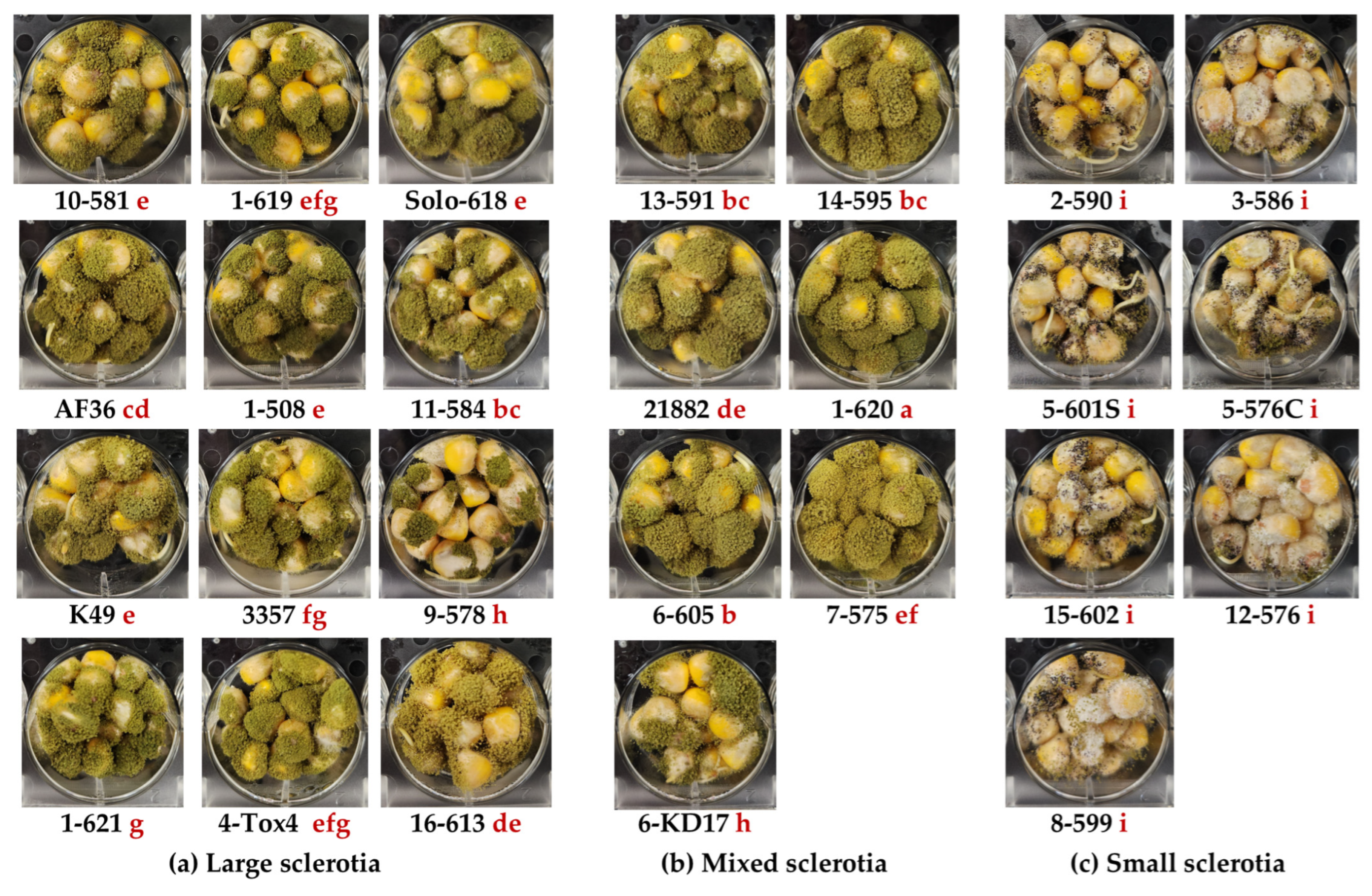
2.1. Isolate Selection
2.2. Kojic Acid Production in Liquid Medium
2.3. Kojic Acid Production by A. flavus During Kernel Infection
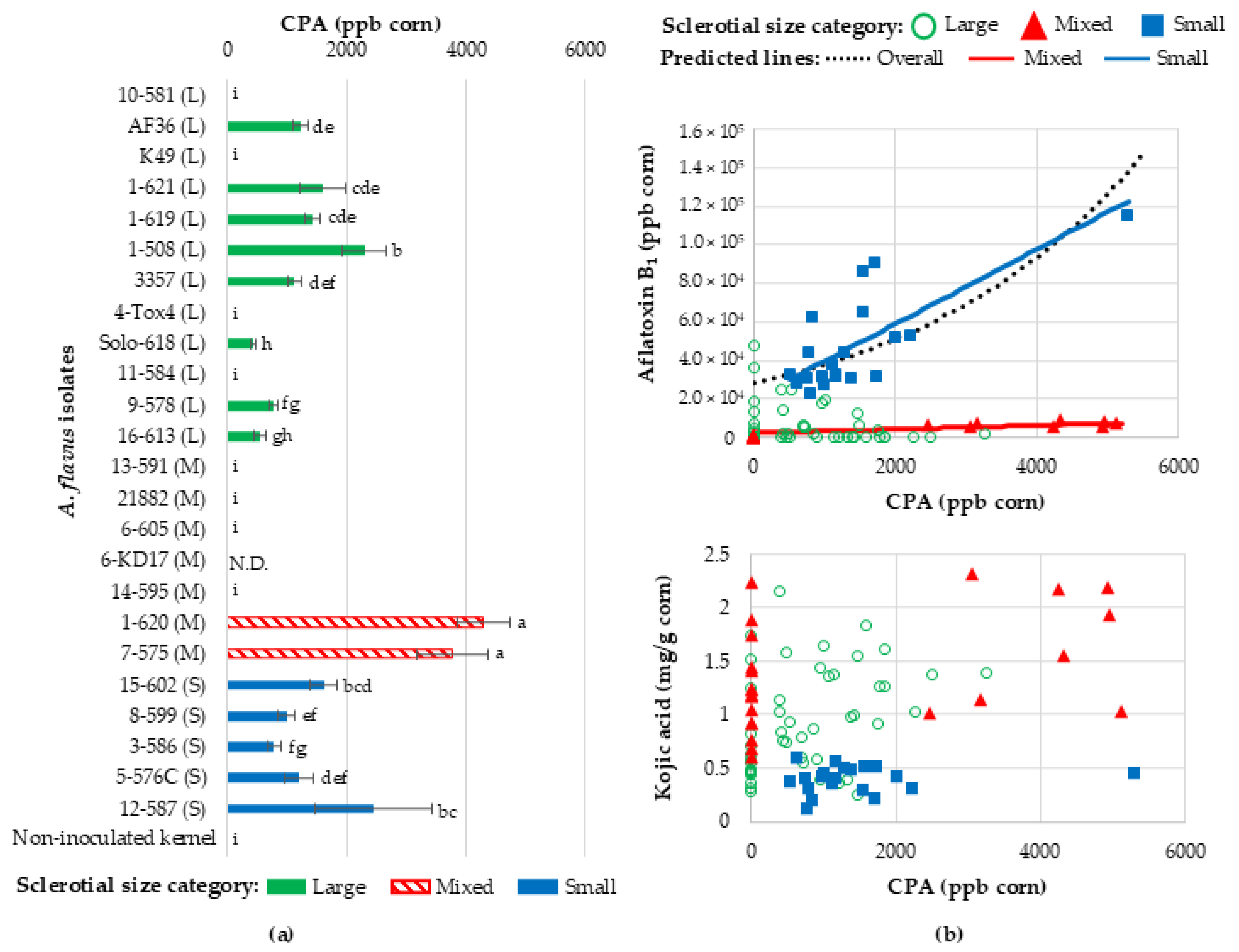
2.4. Aflatoxin B1 Production
Lack of Relationship Between Kojic Acid and Aflatoxin Production
2.5. Cyclopiazonic Acid Production by A. flavus During Corn Kernel Infection
2.6. Conidiospore Production on Corn Kernels
Limited Relationship Between Kojic Acid and Spore Production
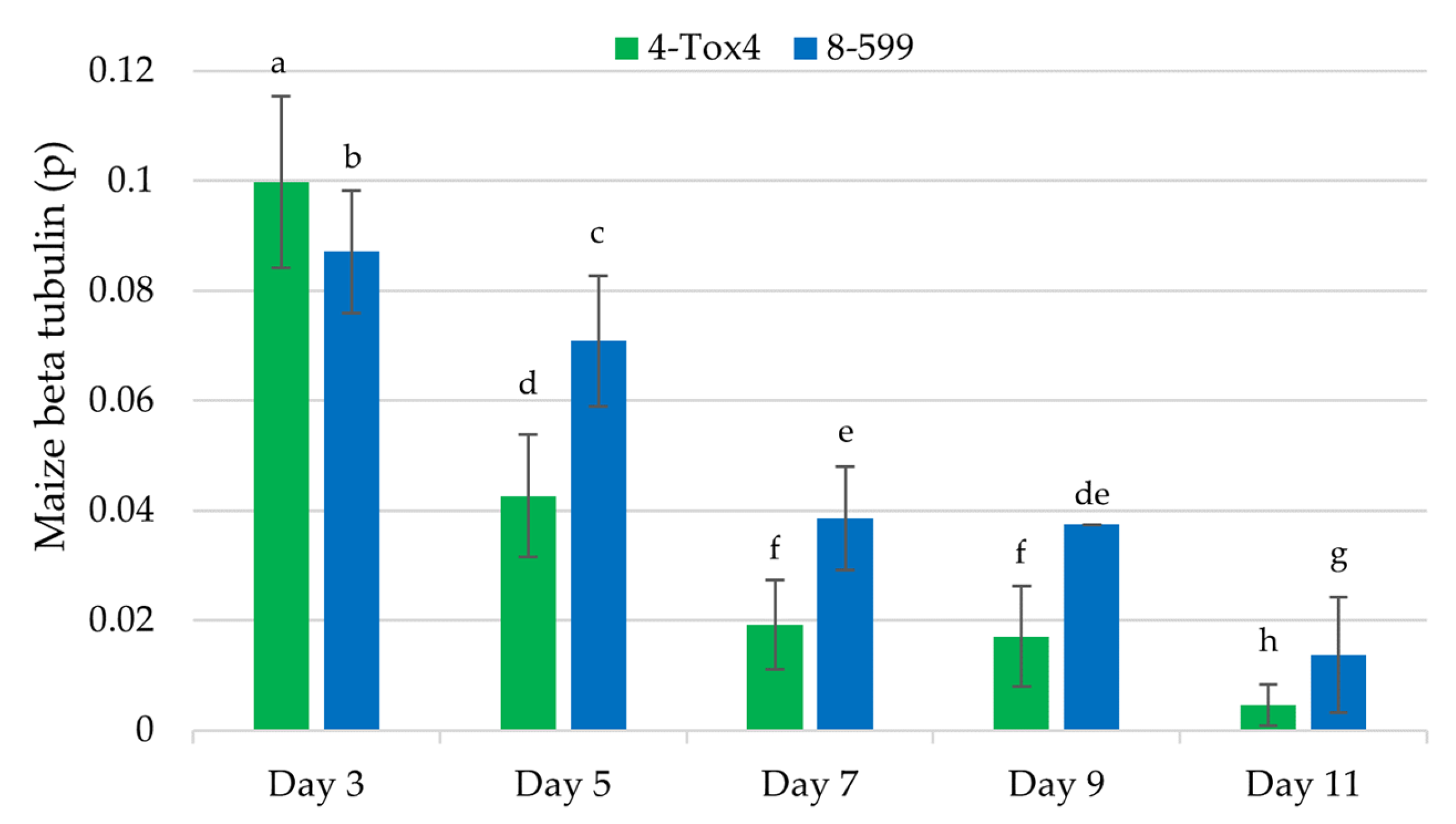
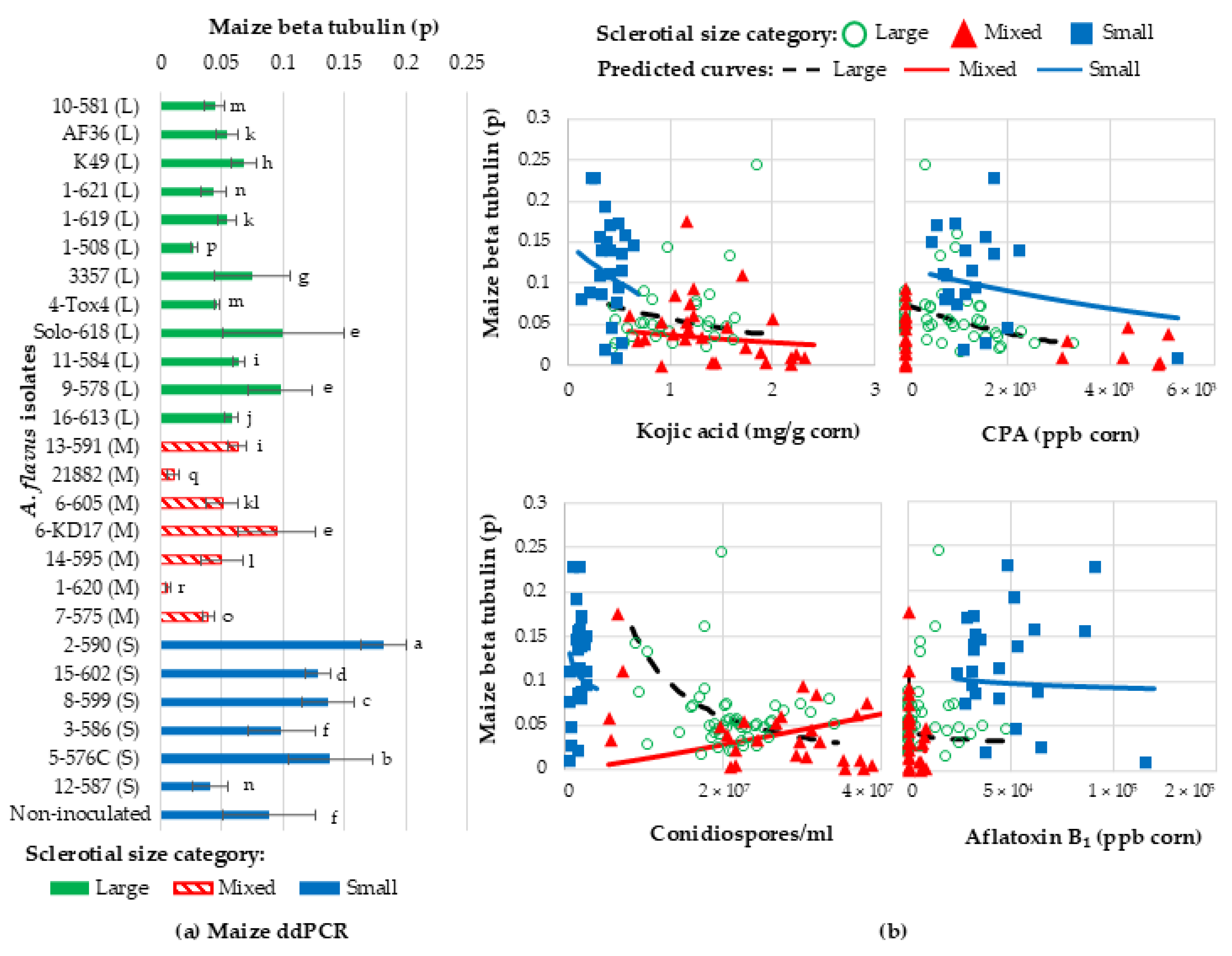
2.7. Quantification of Corn Genomic DNA to Determine the Rate of Kernel Infection by A. flavus
Relating Kojic Acid, CPA, Aflatoxin, and Spore Production with Kernel Infection
3. Discussion
3.1. Kojic Acid Production Is Associated with Greater Corn Colonization
3.2. Kojic Acid Production Among Biocontrol Strains
3.3. Kojic Acid Chemical Properties May Contribute to Virulence on Corn Kernels
3.4. M-Strains Gain Inoculum Potential on Corn Kernels
4. Conclusions
5. Materials and Methods
5.1. Fungal Strains/Isolates
5.2. Growth Conditions for Liquid Medium Experiments
5.3. Growth Conditions for Corn Kernel Experiments
5.4. Kojic Acid Analysis
5.5. Aflatoxin and Cyclopiazonic Acid Analysis
5.6. DNA Analysis Using Droplet Digital PCR
5.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richard, J.L. Discovery of aflatoxins and significant historical features. Toxin Rev. 2008, 27, 171–201. [Google Scholar] [CrossRef]
- Yu, J.; Hennessy, D.A.; Tack, J.; Wu, F. Climate change will increase aflatoxin presence in US corn. Environ. Res. Lett. 2022, 17, 054017. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.L.; Adhikari, B.N.; Cotty, P.J. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016, 9, 771–789. [Google Scholar] [CrossRef]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present status and perspective on the future use of Aflatoxin Biocontrol products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Cotty, P.J.; Antilla, L.; Wakelyn, P.J. Competitive exclusion of aflatoxin producers: Farmer-driven research and development. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovits, G., Eds.; CAB International: Wallingford, UK, 2007; pp. 241–253. [Google Scholar]
- Dorner, J.W.; Cole, R.J.; Connick, W.J.; Daigle, D.J.; McGuire, M.R.; Shasha, B.S. Evaluation of biological control formulations to reduce aflatoxin contamination in peanuts. Biol. Control 2003, 26, 318–324. [Google Scholar] [CrossRef]
