Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity
Abstract
:1. Introduction
2. Results
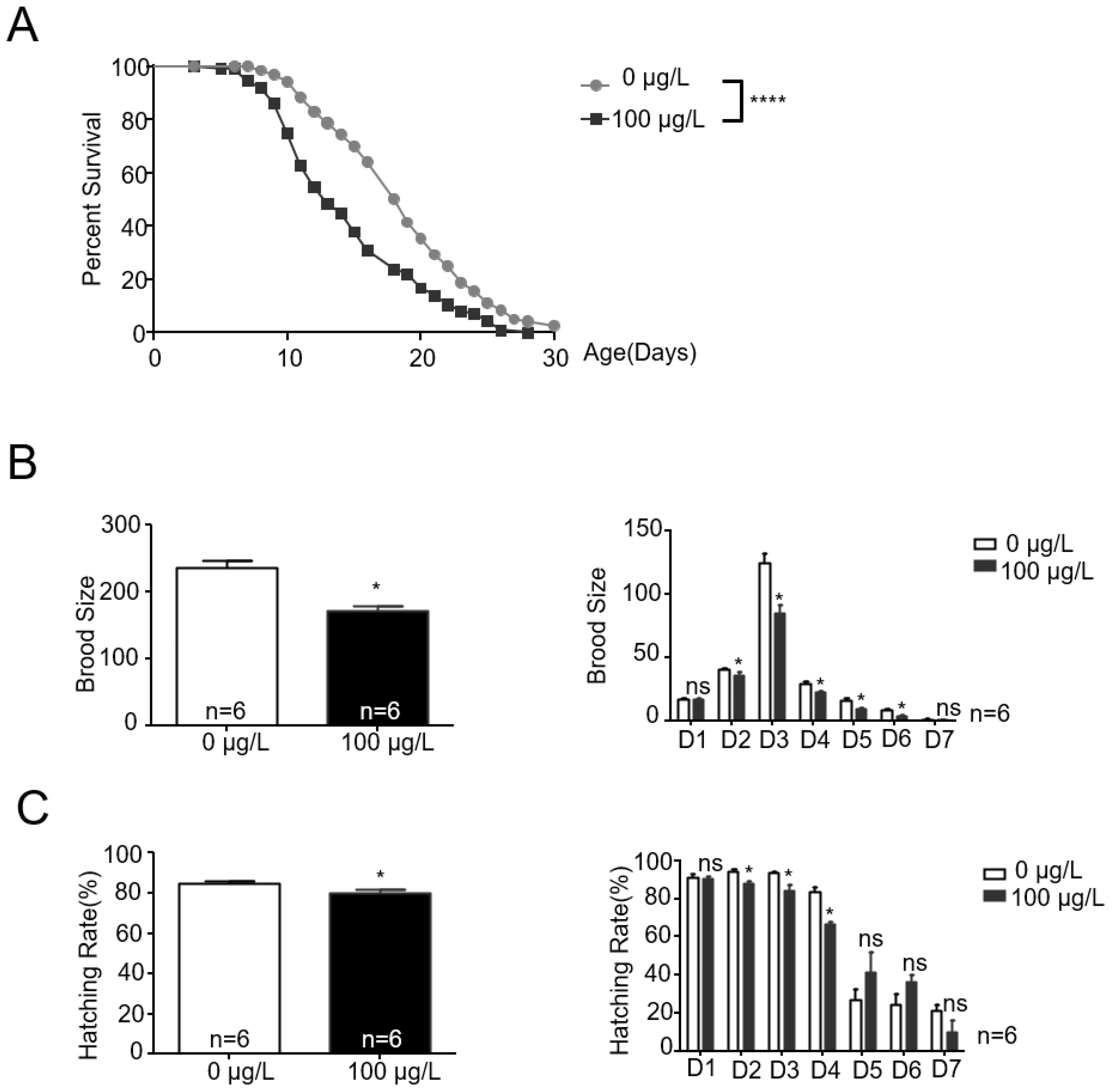
2.1. MC-LR Exposure Alters the Lifespan and Reproduction Ability of Nematodes
2.2. MC-LR Affects the Chemotaxis of Nematodes towards Benzaldehyde
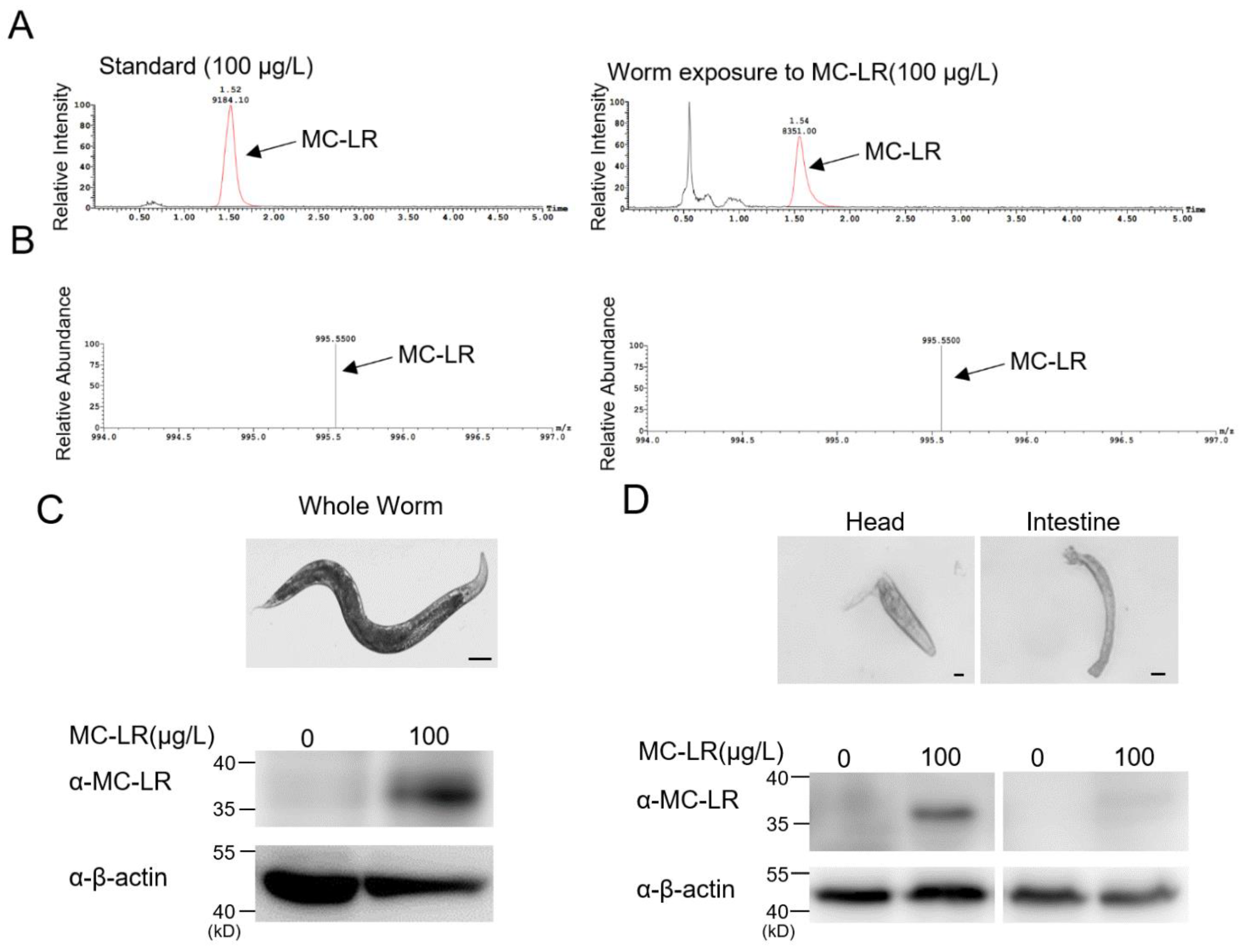
2.3. MC-LR Accumulates within the Nematodes
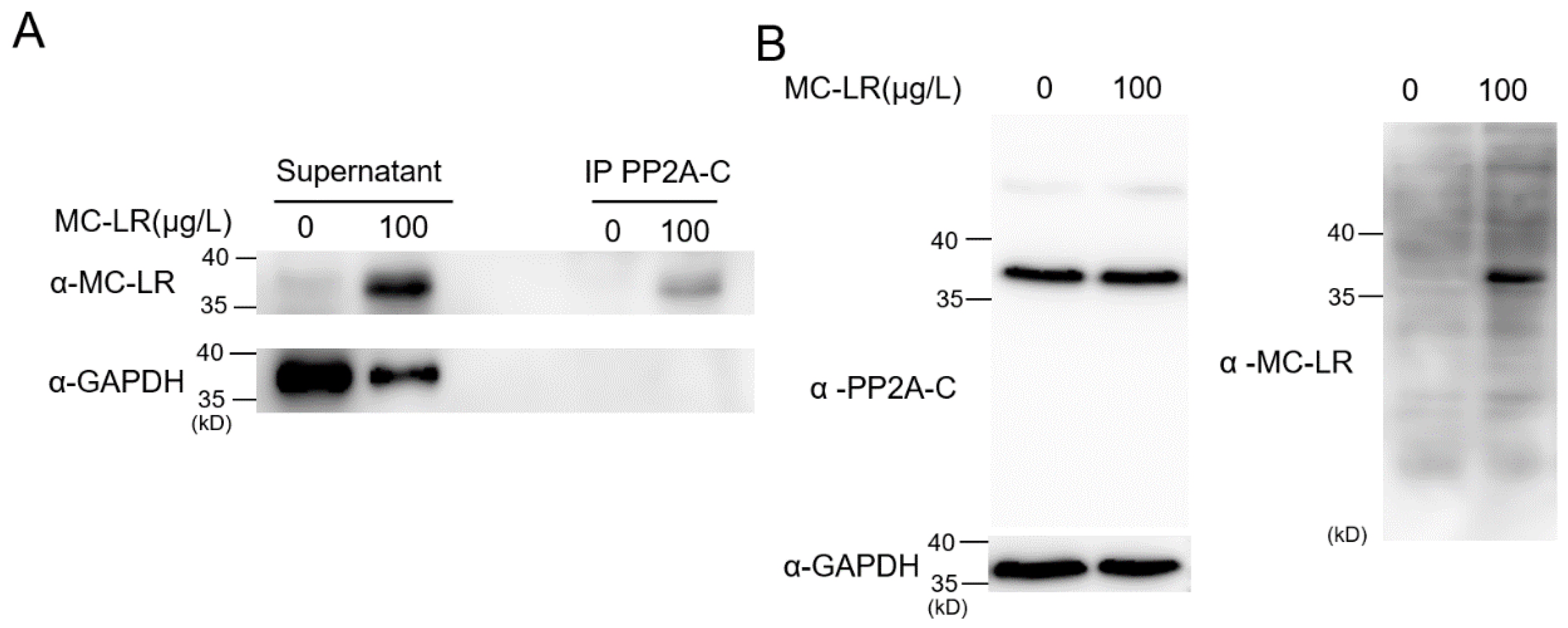
2.4. MC-LR Is Capable of Binding to PP2A-C within Nematodes
2.5. Point Mutations in PP2A-C Prevent the Binding of MC-LR to PP2A-C
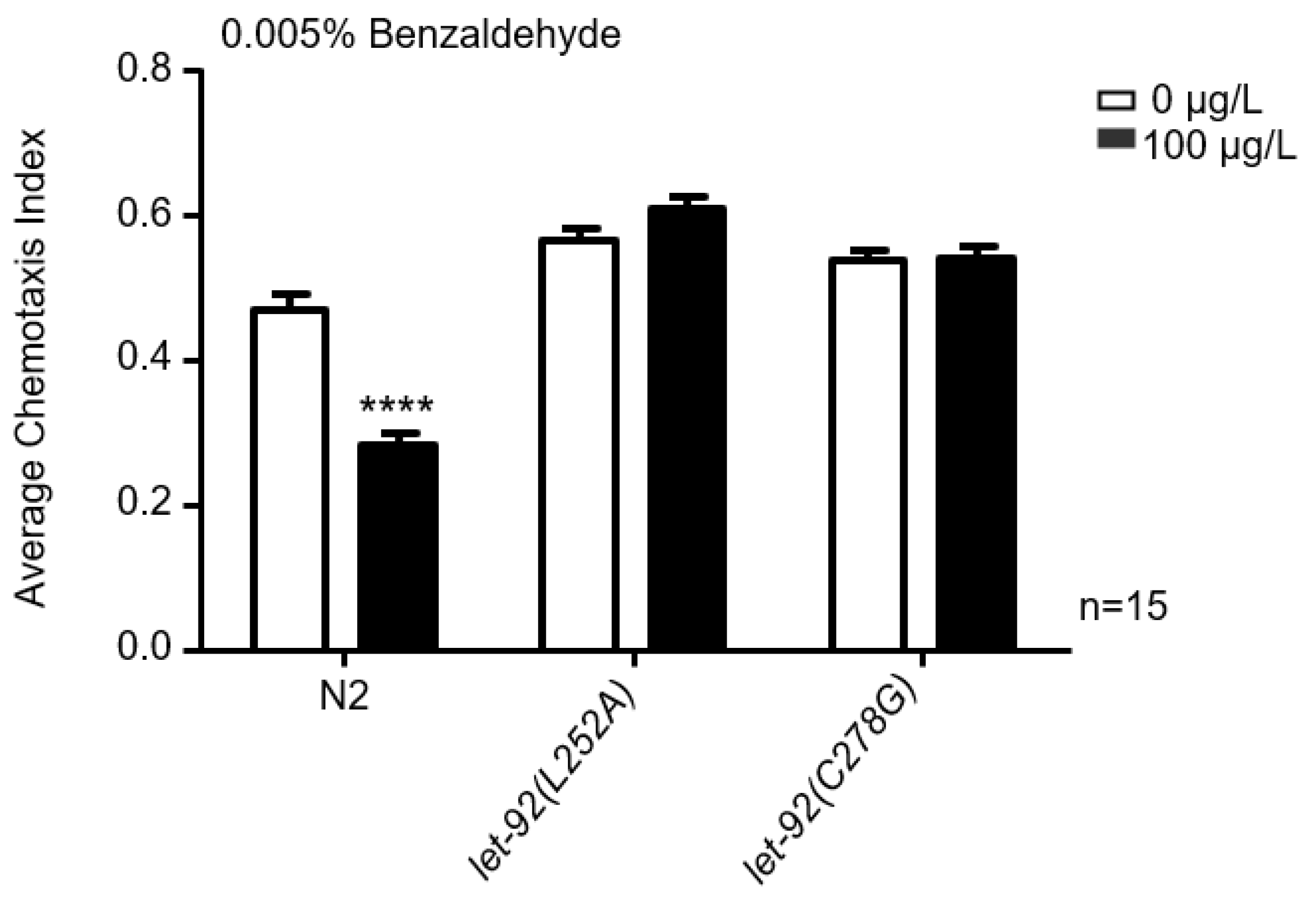
2.6. The Effect of MC-LR on the Response of Point-Mutant Nematodes to Benzaldehyde
2.7. The Impact of MC-LR on Calcium Signaling by Benzaldehyde in Point-Mutant Nematodes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. C. elegans Strains
5.2. Experimental Design
5.3. Behavioral Assays
5.4. CRISPR/Cas9-Mediated Genome Editing
5.5. Lifespan and Brood Assay
5.6. Biochemistry
5.7. LC-MS/MS Analysis
5.8. PP2A Activity Analysis
5.9. Calcium Imaging
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of Generative AI and AI-Assisted Technologies
References
- Carvalho, L.; Mcdonald, C.; de Hoyos, C.; Mischke, U.; Phillips, G.; Borics, G.; Poikane, S.; Skjelbred, B.; Solheim, A.L.; Van Wichelen, J.; et al. Sustaining Recreational Quality of European Lakes: Minimizing the Health Risks from Algal Blooms through Phosphorus Control. J. Appl. Ecol. 2013, 50, 315–323. [Google Scholar] [CrossRef]
- Sangolkar, L.N.; Maske, S.S.; Muthal, P.L.; Kashyap, S.M.; Chakrabarti, T. Isolation and Characterization of Microcystin Producing Microcystis from a Central Indian Water Bloom. Harmful Algae 2009, 8, 674–684. