Meta-Analysis of the Effects of Yeast Cell Wall Extract Supple-Mentation during Mycotoxin Challenges on the Performance of Laying Hens
Abstract
1. Introduction
2. Results
2.1. Research Characteristics
2.2. Body Weight
2.3. Feed Intake
2.4. Egg Production
2.5. Egg Weight
2.6. Between-Study Heterogeneity and Publication Bias
2.7. Economic Assessment
3. Discussion
4. Conclusions
5. Materials and Methods
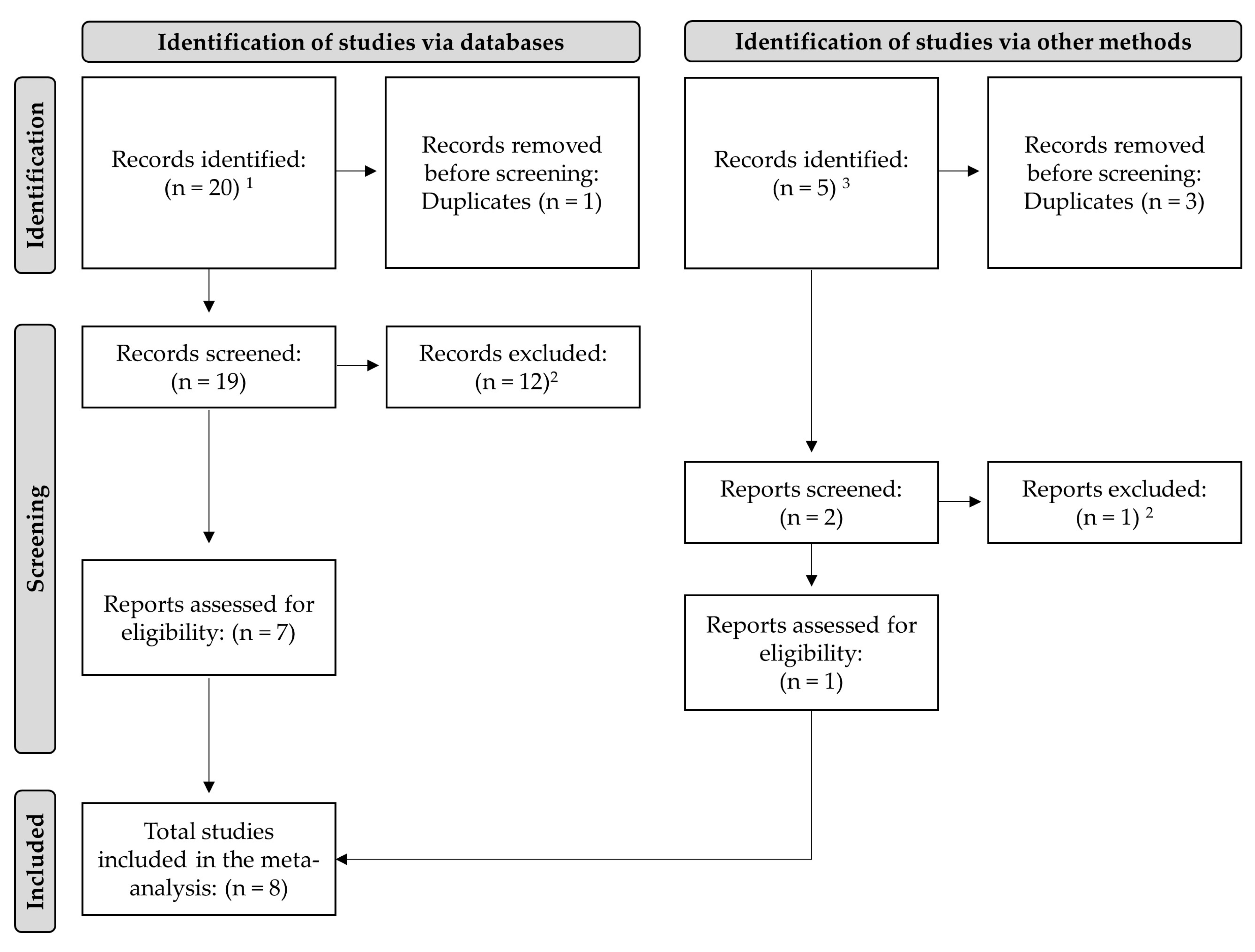
5.1. Literature Search and Selection
5.2. Statistical Analysis
5.3. Economic Assessment
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Kipper, M.I.; Andretta, A.M.L.; Ribeiro, P.G.; da Silva Pires, C.S.; Franceschina, K.M.; Cardinal, P.; de Oliveira Moraes Schroeder, B. Assessing the implications of mycotoxins on productive efficiency of broilers and growing pigs. Sci. Agric. 2020, 77, e20180236. [Google Scholar] [CrossRef]
- Weaver, A.C.; King, W.D.; Verax, M.; Fox, U.; Kudupoje, M.B.; Mathis, G.; Lumpkins, B.; Yiannikouris, A. Impact of chronic levels of naturally multi-contaminated feed with fusarium mycotoxins on broiler chickens and evaluation of the mitigation properties of different titers of yeast cell wall extract. Toxins 2020, 12, 636. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Koletsi, P.; Schrama, J.W.; Graat, E.A.M.; Wiegertjes, G.F.; Lyons, P.; Pietsch, C. The occurrence of mycotoxins in raw materials and fish feeds in Europe and the potential effects of deoxynivalenol (DON) on the health and growth of farmed fish species—A review. Toxins 2021, 13, 403. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Munkvold, G. Crop management practices to minimize the risk of mycotoxins contamination in temperate-zone maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; John Wiley & Sons, Inc.: Ames, IA, USA, 2014; pp. 59–77. [Google Scholar] [CrossRef]
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef]
- Palumbo, R.; Gonçalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.-L.; Dall’asta, C.; Venâncio, A.-D.; Battilani, P. Mycotoxins in maize: Mitigation actions, with chain management approach. Phytopathol. Medeterranea 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.S.; Connolly, L.; Elliott, C. Comparative in vitro assessment of a range of commercial feed additives with multiple mycotoxin binding claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef]
- Kudujope, M.B.; Malathi, V.; Yiannikouris, A. Impact of a natural fusarial multi-mycotoxin challenge on broiler chickens and mitigation properties provided by a yeast cell wall extract and a postbiotic yeast cell wall-based belnd. Toxins 2022, 14, 315. [Google Scholar] [CrossRef]