- Weaver, M.A.; Abbas, H.K. Field displacement of aflatoxigenic Aspergillus flavus strains through repeated biological control applications. Front. Microbiol. 2019, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, K.; Sayler, R.J.; Sickler, C.M.; Majumdar, R.; Jaynes, J.M.; Cary, J.W. Control of Aspergillus flavus growth and aflatoxin production in transgenic maize kernels expressing a tachyplesin-derived synthetic peptide, AGM182. Plant Sci. 2018, 270, 150–156. [Google Scholar] [CrossRef]
- Gilbert, M.K.; Majumdar, R.; Rajasekaran, K.; Chen, Z.-Y.; Wei, Q.; Sickler, C.M.; Lebar, M.D.; Cary, J.W.; Frame, B.R.; Wang, K. RNA interference-based silencing of the alpha-amylase (amy1) gene in Aspergillus flavus decreases fungal growth and aflatoxin production in maize kernels. Planta 2018, 247, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Han, Z.Q.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Host induced gene silencing targeting Aspergillus flavus aflM reduced aflatoxin contamination in transgenic maize under field conditions. Front. Microbiol. 2020, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Raruang, Y.; Omolehin, O.; Hu, D.; Wei, Q.; Promyou, S.; Parakattil, L.J.; Rajasekaran, K.; Cary, J.W.; Wang, K.; Chen, Z.-Y. Targeting the Aspergillus flavus p2c gene through host-induced gene silencing reduces A. flavus infection and aflatoxin contamination in transgenic maize. Front. Plant Sci. 2023, 14, 1150086. [Google Scholar] [CrossRef]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W.; Moore, G.G.; Carbone, I. Sexual reproduction in Aspergillus flavus. Mycologia 2009, 101, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Weaver, M.A.; Zablotowicz, R.M.; Horn, B.W.; Shier, W.T. Relationships between aflatoxin production and sclerotia formation among isolates of Aspergillus section Flavi from the Mississippi Delta. Eur. J. Plant Pathol. 2005, 112, 283–287. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Islam, M.-S.; Callicott, K.A.; Cotty, P.J.; Bandyopadhyay, R. Potential of atoxigenic Aspergillus flavus vegetative compatibility groups associated with maize and groundnut in Ghana as biocontrol agents for aflatoxin management. Front. Microbiol. 2019, 10, 2069. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from Maize kernels in three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Camiletti, B.X.; Moral, J.; Asensio, C.M.; Torrico, A.K.; Lucini, E.I.; Giménez-Pecci, M.P.; Michailides, T.J. Characterization of Argentinian endemic Aspergillus flavus isolates and their potential use as biocontrol agents for mycotoxins in maize. Phytopathology 2018, 108, 818–828. [Google Scholar] [CrossRef]