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A Review of Reproductive Toxicity of Microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Dang, Y.; Xu, Q.; Yu, L.; Liu, C.; Yuan, Y.; Wang, J. Microcystin-LR Induced Developmental Toxicity and Apoptosis in Zebrafish (Danio rerio) Larvae by Activation of ER Stress Response. Chemosphere 2016, 157, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of Using Blooms of Microcystis spp. in Animal Feeds: A Comprehensive Review of Nutritional, Toxicological and Microbial Health Evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P. Microcystin Accumulation in Freshwater Bivalves from Lake Taihu, China, and the Potential Risk to Human Consumption. Environ. Toxicol. Chem. 2007, 26, 1066–1073. [Google Scholar] [CrossRef]
- Abdallah, M.F.; Van Hassel, W.H.R.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and Food Contamination in Developing Countries: Review of Their Types, Toxicity, Analysis, Occurrence and Mitigation Strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef]
- Lahti, K.; Rapala, J.; Färdig, M.; Niemelä, M.; Sivonen, K. Persistence of Cyanobacterial Hepatotoxin, Microcystin-LR in Particulate Material and Dissolved in Lake Water. Water Res. 1997, 31, 1005–1012. [Google Scholar] [CrossRef]
- Cazenave, J.; Wunderlin, D.A.; Bistoni, M.D.L.Á.; Amé, M.V.; Krause, E.; Pflugmacher, S.; Wiegand, C. Uptake, Tissue Distribution and Accumulation of Microcystin-RR in Corydoras paleatus, Jenynsia multidentata and Odontesthes bonariensis: A Field and Laboratory Study. Aquat. Toxicol. 2005, 75, 178–190. [Google Scholar] [CrossRef]
- Martins, J.C.; Vasconcelos, V.M. Microcystin Dynamics in Aquatic Organisms. J. Toxicol. Environ. Heal.-Part B Crit. Rev. 2009, 12, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, H.; Du, M.; Zhang, Y.; Ye, B.; Pu, Y.; Wang, D. Induction of Chemotaxis to Sodium Chloride and Diacetyl and Thermotaxis Defects by Microcystin-LR Exposure in Nematode Caenorhabditis elegans. J. Environ. Sci. 2009, 21, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, L.; Li, G.; Zhao, S. Microcystin-LR Induces Changes in the GABA Neurotransmitter System of Zebrafish. Aquat. Toxicol. 