- Aravind, K.L.; Patil, V.S.; Devegowada, G.; Umakantha, B.; Ganpule, S.P. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult. Sci. 2003, 82, 571–576. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Kettunen, H.; Apajalahti, J.; Pennala, E.; Moran, C.A. Comparison of the sequestering properties of yeast cell wall extract and hydrated sodium calcium aluminosilicate in three in vitro models accounting for the animal physiological bioavailability of zearalenone. Food Addit. Contam. Part A 2013, 30, 1641–1650. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Apajalahti, J.; Kettunen, H.; Ojanperä, S.; Bell, A.N.W.; Keegan, J.D.; Moran, C.A. Efficient aflatoxin B1 sequestration by yeast cell wall extract and hydrated sodium calcium aluminosilicate evaluated using a multimodal in-vitro and ex-vivo methodology. Toxins 2021, 13, 24. [Google Scholar] [CrossRef]
- Sauvant, D.; Schmidely, P.; Daudin, J.; St-Pierre, N. Meta-analysis of experimental data in animal nutrition. Animal 2008, 2, 1203–1214. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Smith, T.K. Effects of feeding blends of grains naturally contaminated with Fusarium mycotoxins on performance and metabolism of laying hens. Poult. Sci. 2004, 83, 1849–1856. [Google Scholar] [CrossRef]
- Manoj, K.B. Effect of dietary T-2 toxin level and mycosorb on health and productivity of commercial layers. M.S. Thesis, University of Agricultural Sciences, Bangalore, India, 1999. [Google Scholar]
- Tran, S.T.; Bowman, M.E.; Smith, T.K. Effects of feeding diets naturally contaminated with low levels of Fusarium mycotoxins on performance and egg quality in laying hens. Unpublished work. 2016. [Google Scholar]
- Siloto, E.V.; Oliveria, E.F.A.; Sartori, J.R.; Fascina, V.B.; Martins, B.A.B.; Ledoux, D.R.; Rottinghaus, G.E.; Sartori, D.R. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult. Sci. 2013, 92, 2077–2083. [Google Scholar] [CrossRef]
- Rizzi, L.; Simioli, M.; Altafini, A.; Zaghini, A. Egg quality and mycotoxin residues of laying hens fed a diet containing aflatoxin B1 and esterified glucomannans. In Proceedings of the XVIth European Symposium on the Quality of Poultry Meat and the Xth European Symposium on the Quality of Eggs and Egg Products, Saint-Brieuc, France, 23–26 September 2003. [Google Scholar]
- Roncada, P.; Simioli, M.; Altafini, A.; Zaghini, A.; Mancini, G.; Rizzi, L. Ochratoxin A, esterified glucomannan and Saccharomyces cerevisiae in diet for laying hens. In Proceedings of the EPC 2006—12th European Poultry Conference, Verona, Italy, 10–14 September 2006. [Google Scholar]
- Spalević, L.; Maslić-Strižak, D.; Pavlović, I. Influence of modified clinoptilolite and esterified glucomannan on performance of laying hens. Lucr. Stiintifice Med. Vet. 2013, 46, 191–196. [Google Scholar]
- Stanley, V.G.; Winsman, M.; Dunkley, C.; Ogunleye, T.; Daley, M.; Krueger, W.F.; Sefton, A.E.; Hinton, A., Jr. The impact of yeast culture residue on the suppression of dietary aflatoxin on the performance of broiler breeder hens. J. Appl. Poult. Res. 2004, 13, 533–539. [Google Scholar] [CrossRef]