- Cotty, P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 1989, 79, 808–814. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Battilani, P.; Callicott, K.A.; Giorni, P.; Pietri, A.; Cotty, P.J. Structure of an Aspergillus flavus population from maize kernels in northern Italy. Int. J. Food Microbiol. 2013, 162, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Novas, M.V.; Cabral, D. Association of mycotoxin and sclerotia production with compatibility groups in Aspergillus flavus from peanut in Argentina. Plant Dis. 2002, 86, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Pildain, M.B.; Vaamonde, G.; Cabral, D. Analysis of population structure of Aspergillus flavus from peanut based on vegetative compatibility, geographic origin, mycotoxin and sclerotia production. Int. J. Food Microbiol. 2004, 93, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sweany, R.R.; Damann, K.E., Jr.; Kaller, M.D. Comparison of soil and corn kernel Aspergillus flavus populations: Evidence for niche specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Vaamonde, G.; Patriarca, A.; Fernández Pinto, V.; Comerio, R.; Degrossi, C. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section flavi from different substrates in Argentina. Int. J. Food Microbiol. 2003, 88, 79–84. [Google Scholar] [CrossRef]
- Chang, P.-K.; Abbas, H.K.; Weaver, M.A.; Ehrlich, K.C.; Scharfenstein, L.L.; Cotty, P.J. Identification of genetic defects in the atoxigenic biocontrol strain Aspergillus flavus K49 reveals the presence of a competitive recombinant group in field populations. Int. J. Food Microbiol. 2012, 154, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Horn, B.W.; Dorner, J.W. Sequence breakpoints in the Aflatoxin Biosynthesis gene cluster and flanking regions in nonaflatoxigenic Aspergillus flavus isolates. Fungal Genet. Biol. 2005, 42, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Cotty, P.J. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase gene. Appl. Microbiol. Biotechnol. 2004, 65, 473–478. [Google Scholar] [CrossRef]
- Sweany, R.R.; Mack, B.M.; Gebru, S.T.; Mammel, M.K.; Cary, J.W.; Moore, G.G. Divergent Aspergillus flavus corn population is composed of prolific conidium producers: Implications for saprophytic disease cycle. Mycologia 2024, 116, 536–575. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Callicott, K.A.; Cotty, P.J. Deadly strains of Kenyan Aspergillus are distinct from other aflatoxin producers. Eur. J. Plant Pathol. 2012, 132, 419–429. [Google Scholar] [CrossRef]