2017, 188, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; El Herry, S.; Bouaïcha, N. First Reported Case of Turtle Deaths during a Toxic Microcystis Spp. Bloom in Lake Oubeira, Algeria. Ecotoxicol. Environ. Saf. 2008, 71, 535–544. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, W.A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a Novel Marine Harmful Algal Bloom: Cyanotoxin (Microcystin) Transfer from Land to Sea Otters. PLoS ONE 2010, 5, e12576. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Frenken, T.; Rudman, S.M.; van West, P.; Ibelings, B.W.; Trainer, V.L. Diseases and Disorders in Fish Due to Harmful Algal Blooms. In Climate Change on Diseases and Disorders of Finfish in Cage Culture; CABI: Wallingford, UK, 2023; pp. 387–429. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Bhavsar, S.P.; Ortiz Almirall, X.; Marklevitz, S.A.C.; Rudman, S.M.; Sibley, P.K. Lake Erie Fish Safe to Eat yet Afflicted by Algal Hepatotoxins. Sci. Total Environ. 2023, 861, 160474. [Google Scholar] [CrossRef]
- Nonga, H.E.; Sandvik, M.; Miles, C.O.; Lie, E.; Mdegela, R.H.; Mwamengele, G.L.; Semuguruka, W.D.; Skaare, J.U. Possible Involvement of Microcystins in the Unexplained Mass Mortalities of Lesser Flamingo (Phoeniconaias Minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678, 167–178. [Google Scholar] [CrossRef]
- Matsunaga, H.; Harada, K.I.; Senma, M.; Ito, Y.; Yasuda, N.; Ushida, S.; Kimura, Y. Possible Cause of Unnatural Mass Death of Wild Birds in a Pond in Nishinomiya, Japan: Sudden Appearance of Toxic Cyanobacteria. Nat. Toxins 1999, 7, 81–84. [Google Scholar] [CrossRef]
- Alonso-Andicoberry, C.; García-Villada, L.; Lopez-Rodas, V.; Costas, E. Catastrophic Mortality of Flamingos in a Spanish National Park Caused by Cyanobacteria. Vet. Rec. 2002, 151, 706. [Google Scholar] [CrossRef]
- Lopez-Rodas, V.; Maneiro, E.; Lanzarot, M.P.; Perdigones, N.; Costas, E. Mass Wildlife Mortality Due to Cyanobacteria in the Doñana National Park, Spain. Vet. Rec. 2008, 162, 317–318. [Google Scholar] [CrossRef]
