Actinobacteria as Promising Biocontrol Agents for In Vitro and In Planta Degradation and Detoxification of Zearalenone
Abstract
:1. Introduction
2. Results
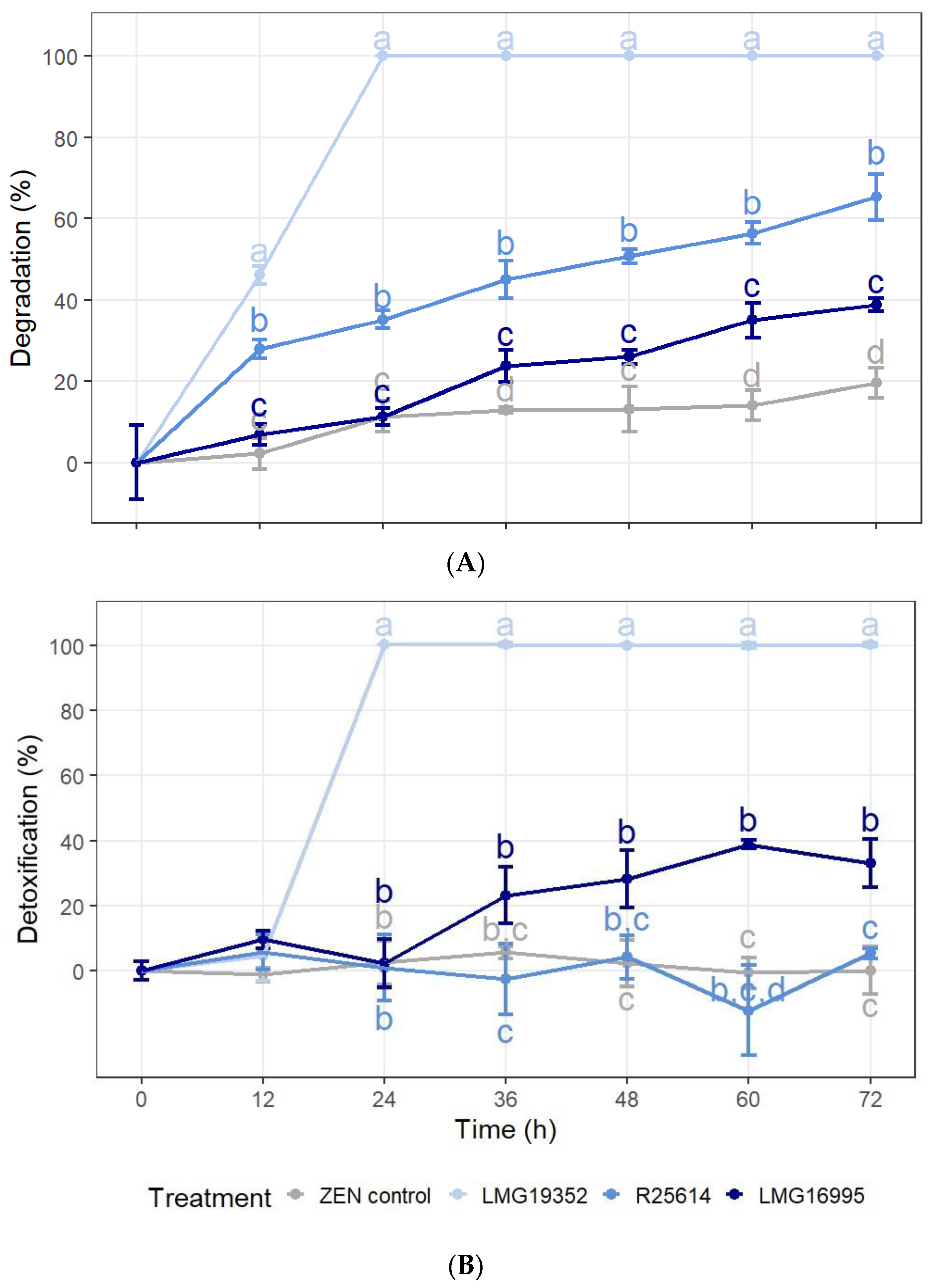
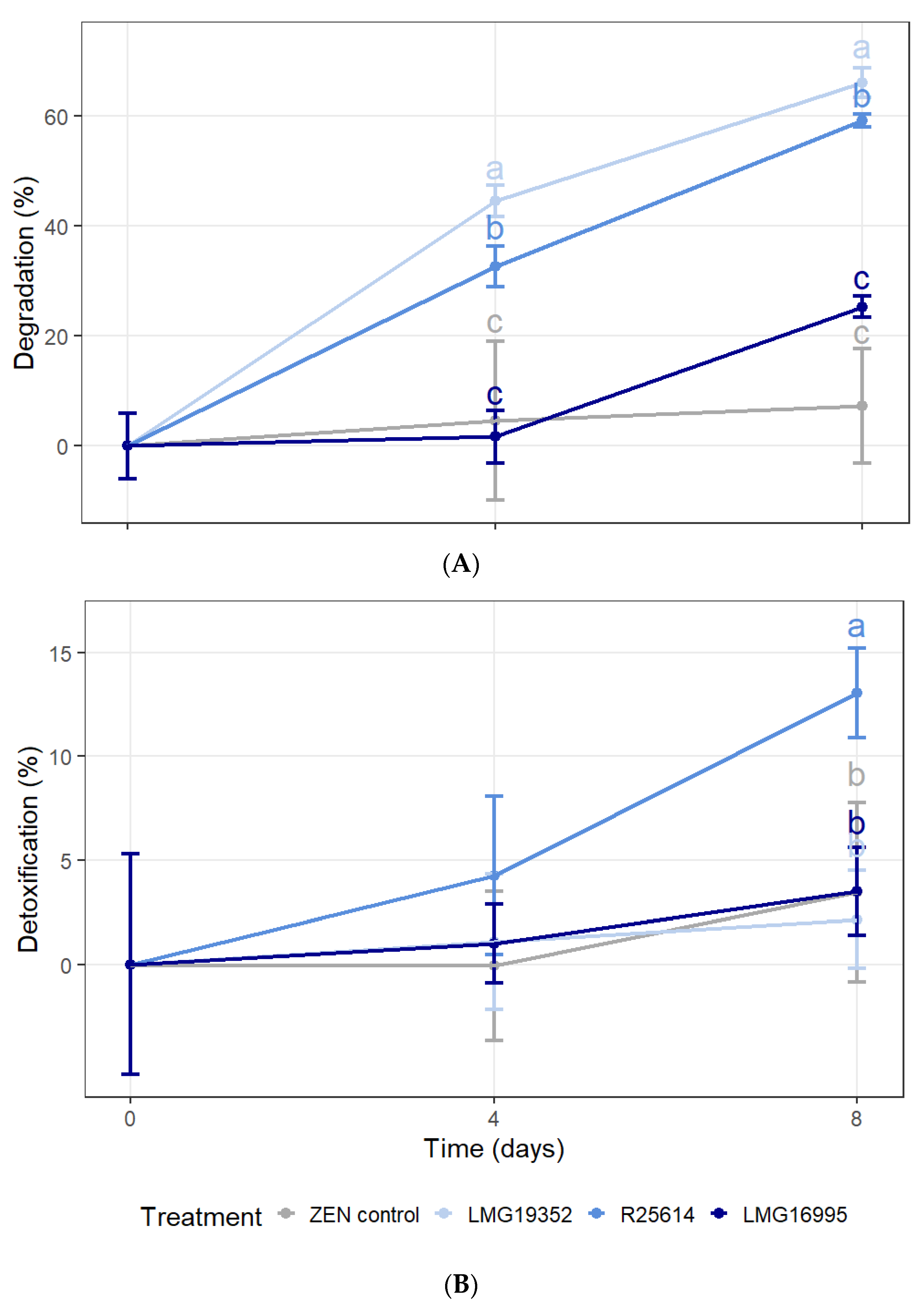
2.1. In Vitro Degradation and Detoxification of ZEN
2.1.1. Degradation, Detoxification, and Adsorption of ZEN in LB Broth
2.1.2. Degradation and Detoxification of ZEN in MM with ZEN as the Only Carbon Source
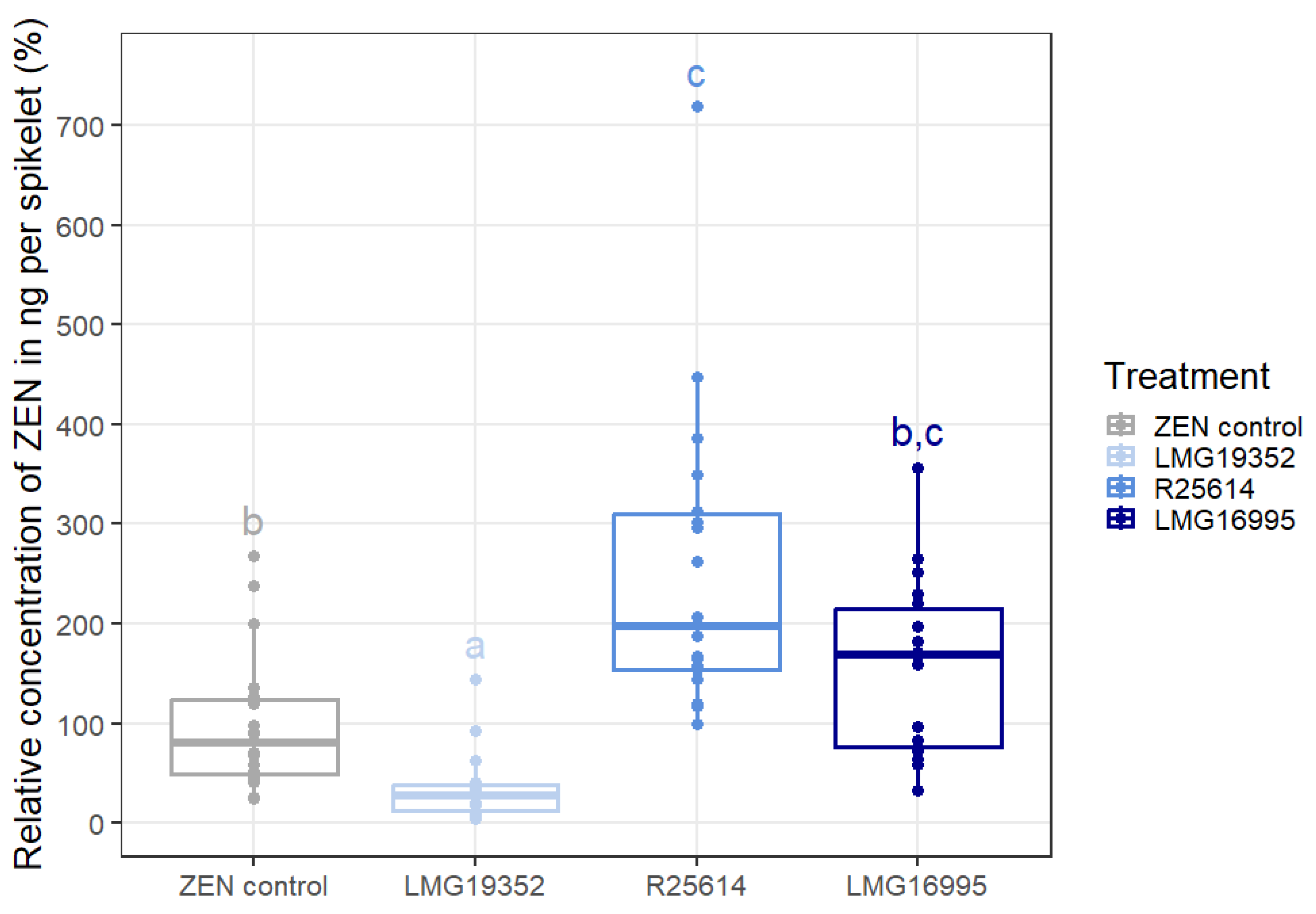
2.2. Colony-Forming Units of Actinobacteria on Wheat Ears
2.3. Degradation of ZEN on Wheat Ears
3. Discussion
3.1. Streptomyces rimosus subsp. rimosus LMG19352
3.2. Rhodococcus sp. R25614
3.3. Streptomyces sp. LMG16995
3.4. Future Perspectives
4. Conclusions
5. Materials and Methods
5.1. Actinobacterial Strains
5.2. Chemicals and Mycotoxin Standards
5.3. Biotransformation and Adsorption of ZEN
5.3.1. In Vitro Biotransformation Experiments
5.3.2. In Vitro Adsorption Experiment
5.3.3. Detached Ear Assay
5.4. Survival of Actinobacteria on Wheat Ears
5.5. Detection of ZEN by UHPLC-MS/MS
5.5.1. Sample Preparation and Mycotoxin Extraction
5.5.2. LC-MS/MS Analysis of In Vitro Degradation
5.5.3. LC-MS/MS Analysis of In Planta Degradation
5.6. Biodetoxification of ZEN: A BLYES Assay to Monitor the Residual Oestrogenicity
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maaroufi, K.; Chekir, L.; Creppy, E.E.; Ellouz, F.; Bacha, H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon 1996, 34, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Čonková, E.; Laciaková, A.; Pástorová, B.; Seidel, H.; Kováč, G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001, 121, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Berek, L.; Petri, I.B.; Mesterházy, Á.; Téren, J.; Molnár, J. Effects of mycotoxins on human immune functions in vitro. Toxicol. Vitr. 2001, 15, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ouanes, Z.; Abid, S.; Ayed, I.; Anane, R.; Mobio, T.; Creppy, E.E.; Bacha, H. Induction of micronuclei by Zearalenone in Vero monkey kidney cells and in bone marrow cells of mice: Protective effect of Vitamin E. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2003, 538, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.; Bacha, H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol. Vitr. 2004, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.; Santoro, A.; Barbieri, R.; Salzano, S.; Ursini, M. Ochratoxin A and zearalenone: A comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2003, 557, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.-Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Palacios, S.; Del Canto, A.; Erazo, J.; Torres, A. Fusarium cerealis causing Fusarium head blight of durum wheat and its associated mycotoxins. Int. J. Food Microbiol. 2021, 346, 109161. [Google Scholar] [CrossRef] [PubMed]
- Neuhof, T.; Koch, M.; Rasenko, T.; Nehls, I. Occurrence of zearalenone in wheat kernels infected with Fusarium culmorum. World Mycotoxin J. 2008, 1, 429–435. [Google Scholar] [CrossRef]
- Barros, G.; Zanon, M.A.; Palazzini, J.; Haidukowski, M.; Pascale, M.; Chulze, S. Trichothecenes and zearalenone production by Fusarium equiseti and Fusarium semitectum species isolated from Argentinean soybean. Food Addit. Contam. Part A 2012, 29, 1436–1442. [Google Scholar] [CrossRef]
- Thapa, A.; Horgan, K.A.; White, B.; Walls, D. Deoxynivalenol and zearalenone—Synergistic or antagonistic agri-food chain co-contaminants? Toxins 2021, 13, 561. [Google Scholar] [CrossRef]
- Richardson, K.E.; Hagler, W.M.; Mirocha, C.J. Production of zearalenone, .alpha.- and .beta.-zearalenol, and .alpha.- and .beta.-zearalanol by Fusarium spp. in rice culture. J. Agric. Food Chem. 1985, 33, 862–866. [Google Scholar] [CrossRef]
- Köppen, R.; Riedel, J.; Proske, M.; Drzymala, S.; Rasenko, T.; Durmaz, V.; Weber, M.; Koch, M. Photochemical trans-/cis-Isomerization and Quantitation of Zearalenone in Edible Oils. J. Agric. Food Chem. 2012, 60, 11733–11740. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Rolli, E.; Righetti, L.; Galaverna, G.; Suman, M.; Dall’asta, C.; Bruni, R. Zearalenone Uptake and Biotransformation in Micropropagated Triticum durum Desf. Plants: A Xenobolomic Approach. J. Agric. Food Chem. 2018, 66, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.; Pettersson, H.; Kiessling, K.