- Geiser, D.M.; Pitt, J.I.; Taylor, J.W. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 1998, 95, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Drott, M.T.; Fessler, L.M.; Milgroom, M.G. Population subdivision and the frequency of aflatoxigenic isolates in Aspergillus flavus in the United States. Phytopathology 2019, 109, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Drott, M.T.; Satterlee, T.R.; Skerker, J.M.; Pfannenstiel, B.T.; Glass, N.L.; Keller, N.P.; Milgroom, M.G. The frequency of sex: Population genomics reveals differences in recombination and population structure of the aflatoxin-producing fungus Aspergillus flavus. mBio 2020, 11, e00963-20. [Google Scholar] [CrossRef]
- Weaver, M.A.; Callicott, K.A.; Mehl, H.L.; Opoku, J.; Park, L.C.; Fields, K.S.; Mandel, J.R. Characterization of the Aspergillus flavus population from highly aflatoxin-contaminated corn in the United States. Toxins 2022, 14, e755. [Google Scholar] [CrossRef] [PubMed]
- Bayman, P.; Cotty, P.J. Vegetative compatibility and genetic diversity in the Aspergillus flavus population of a single field. Can. J. Botechnol. 1991, 69, 1707–1711. [Google Scholar] [CrossRef]
- Molo, M.S.; White, J.B.; Cornish, V.; Gell, R.M.; Baars, O.; Singh, R.; Carbone, M.A.; Isakeit, T.; Wise, K.A.; Woloshuk, C.P.; et al. Asymmetrical lineage introgression and recombination in populations of Aspergillus flavus: Implications for biological control. PLoS ONE 2022, 17, e0276556. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Cotty, P.J. Frequent shifts in Aspergillus flavus populations associated with maize production in Sonora, Mexico. Phytopathology 2018, 108, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wei, C.-I.; Marshall, M.R. Inhibition mechanism of kojic acid on polyphenol oxidase. J. Agric. Food Chem. 1991, 39, 1897–1901. [Google Scholar] [CrossRef]
- Murakami, Y. Complexing behaviour of kojic acid with metal ions—II: Fe(III) chelates. J. Inorg. Nucl. Chem. 1962, 39, 679–688. [Google Scholar] [CrossRef]
- Watarai, N.; Yamamoto, N.; Sawada, K.; Yamada, T. Evolution of Aspergillus oryzae before and after domestication inferred by large-scale comparative genomic analysis. DNA Res. 2019, 26, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Scharfenstein, L.L.; Mahoney, N.; Kong, Q. Kojic acid gene clusters and the transcriptional activation mechanism of Aspergillus flavus KojR on expression of clustered genes. J. Fungi 2023, 9, 259. [Google Scholar] [CrossRef]