- Breinlinger, S.; Phillips, T.J.; Haram, B.N.; Mareš, J.; Martínez Yerena, J.A.; Hrouzek, P.; Sobotka, R.; Henderson, W.M.; Schmieder, P.; Williams, S.M.; et al. Hunting the Eagle Killer: A Cyanobacterial Neurotoxin Causes Vacuolar Myelinopathy. Science 2021, 371, eaax9050. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An Acute Case of Intoxication with Cyanobacteria and Cyanotoxins in Recreational Water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First Identification of the Hepatotoxic Microcystins in the Serum of a Chronically Exposed Human Population Together with Indication of Hepatocellular Damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Watanabe, M.F.; Park, H.D.; Chen, G.C.; Chen, G.; Yu, S.Z. Detection of Microcystins, a Blue-Green Algal Hepatotoxin, in Drinking Water Sampled in Haimen and Fusui, Endemic Areas of Primary Liver Cancer in China, by Highly Sensitive Immunoassay. Carcinogenesis 1996, 17, 1317–1321. [Google Scholar] [CrossRef]
- Olson, N.E.; Cooke, M.E.; Shi, J.H.; Birbeck, J.A.; Westrick, J.A.; Ault, A.P. Harmful Algal Bloom Toxins in Aerosol Generated from Inland Lake Water. Environ. Sci. Technol. 2020, 54, 4769–4780. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.A.; Dietrich, D.R. Quantitative Assessment of Aerosolized Cyanobacterial Toxins at Two New Zealand Lakes. J. Environ. Monit. 2011, 13, 1617–1624. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y. The Role of PP2A-Associated Proteins and Signal Pathways in Microcystin-LR Toxicity. Toxicol. Lett. 2015, 236, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mumby, M. Regulation by Tumour Antigens Defines a Role for PP2A in Signal Transduction. Semin. Cancer Biol. 1995, 6, 229–237. [Google Scholar] [CrossRef]
- Schönthal, A.H. Role of PP2A in Intracellular Signal Transduction Pathways. Front. Biosci. 1998, 3, 1262–1273. [Google Scholar] [CrossRef]