- Rodney, R.M.; Celi, P.; Scott, W.; Breinhild, K.; Lean, I.J. Effects of dietary fat on fertility of dairy cattle: A meta-analysis and meta-regression. J. Dairy Sci. 2015, 98, 5601–5620. [Google Scholar] [CrossRef]
- Salami, S.A.; Ross, S.A.; Patsiogiannis, A.; Moran, C.A.; Taylor-Pickard, J. Performance and environmental impact of egg production in response to dietary supplementation of mannan oligosaccharide in laying hens: A meta-analysis. Poult. Sci. 2022, 4, 101745. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Yiannikouris, A.; Adams, N. Meta-analysis of the effects of mycotoxins and yeast cell wall extract supplementation on the performance, livability, and environmental sustainability of broiler production. Poult. Sci. 2022, 101, 102043. [Google Scholar] [CrossRef]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Use of yeast cell wall extract for growing pigs consuming feed contaminated with mycotoxins below or above regulatory guidelines: A meta-analysis with meta-regression. Toxins 2023, 15, 596. [Google Scholar] [CrossRef]
- Danicke, S.; Ueberschär, K.-H.; Halle, S.; Matthes, S.; Valenta, H.; Flachowsky, G. Effect of addition of a detoxifying agent to laying hen diets containing uncontaminated or Fusarium toxin-contaminated maize on performance of hens and on carryover of zearalenone. Poult. Sci. 2002, 81, 1671–1680. [Google Scholar] [CrossRef]
- Yegani, M.; Smith, T.K.; Leeson, S.; Boermans, H.J. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on performance and metabolism of broiler breeders. Poult. Sci. 2006, 85, 1541–1549. [Google Scholar] [CrossRef]
- Kulcsár, S.; Kövesi, B.; Balogh, K.; Zándoki, E.; Ancsin, Z.; Erdélyi, M.; Mézes, M. The Co-Occurrence of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 Activated the Glutathione Redox System in the EU-Limiting Doses in Laying Hens. Toxins 2023, 15, 305. [Google Scholar] [CrossRef]
- Yuan, T.; Li, J.; Wang, Y.; Li, M.; Yang, A.; Ren, C.; Qi, D.; Zhang, N. Effects of Zearalenone on Production Performance, Egg Quality, Ovarian Function and Gut Microbiota of Laying Hens. Toxins 2022, 14, 653. [Google Scholar] [CrossRef]
- Zhai, X.; Qiu, Z.; Wang, L.; Luo, Y.; He, W.; Yang, J. Possible Toxic Mechanisms of Deoxynivalenol (DON) Exposure to Intestinal Barrier Damage and Dysbiosis of the Gut Microbiota in Laying Hens. Toxins 2022, 14, 682. [Google Scholar] [CrossRef]
- Putra, R.P.; Astuti, D.; Respati, A.N.; Triswanto, T.; Yano, A.A.; Gading, B.M.W.T.; Jayanegara, A.; Sholikin, M.; Hassim, H.A.; Adil, D.N.; et al. Protective effects of various feed additives on broiler chickens exposed to mycotoxin-contaminated feed: A systematic review and meta-analysis. Vet. Res. Commun. 2023, 48, 225–244. [Google Scholar] [CrossRef]