-H. Reduction of Zearalenone to Zearalenol in Female Rat Liver by 3α-Hydroxysteroid Dehydrogenase. Acta Pharmacol. Toxicol. 1981, 48, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Devreese, M.; Antonissen, G.; Broekaert, N.; De Baere, S.; Vanhaecke, L.; De Backer, P.; Croubels, S. Comparative Toxicokinetics, Absolute Oral Bioavailability, and Biotransformation of Zearalenone in Different Poultry Species. J. Agric. Food Chem. 2015, 63, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Drzymala, S.S.; Herrmann, A.J.; Maul, R.; Pfeifer, D.; Garbe, L.-A.; Koch, M. In Vitro Phase I Metabolism of cis-Zearalenone. Chem. Res. Toxicol. 2014, 27, 1972–1978. [Google Scholar] [CrossRef]
- Drzymala, S.S.; Binder, J.; Brodehl, A.; Penkert, M.; Rosowski, M.; Garbe, L.-A.; Koch, M. Estrogenicity of novel phase I and phase II metabolites of zearalenone and cis-zearalenone. Toxicon 2015, 105, 10–12. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1055–1063. [Google Scholar] [CrossRef]
- Prelusky, D.B.; Scott, P.M.; Trenholm, H.L.; Lawrence, G.A. Minimal transmission of zearalenone to milk of dairy cows. J. Environ. Sci. Health Part B 1990, 25, 87–103. [Google Scholar] [CrossRef]
- European Commission. Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Alexandre, A.P.S.; Vela-Paredes, R.S.; Santos, A.S.; Costa, N.S.; Canniatti-Brazaca, S.G.; Calori-Domingues, M.A.; Augusto, P.E.D. Ozone treatment to reduce deoxynivalenol (DON) and zearalenone (ZEN) contamination in wheat bran and its impact on nutritional quality. Food Addit. Contam. Part A 2018, 35, 1189–1199. [Google Scholar] [CrossRef]
- Poór, M.; Zand, A.; Szente, L.; Lemli, B.; Kunsági-Máté, S. Interaction of α- and β-zearalenols with β-cyclodextrins. Molecules 2017, 22, 1910. [Google Scholar] [CrossRef]