- Wlodarchak, N.; Xing, Y. PP2A as a Master Regulator of the Cell Cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef]
- Janssens, V.; Goris, J. Protein Phosphatase 2A: A Highly Regulated Family of Serine/Threonine Phosphatases Implicated in Cell Growth and Signalling. Biochem. J. 2001, 353, 417–439. [Google Scholar] [CrossRef]
- Ramos, F.; Villoria, M.T.; Alonso-Rodríguez, E.; Clemente-Blanco, A. Role of Protein Phosphatases PP1, PP2A, PP4 and Cdc14 in the DNA Damage Response. Cell Stress 2019, 3, 70–85. [Google Scholar] [CrossRef]
- Shi, Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell 2009, 139, 468–484. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Neurotoxic Action of Microcystin-LR Is Reflected in the Transcriptional Stress Response of Caenorhabditis elegans. Chem. Biol. Interact. 2014, 223, 51–57. [Google Scholar] [CrossRef]
- Ogura, K.I.; Okada, T.; Mitani, S.; Gengyo-Ando, K.; Baillie, D.L.; Kohara, Y.; Goshima, Y. Protein Phosphatase 2A Cooperates with the Autophagyrelated Kinase UNC-51 to Regulate Axon Guidance in Caenorhabditis elegans. Development 2010, 137, 1657–1667. [Google Scholar] [CrossRef]
- Sieburth, D.S.; Sundaram, M.; Howard, R.M.; Han, M. A PP2A Regulatory Subunit Positively Regulates Ras-Mediated Signaling during Caenorhabditis elegans Vulval Induction. Genes Dev. 1999, 13, 2562–2569. [Google Scholar] [CrossRef]
- Haselkorn, R. Cyanobacteria. Curr. Biol. 2009, 19, R277–R278. [Google Scholar] [CrossRef]
- Grauer, F.H.; Arnold, H.L. Seaweed Dermatitis: First Report of a Dermatitis-Producing Marine Alga. Arch. Dermatol. 1961, 84, 720–732. [Google Scholar] [CrossRef]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Shaw, G.R. Recreational and Occupational Field Exposure to Freshwater Cyanobacteria—A Review of Anecdotal and Case Reports, Epidemiological Studies and the Challenges for Epidemiologic Assessment. Environ. Health 2006, 5, 6. [Google Scholar] [CrossRef]