- He, M.; Zhang, J.; Li, N.; Chen, L.; He, Y.; Peng, Z.; Wang, G. Synthesis, anti-browning effect and mechanism research of kojic acid-coumarin derivatives as anti-tyrosinase inhibitors. Food Chem. 2024, 21, 101128. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Son, S.M.; Moon, K.D.; Lee, C.Y. Inhibitory effects of various anti browning agents on apple slices. Food Chem. 2001, 73, 23–30. [Google Scholar] [CrossRef]
- Terabayashi, Y.; Sano, M.; Yamane, N.; Marui, J.; Tamano, K.; Higa, Y.; Ohashi, S.; Koike, H.; Machida, M. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 953–961. [Google Scholar] [CrossRef]
- Sweany, R.R.; Mack, B.M.; Moore, G.G.; Gilbert, M.K.; Cary, J.W.; Lebar, M.D.; Rajasekaran, K.; Damann, K.E., Jr. Genetic responses and aflatoxin inhibition during co-culture of aflatoxigenic and non-aflatoxigenic Aspergillus flavus. Toxins 2021, 13, 794. [Google Scholar] [CrossRef]
- Baidya, S.; Duran, R.M.; Lohmar, J.M.; Harris-Coward, P.Y.; Cary, J.W.; Hong, S.-Y.; Roze, L.V.; Linz, J.E.; Calvo, A.M. VeA is associated with the response to oxidative stress in the aflatoxin producer Aspergillus flavus. Eukaryot. Cell 2014, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Fountain, J.C.; Scully, B.T.; Chen, Z.-Y.; Gold, S.E.; Glenn, A.E.; Abbas, H.K.; Lee, R.D.; Kemerait, R.C.; Guo, B. Effects of hydrogen peroxide on different toxigenic and atoxigenic isolates of Aspergillus flavus. Toxins 2015, 7, 2985–2999. [Google Scholar] [CrossRef]
- Zhang, N.; NurAinIzzati, M.Z.; Scher, K.; Condon, B.J.; Horwitz, B.A.; Turgeon, B.G. Iron, oxidative stress, and virulence: Roles of iron-sensitive transcription factor Sre1 and the redox sensor ChAp1 in the maize pathogen Cochliobolus heterostrophus. Mol. Plant-Microbe Interact. 2013, 26, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; Battilani, P.; Marocco, A. Infection with toxigenic and atoxigenic strains of Aspergillus flavus induces different transcriptional signatures in maize kernels. J. Plant Interact. 2017, 12, 21–30. [Google Scholar]
- Chalivendra, S.C.; DeRobertis, C.; Chang, P.-K.; Damann, K.E. Cyclopiazonic acid is a pathogenicity factor for Aspergillus flavus and a promising target for screening germplasm for ear rot resistance. Mol. Plant-Microbe Interact. 2017, 30, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Prado, J.H.; Moore, G.G.; Horn, B.W.; Carbone, I. Characterization and population analysis of the mating-type genes in Aspergillus flavus and Aspergillus parasiticus. Fungal Genet. Biol. 2008, 45, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Uka, V.; Cary, J.W.; Lebar, M.D.; Puel, O.; De Saeger, S.; Diana Di Mavungu, J. Chemical repertoire and biosynthetic machinery of the Aspergillus flavus secondary metabolome: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2797–2842. [Google Scholar] [CrossRef]