- Ma, C.-G.; Wang, Y.-D.; Huang, W.-F.; Liu, J.; Chen, X.-W. Molecular reaction mechanism for elimination of zearalenone during simulated alkali neutralization process of corn oil. Food Chem. 2019, 307, 125546. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, W.; Pan, J.; Long, M. Detoxification strategies for zearalenone using microorganisms: A review. Microorganisms 2019, 7, 208. [Google Scholar] [CrossRef]
- Murtaza, B.; Li, X.; Dong, L.; Javed, M.T.; Xu, L.; Saleemi, M.K.; Li, G.; Jin, B.; Cui, H.; Ali, A.; et al. Microbial and enzymatic battle with food contaminant zearalenone (ZEN). Appl. Microbiol. Biotechnol. 2022, 106, 4353–4365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y. Review on microbial degradation of zearalenone and aflatoxins. Grain Oil Sci. Technol. 2020, 3, 117–125. [Google Scholar] [CrossRef]
- Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins 2022, 14, 729. [Google Scholar] [CrossRef]
- Ji, J.; Yu, J.; Ye, Y.; Sheng, L.; Fang, J.; Yang, Y.; Sun, X. Biodegradation methods and product analysis of zearalenone and its future development trend: A review. Food Control 2023, 145, 109469. [Google Scholar] [CrossRef]
- El-Sharkawy, S.H.; Abul-Hajj, Y.J. Microbial transformation of zearalenone. 2. Reduction, hydroxylation, and methylation products. J. Org. Chem. 1988, 53, 515–519. [Google Scholar] [CrossRef]
- Alkahtani, M.; Abdel-Kare, M.E.; El-Naggar, M.; Sarhan, E. Some of Soil Streptomyces Isolates Decrease Toxigenic Capability of Fusarium verticillioides in vitro. Am. J. Biochem. Mol. Biol. 2011, 1, 389–398. [Google Scholar] [CrossRef]
- Krifaton, C.; Kriszt, B.; Risa, A.; Szoboszlay, S.; Cserháti, M.; Harkai, P.; Eldridge, M.; Wang, J.; Kukolya, J. Application of a yeast estrogen reporter system for screening zearalenone degrading microbes. J. Hazard. Mater. 2013, 244–245, 429–435. [Google Scholar] [CrossRef]
- Cserháti, M.; Kriszt, B.; Krifaton, C.; Szoboszlay, S.; Háhn, J.; Tóth, S.; Nagy, I.; Kukolya, J. Mycotoxin-degradation profile of Rhodococcus strains. Int. J. Food Microbiol. 2013, 166, 176–185. [Google Scholar] [CrossRef]
- Harkai, P.; Szabó, I.; Cserháti, M.; Krifaton, C.; Risa, A.; Radó, J.; Balázs, A.; Berta, K.; Kriszt, B. Biodegradation of aflatoxin-B1 and zearalenone by Streptomyces sp. collection. Int. Biodeterior. Biodegradation 2016, 108, 48–56. [Google Scholar] [CrossRef]