- Ju, J.; Ruan, Q.; Li, X.; Liu, R.; Li, Y.; Pu, Y.; Yin, L.; Wang, D. Neurotoxicological Evaluation of Microcystin-LR Exposure at Environmental Relevant Concentrations on Nematode Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2013, 20, 1823–1830. [Google Scholar] [CrossRef]
- Wang, S.C.; Geng, Z.Z.; Wang, Y.; Tong, Z.H.; Yu, H.Q. Essential Roles of P53 and MAPK Cascades in Microcystin-LR-Induced Germline Apoptosis in Caenorhabditis elegans. Environ. Sci. Technol. 2012, 46, 3442–3448. [Google Scholar] [CrossRef]
- Moore, C.E.; Lein, P.J.; Puschner, B. Microcystins Alter Chemotactic Behavior in Caenorhabditis elegans by Selectively Targeting the AWA Sensory Neuron. Toxins 2014, 6, 1813–1836. [Google Scholar] [CrossRef]
- Christoffersen, K.; Kaas, H. Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring, and Management. Limnol. Oceanogr. 2000, 45, 1212. [Google Scholar] [CrossRef]
- Antoniou, M.G.; de la Cruz, A.A.; Dionysiou, D.D. Cyanotoxins: New Generation of Water Contaminants. J. Environ. Eng. 2005, 131, 1239–1243. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Drobac Backović, D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global Geographical and Historical Overview of Cyanotoxin Distribution and Cyanobacterial Poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of Microcystins with Reference to Cases of Human Intoxications and Epidemiological Investigations of Exposures to Cyanobacteria and Cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Lei, H.; Xie, P.; Chen, J.; Liang, G.; Dai, M.; Zhang, X. Distribution of Toxins in Various Tissues of Crucian Carp Intraperitoneally Injected with Hepatotoxic Microcystins. Environ. Toxicol. Chem. 2008, 27, 1167–1174. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of Microcystin Distribution and Biomagnification in Tissues of Aquatic Food Web Compartments from a Shallow Lake and Evaluation of Potential Risks to Public Health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Xie, P.; Wang, Q.; Ma, Z. Simultaneous Determination of Microcystin Contaminations in Various Vertebrates (Fish, Turtle, Duck and Water Bird) from a Large Eutrophic Chinese Lake, Lake Taihu, with Toxic Microcystis Blooms. Sci. Total Environ. 