- Kollar, R.; Reinhold, B.B.; Petrakova, E.; Yeh, H.J.C.; Ashwell, G.; Drgonova, J.; Kapteyn, J.C.; Klis, F.M.; Cabib, E. Architecture of the yeast cell wall. Β(1-6)-gluten interconnects mannoprotein, β(1-3)-glucan, and chitin. J. Biol. Chem. 1997, 272, 17762–17775. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Xu, R.; Yiannikouris, A.; Shandilya, U.K.; Karrow, N.A. Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach. Toxins 2023, 15, 104. [Google Scholar] [CrossRef]
- Ledvinka, Z.; Zita, L.; Klesalová, L. Egg quality and some factors influencing it: A review. Sci. Agric. Bohem. 2012, 43, 46–52. [Google Scholar]
- Egg Safety and Quality Management Program. Shell Egg Inspection Manual 2018/2019. California Department of Food and Agriculture, Animal Health and Food Safety Services: Meat, Poultry and Egg Safety Branch. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.cdfa.ca.gov/AHFSS/mpes/pdfs/ESQM/2018InspectionManualFullManual.pdf&ved=2ahUKEwiToN_sqpmFAxWpv4kEHecWAX8QFnoECA4QAQ&usg=AOvVaw3nGOl09VA8P8D_Lle4WzSK (accessed on 15 February 2024).
- U.S. Department of Agriculture Agricultural Marketing Service Livestock, Poultry & Grain Market News. In Egg market news report. 12 February 2024. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.ams.usda.gov/mnreports/pybshellegg.pdf&ved=2ahUKEwjhhcnZ_5aFAxWHj4kEHc_RCdAQFnoECBkQAQ&usg=AOvVaw3CJJ7AfEY7EhGz4A_QYdo_ (accessed on 12 February 2024).
- Sypecka, Z.; Kelly, M.; Brereton, P. Deoxynivalenol and zearalenone residues in eggs of laying hens fed with a naturally contaminated diet: Effects on egg production and estimation of transmission rates from feed to eggs. J. Agric. Food Chem. 2004, 52, 5463–5471. [Google Scholar] [CrossRef]
- Jia, R.; Ma, Q.; Fan, Y.; Ji, C.; Zhang, J.; Liu, T.; Zhao, L. The toxic effects of combined aflatoxins and zearalenone in naturally contaminated diets on laying performance, egg quality and mycotoxin residues in eggs of layers and the protective effect of Bacillus subtilis biodegradation product. Food Chem. Toxicol. 2016, 90, 142–150. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, L.; Xu, J.; Wang, Y.; Wang, Y. Effects of moldy corn on the performance, antioxidant capacity, immune function, metabolism and residues of mycotoxins in eggs, muscle, and edible viscera of laying hens. Poult. Sci. 2023, 102, 102502. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Wei, J.-T.; Zhu, M.-X.; Zhang, L.; Zhang, J.-C.; Karrow, N.A.; Han, Y.-M.; Wu, Y.-Y.; Guo, Y.-M.; et al. Mitigation Effects of Bentonite and Yeast Cell Wall Binders on AFB1, DON, and OTA Induced Changes in Laying Hen Performance, Egg Quality, and Health. Toxins 2021, 13, 156. [Google Scholar] [CrossRef]
- Dvorska, J.E.; Surai, P.F.; Speake, B.K.; Sparks, N.H.C. Protective effect of modified glucomannans against aurofusarin-induced changes in quail egg and embryo. Comp. Biochem. Physiol. Part C 2003, 135, 337–343. [Google Scholar] [CrossRef]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional impact of mycotoxins in food animal production and strategies for mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef]