- Hruska, Z.; Yao, H.; Kincaid, R.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Temporal effects on internal fluorescence emissions associated with aflatoxin contamination from corn kernel cross-sections inoculated with toxigenic and atoxigenic Aspergillus flavus. Front. Microbiol. 2017, 8, 1718. [Google Scholar] [CrossRef]
- Kaklan, H.; Güneş, A.; Durmuş, E.; Kuşcu, A. Non-invasive detection of aflatoxin-contaminated figs using fluorescence and multispectral imaging. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1414–1421. [Google Scholar]
- Sweany, R.R.; DeRobertis, C.D.; Kaller, M.D.; Damman, K.E., Jr. Intraspecific growth and aflatoxin inhibition responses to atoxigenic Aspergillus flavus: Evidence of secreted, inhibitory substances in biocontrol. Phytopathology 2022, 112, 2084–2098. [Google Scholar] [CrossRef]
- Moore, G.G.; Lebar, M.D.; Sweany, R.R.; Lohmar, J.M.; Carter-Wientjes, C.H. Production of inhibitory extrolites is a shared trait among non-aflatoxigenic Aspergillus flavus. J. Appl. Microbiol. 2024; submitted. [Google Scholar]
- Uka, V.; Moore, G.G.; Arroyo-Manzanares, N.; Nebija, D.; De Saeger, S.; Diana Di Mavungu, J. Secondary metabolite dereplication and phylogenetic analysis identify various emerging mycotoxins and reveal the high intra-species diversity in Aspergillus flavus. Front. Microbiol. 2019, 10, e435411. [Google Scholar] [CrossRef]
- Mehl, H.L.; Cotty, P.J. Influence of plant host species on intraspecific competition during infection by Aspergillus flavus. Plant Pathol. 2013, 62, 1195–1428. [Google Scholar] [CrossRef]
- Probst, C.; Cotty, P.J. Relationships between in vivo and in vitro aflatoxin production: Reliable prediction of fungal ability to contaminate maize with aflatoxins. Fungal Biol. 2012, 116, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jha, A.; Sweany, R.; DeRobertis, C.; Damann, K.E., Jr. Intraspecific aflatoxin inhibition in Aspergillus flavus is thigmoregulated, independent of vegetative compatibility group and is strain dependent. PLoS ONE 2011, 6, e23470. [Google Scholar] [CrossRef] [PubMed]
- Chanda, A.; Roze, L.V.; Linz, J.E. A possible role for exocytosis in aflatoxin export in A. parasiticus. Eukaryote Cell 2010, 9, 1724–1727. [Google Scholar] [CrossRef]