2009, 407, 3317–3322. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, W.; Liu, C.; Li, L.; Yu, L.; Zhao, S.; Li, G. Microcystin-LR Exposure Induces Developmental Neurotoxicity in Zebrafish Embryo. Environ. Pollut. 2016, 213, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yan, W.; Cheng, H.; Liu, C.; Hung, T.C.; Guo, X.; Li, G. Parental Transfer of Microcystin-LR Induced Transgenerational Effects of Developmental Neurotoxicity in Zebrafish Offspring. Environ. Pollut. 2017, 231, 471–478. [Google Scholar] [CrossRef]
- Culetto, E.; Sattelle, D.B. A Role for Caenorhabditis elegans in Understanding the Function and Interactions of Human Disease Genes. Hum. Mol. Genet. 2000, 9, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.B.; Sengupta, P. How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli. Genetics 2019, 212, 25. [Google Scholar] [CrossRef] [PubMed]
- Somdee, T.; Wibuloutai, J.; Somdee, T.; Somdee, A. Biodegradation of the Cyanobacterial Hepatotoxin [Dha7] Microcystin-LR within a Biologically Active Sand Filter. Water Sci. Technol. Water Supply 2014, 14, 672–680. [Google Scholar] [CrossRef]
- Sontag, J.-M.; Sontag, E.; Wang, H. Protein Phosphatase 2A Dysfunction in Alzheimer’s Disease. Front. Mol. Neurosci. 2014, 7, 16. [Google Scholar] [CrossRef]
- Fujiwara, N.; Usui, T.; Ohama, T.; Sato, K. Regulation of Beclin 1 Protein Phosphorylation and Autophagy by Protein Phosphatase 2A (PP2A) and Death-Associated Protein Kinase 3 (DAPK3). J. Biol. Chem. 2016, 291, 10858–10866. [Google Scholar] [CrossRef]
- Wong, P.-M.; Feng, Y.; Wang, J.; Shi, R.; Jiang, X. ARTICLE Regulation of Autophagy by Coordinated Action of MTORC1 and Protein Phosphatase 2A. Nat. Commun. 2015, 6, 8048. [Google Scholar] [CrossRef]