- Zaheer, K. An Updated Review on Chicken Eggs: Production, Consumption, Management Aspects and Nutritional Benefits to Human Health. Food Nutr. Sci. 2015, 6, 1208–1220. [Google Scholar] [CrossRef]
- De Vries, M.; de Boer, I.J.M. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Guillaume, A.; Hubatová-Vacková, A.; Koáí, V. Environmental impacts of egg production from a life cycle perspective. Agriculture 2022, 12, 355. [Google Scholar] [CrossRef]
- Pelletier, N. Changes in the life cycle environmental footprint of egg production in Canada from 1962 to 2012. J. Clean. Prod. 2018, 176, 1144–1153. [Google Scholar] [CrossRef]
- Girgis, G.N.; Barta, J.R.; Girish, C.K.; Karrow, N.A.; Boermans, H.J.; Smith, T.K. Effects of feed-borne Fusarium mycotoxins and an organic mycotoxin adsorbent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Vet. Immunol. Immunopathol. 2010, 138, 218–223. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- R Development Core Team. 2023 R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; Version 1.4.1106; RStudio, PBC: Boston, MA, USA, 2023; Available online: http://www.rstudio.com/ (accessed on 30 April 2023).
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Von Hippel, P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Statist. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Ibarburu, M. U.S. Egg Cost of Production and Prices; Egg Industry Center: Ames, IA, USA, 2024. [Google Scholar]
- Puglisi, M.J.; Fernandez, M.L. The health benefits of egg protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]


| Mycotoxin, mg/kg 6 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. 1 | Location | Breed | Hens/trt 2 | Start Age, Weeks | Trial Weeks | Number of MT Levels 3 | YCWE, kg/t 4 | Source 5 | AF, AFB1 | OTA | DON | 15-A-DON | DON-3-G | T2 | FUM, FB1 | ZEA |
| [18] | Canada | ISA Brown | 48 | 45 | 12 | 1 | 2 | Natural | 12.1 | 0.5 | 0.60 | |||||
| [19] | India | - | 24 | 26 | 12 | 1 | 1 | Pure | 0.5 | |||||||
| 2 | 1 | 1.0 | ||||||||||||||
| 3 | 1 | 2.0 | ||||||||||||||
| [20] | Canada | Lohmann LS-LITE | 36 | 48 | 12 | 1 | 2 | Natural | 2.53 | 0.31 | 0.33 | |||||
| [21] | Brazil | Hisex Brown | 24 | 37 | 8 | 1 | 2 | Culture | 1.0 | |||||||
| 2 | 2 | 25 | ||||||||||||||
| 3 | 2 | 1.0 | 25 | |||||||||||||
| [22] | Italy | Warren | 18 | - | 4 | 1 | 1.1 | - | 0.893 | 0.171 | 10.36 | |||||
| [23] | Italy | Warren-ISA Brown | 14 | - | 12 | 1 | 2 | Pure | 0.20 | |||||||
| [24] 7 | Serbia | Shaver 579 | 500 | 18 | 12 | 1 | 2 | Natural | 0.005 | 0.19 | 3.14 | |||||
| [25] | USA | Cobb | 30 | 35 | 4 | 1 | 0.91 | Culture | 3.0 | |||||||
| Heterogeneity Test | |||||||
|---|---|---|---|---|---|---|---|
| Item 1 | No. Comp. 2 | Mean Effect Size | 95% CI 3 | p-Value | I2 (%) 4 | p-Value | Eggar p-Value 5 |
| Body weight, g | |||||||
| MT − CTRL | 6 | −49.98 | −88.77, −11.20 | 0.012 | 99.96 | <0.0001 | 0.871 |
| YCWE + MT − MT | 7 | 12.47 | −19.25, 44.19 | 0.441 | 99.97 | <0.0001 | 0.121 |