- Zhang, J.D.; Han, L.; Yan, S.; Liu, C.M. The non-metabolizable glucose analog D-glucal inhibits Aflatoxin Biosynthesis and promotes kojic acid production in Aspergillus flavus. BMC Microbiol. 2014, 14, 95. [Google Scholar] [CrossRef]
- Dao, T.T.; de Mattos-Shipley, K.M.J.; Prosser, I.M.; Williams, K.; Zacharova, M.K.; Lazarus, C.M.; Willis, C.L.; Bailey, A.M. Cleaning the cellular Factory–Deletion of McrA in Aspergillus oryzae NSAR1 and the generation of a novel kojic acid deficient strain for cleaner heterologous production of secondary metabolites. Front. Fungal Biol. 2021, 2, 632542. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops; biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X. Recent advances in polyphenol oxidase-mediated plant stress responses. Phytochemistry 2021, 181, 112588. [Google Scholar] [CrossRef]
- Chang, P.-K.; Horn, B.W.; Dorner, J.W. Clustered genes involved in cyclopiazonic acid production are next to the Aflatoxin Biosynthesis gene cluster in Aspergillus flavus. Fungal Genet. Biol. 2009, 46, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. Cumulative Effects of Non-Aflatoxigenic Aspergillus flavus Volatile Organic Compounds to Abate Toxin Production by Mycotoxigenic Aspergilli. Toxins 2022, 14, 340. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E., Jr. Influence of neighboring clonal-colonies on aflatoxin production by Aspergillus flavus. Front. Microbiol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Aime, M.C.; Phillips-Mora, W. The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia 2005, 97, 1012–1022. [Google Scholar] [PubMed]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons: Practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef] [PubMed]
- Matthijs Vynck, M.; Vandesompele, J.; Nijs, N.; Menten, B.; De Ganck, A.; Olivier Thas, O. Flexible analysis of digital PCR experiments using generalized linear mixed models. Biomol. Detect. Quantif. 2016, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]


| Study Names 1 | Isolate 2 | SRRC 3 | Parish 4 | Source 5 | VCG 6 | Sclerotia 7 | MAT 8 | AFB1 9 |
|---|---|---|---|---|---|---|---|---|
| 1-508 | 07-C-7-10-3 | 580 | St. Landry | Corn | 1 (483:4) | Large | M1-2 | 0 |
| 1-620 | 07-M-S-3-3-2 | 620 | St. Landry | Soil | 1 (483:4) | Mixed | M1-1 | 3 |
| 1-619 | 07-C-1-1-4 | 619 | Franklin | Corn | 1 (483:4) | Large | M1-2 | 40 |
| 1-621 | 07-C-7-6-4 | 621 | St. Landry | Corn | 1 (483:4) | Large | M1-2 | 2 |
| 2-590 | 07-S-2-5-7 | 590 | Franklin | Soil | 2 (0:29) | Small | M1-2 | 14,793 |
| 3-586 | 07-S-2-1-1 | 586 | Franklin | Soil | 3 (0:6) | Small | M1-1 | 24,241 |