- Bye-A-Jee, H.; Zaru, R.; Magrane, M.; Orchard, S. Caenorhabditis elegans Phosphatase Complexes in UniProtKB and Complex Portal. FEBS J. 2020, 287, 2664–2684. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, Y.; Chen, Y.; Jeffrey, P.D.; Chao, Y.; Lin, Z.; Li, Z.; Strack, S.; Stock, J.B.; Shi, Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell 2006, 127, 341–353. [Google Scholar] [CrossRef]
- WHO. Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2006.
- Pan, C.; Qin, H.; Yan, M.; Qiu, X.; Gong, W.; Luo, W.; Guo, H.; Han, X. Environmental Microcystin Exposure Triggers the Poor Prognosis of Prostate Cancer: Evidence from Case-Control, Animal, and in Vitro Studies. J. Environ. Sci. 2023, 127, 69–81. [Google Scholar] [CrossRef] [PubMed]



| Strains | Source | Identifier |
|---|---|---|
| N2 | CGC (University of Minnesota-Twin Cities, Minneapolis, MN, USA) | |
| let-92(syb6505[C278G]) | SunyBiotech (FuZhou, China) | PHX6506 |
| let-92(syb6455[L252A]) | SunyBiotech | PHX6455 |
| let-92(syb6542[I132V]) | SunyBiotech | PHX6542 |
| Ex [Podr-1:Gcamp6s+Pstr-2:mNepturn] | Yanxun V. Yu | YVY150 |
| gonEx 111 [Podr-1:Gcamp6s+Pstr-2:mNepturn];let-92(syb6505) | This study | JKG 712 |
| gonEx 112 [Podr-1:Gcamp6s+Pstr-2:mNepturn];let-92(syb6455) | This study | JKG 713 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, C.; Gong, J. Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity. Toxins 2024, 16, 145. https://doi.org/10.3390/toxins16030145
Zhan C, Gong J. Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity. Toxins. 2024; 16(3):145. https://doi.org/10.3390/toxins16030145
Chicago/Turabian StyleZhan, Chunhua, and Jianke Gong. 2024. "Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity" Toxins 16, no. 3: 145. https://doi.org/10.3390/toxins16030145
APA StyleZhan, C., & Gong, J. (2024). Mutations at Two Key Sites in PP2A Safeguard Caenorhabditis elegans Neurons from Microcystin-LR Toxicity. Toxins, 16(3), 145. https://doi.org/10.3390/toxins16030145