- Risa, A.; Krifaton, C.; Kukolya, J.; Kriszt, B.; Cserháti, M.; Táncsics, A. Aflatoxin B1 and Zearalenone-Detoxifying Profile of Rhodococcus Type Strains. Curr. Microbiol. 2018, 75, 907–917. [Google Scholar] [CrossRef]
- Hu, J.; Du, S.; Qiu, H.; Wu, Y.; Hong, Q.; Wang, G.; Mohamed, S.R.; Lee, Y.-W.; Xu, J. A Hydrolase Produced by Rhodococcus erythropolis HQ Is Responsible for the Detoxification of Zearalenone. Toxins 2023, 15, 688. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hu, J.; Chen, X.; Qiu, Y.; Shi, J.; Wang, G.; Xu, J. Isolation and characterization of Bacillus amyloliquefaciens MQ01, a bifunctional biocontrol bacterium with antagonistic activity against Fusarium graminearum and biodegradation capacity of zearalenone. Food Control 2021, 130, 108259. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Zhu, Z.; Ji, F.; Yin, X.; Hong, Q.; Shi, J. Isolation and characterization of Bacillus amyloliquefaciens ZDS-1: Exploring the degradation of Zearalenone by Bacillus spp. Food Control 2016, 68, 244–250. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, J.; Tang, Y.; Ma, Q.; Zhang, J.; Ji, C.; Zhao, L. Characterization and Genome Analysis of a Zearalenone-Degrading Bacillus velezensis Strain ANSB01E. Curr. Microbiol. 2020, 77, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of Zearalenone (ZEN)-Detoxifying Bacillus in Animal Feed Decontamination through Fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef]
- Abdallah, M.F.; De Boevre, M.; Landschoot, S.; De Saeger, S.; Haesaert, G.; Audenaert, K. Fungal endophytes control Fusarium graminearum and reduce trichothecenes and zearalenone in maize. Toxins 2018, 10, 493. [Google Scholar] [CrossRef]
- Gimeno, A.; Kägi, A.; Drakopoulos, D.; Bänziger, I.; Lehmann, E.; Forrer, H.; Keller, B.; Vogelgsang, S. From laboratory to the field: Biological control of Fusarium graminearum on infected maize crop residues. J. Appl. Microbiol. 2020, 129, 680–694. [Google Scholar] [CrossRef]