| 4-Tox4 | 07-C-1-1-1 (Tox4/LA2) | 573 | Franklin | Corn | 4 (56:5) | Large | M1-2 | 43,145 |
| 5-576C | 07-C-3-5-5 | 576 | Tensas | Corn | 5 (1:10) | Small | M1-2 | ND |
| 5-601S | 07-S-4-4-1 | 601 | Tensas | Soil | 5 (1:10) | Small | M1-1 | 26,846 |
| 6-KD17 | 07-S-3-1-6 (KD17/LA1) | 1588 | Tensas | Soil | 6 (0:5) | Mixed | M1-2 | 0 |
| 6-605 | 07-S-6-4-1 | 605 | Point Coupee | Soil | 6 (0:5) | Mixed | M1-2 | 4 |
| 7-575 | 07-C-3-2-5 | 575 | Tensas | Corn | 7 (10:2) | Mixed | M1-1 | 6 |
| 8-599 | 07-S-3-2-4 (LA3) | 599 | Tensas | Soil | 8 (0:14) | Small | M1-1 | 14,653 |
| 9-578 | 07-C-5-1-5 | 578 | Concordia | Corn | 9 (2:14) | Large | M1-1 | 1884 |
| 10-581 | 07-M-C-3-1-7 | 581 | Point Coupee | Corn | 10 (10:2) | Large | M1-2 | 0 |
| 11-584 | 07-S-1-1-7 | 584 | Franklin | Soil | 11 (0:2) | Large | M1-2 | 11,174 |
| 12-587 | 07-S-2-1-2 | 587 | Franklin | Soil | 12 (0:4) | Small | M1-2 | 50,050 |
| 13-591 | 07-S-3-1-1 | 591 | Tensas | Soil | 13 (0:3) | Mixed | M1-1 | 13 |
| 14-595 | 07-S-3-1-8 | 595 | Tensas | Soil | 14 (0:2) | Mixed | M1-2 | 2 |
| 15-602 | 07-S-5-1-6 | 602 | Concordia | Soil | 15 (0:3) | Small | M1-2 | 29,236 |
| 16-613 | 07-M-S-2-4-2 | 613 | Rapides | Soil | 16 (0:2) | Large | M1-2 | 10,317 |
| Solo-618 | 07-M-S-3-6-1 | 618 | Point Coupee | Soil | Solo (0:1) | Large | M1-1 | 24,236 |
| 3357 | NRRL 3357 | 167 | Georgia | Peanut | Large | M1-1 | ND | |
| 21882 | NRRL 21882 | 1534 | Georgia | Peanut | Mixed | M1-2 | 0 | |
| AF36 | AF36 | 1533 | Arizona | Cotton | Af36 | Large | M1-2 | 0 |
| K49 | K49 | 1587 | Mississippi | Corn | Af36 | Large | M1-2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweany, R.R.; Gilbert, M.K.; Carter-Wientjes, C.H.; Moore, G.G.; Lebar, M.D. Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor. Toxins 2024, 16, 539. https://doi.org/10.3390/toxins16120539
Sweany RR, Gilbert MK, Carter-Wientjes CH, Moore GG, Lebar MD. Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor. Toxins. 2024; 16(12):539. https://doi.org/10.3390/toxins16120539
Chicago/Turabian StyleSweany, Rebecca R., Matthew K. Gilbert, Carol H. Carter-Wientjes, Geromy G. Moore, and Matthew D. Lebar. 2024. "Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor" Toxins 16, no. 12: 539. https://doi.org/10.3390/toxins16120539
APA StyleSweany, R. R., Gilbert, M. K., Carter-Wientjes, C. H., Moore, G. G., & Lebar, M. D. (2024). Variations in Kojic Acid Production and Corn Infection Among Aspergillus flavus Isolates Suggest a Potential Role as a Virulence Factor. Toxins, 16(12), 539. https://doi.org/10.3390/toxins16120539