| YCWE + MT − CTRL | 6 | −25.57 | −52.60, −2.54 | 0.031 | 99.89 | <0.0001 | 0.202 |
| Feed intake, g/d | |||||||
| MT − CTRL | 8 | −1.21 | −8.06, 5.65 | 0.730 | 99.78 | <0.0001 | 0.003 |
| YCWE + MT − MT | 8 | −0.80 | −4.41, 2.82 | 0.666 | 99.07 | <0.0001 | 0.009 |
| YCWE + MT − CTRL | 8 | −2.47 | −5.91, 0.96 | 0.158 | 99.26 | <0.0001 | 0.064 |
| Egg production, % | |||||||
| MT − CTRL | 6 | −6.30 | −10.74, −1.85 | 0.006 | 99.91 | <0.0001 | 0.732 |
| YCWE + MT − MT | 6 | 4.24 | 1.98, 6.50 | <0.001 | 99.52 | <0.0001 | 0.779 |
| YCWE + MT − CTRL | 6 | −5.01 | −10.40, 0.39 | 0.069 | 99.94 | <0.0001 | 0.482 |
| Egg weight, g | |||||||
| MT − CTRL | 6 | −1.95 | −2.69, −1.21 | <0.001 | 94.30 | <0.0001 | 0.292 |
| YCWE + MT − MT | 6 | 1.37 | 0.74, 1.99 | <0.001 | 93.59 | <0.0001 | 0.406 |
| YCWE + MT − CTRL | 6 | −0.67 | −1.16, −0.18 | 0.008 | 87.86 | <0.0001 | 0.745 |
| Treatments 1 | ||||
|---|---|---|---|---|
| Item | CTRL | MT | YCWE + MT | YCWE + MT vs. MT 2 |
| Number of weeks laying | 9.5 | 9.5 | 9.5 | - |
| Cumulative egg counts per hen | 62.51 | 58.52 | 61.18 | 2.66 |
| Egg mass per hen, kg | 3.72 | 3.37 | 3.60 | 0.24 |
| Edible protein per egg, g | 7.49 | 7.25 | 7.42 | 0.17 |
| Edible protein output, g | 468.20 | 424.27 | 453.96 | 29.69 |
| Cumulative feed intake per hen, kg | 7.59 | 7.53 | 7.45 | −0.08 |
| Grams of feed per egg, g | 121.42 | 128.67 | 121.77 | −6.90 |
| Average egg weight, g | 59.48 | 57.54 | 58.91 | 1.37 |
| Egg price per egg produced, USD | 0.125 | 0.125 | 0.125 | - |
| Total value of eggs sold per hen, USD | 7.81 | 7.32 | 7.65 | 0.33 |
| Feed cost per ton, USD | 316.09 | 316.09 | 323.99 | 7.90 |
| Product inclusion rate, kg | - | - | 1.58 | - |
| Feed cost per hen, USD | 2.40 | 2.38 | 2.41 | 0.03 |
| Return on investment (ROI) | 4.65:1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Meta-Analysis of the Effects of Yeast Cell Wall Extract Supple-Mentation during Mycotoxin Challenges on the Performance of Laying Hens. Toxins 2024, 16, 171. https://doi.org/10.3390/toxins16040171
Weaver AC, Weaver DM, Adams N, Yiannikouris A. Meta-Analysis of the Effects of Yeast Cell Wall Extract Supple-Mentation during Mycotoxin Challenges on the Performance of Laying Hens. Toxins. 2024; 16(4):171. https://doi.org/10.3390/toxins16040171
Chicago/Turabian StyleWeaver, Alexandra C., Daniel M. Weaver, Nicholas Adams, and Alexandros Yiannikouris. 2024. "Meta-Analysis of the Effects of Yeast Cell Wall Extract Supple-Mentation during Mycotoxin Challenges on the Performance of Laying Hens" Toxins 16, no. 4: 171. https://doi.org/10.3390/toxins16040171
APA StyleWeaver, A. C., Weaver, D. M., Adams, N., & Yiannikouris, A. (2024). Meta-Analysis of the Effects of Yeast Cell Wall Extract Supple-Mentation during Mycotoxin Challenges on the Performance of Laying Hens. Toxins, 16(4), 171. https://doi.org/10.3390/toxins16040171