- Balázs, A.; Faisal, Z.; Csepregi, R.; Kőszegi, T.; Kriszt, B.; Szabó, I.; Poór, M. In Vitro Evaluation of the individual and combined cytotoxic and estrogenic effects of zearalenone, its reduced metabolites, alternariol, and genistein. Int. J. Mol. Sci. 2021, 22, 6281. [Google Scholar] [CrossRef]
- Horvath, R.S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol. Rev. 1972, 36, 146–155. [Google Scholar] [CrossRef]
- EFSA Panel on Additives, Products or Substances Used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; et al. Safety and efficacy of a feed additive consisting of zearalenone hydrolase produced by Escherichia coli DSM 32731 for all terrestrial animal species (Biomin GmbH). EFSA J. 2022, 20, e07157. [Google Scholar] [CrossRef]
- Tan, J.; De Zutter, N.; De Saeger, S.; De Boevre, M.; Tran, T.M.; van der Lee, T.; Waalwijk, C.; Willems, A.; Vandamme, P.; Ameye, M.; et al. Presence of the Weakly Pathogenic Fusarium poae in the Fusarium Head Blight Disease Complex Hampers Biocontrol and Chemical Control of the Virulent Fusarium graminearum Pathogen. Front. Plant Sci. 2021, 12, 641890. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, L.; Wu, H.; Tang, Y.; Yu, Y.; Li, X.; Liu, D. Degradation of zearalenone by the extracellular extracts of Acinetobacter sp. SM04 liquid cultures. Biodegradation 2011, 22, 613–622. [Google Scholar] [CrossRef]
- Ahad, R.; Zhou, T.; Lepp, D.; Pauls, K.P. Microbial detoxification of eleven food and feed contaminating trichothecene mycotoxins. BMC Biotechnol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Cetin, Y.; Bullerman, L.B. Evaluation of Reduced Toxicity of Zearalenone by Extrusion Processing as Measured by the MTT Cell Proliferation Assay. J. Agric. Food Chem. 2005, 53, 6558–6563. [Google Scholar] [CrossRef]
- Blackwell, B.A.; Miller, J.D.; Greenhalgh, R. 13C NMR study of the biosynthesis of toxins by Fusarium graminearum. J. Biol. Chem. 1985, 260, 4243–4247. [Google Scholar] [CrossRef]
- Stadler, D.; Lambertini, F.; Bueschl, C.; Wiesenberger, G.; Hametner, C.; Schwartz-Zimmermann, H.; Hellinger, R.; Sulyok, M.; Lemmens, M.; Schuhmacher, R.; et al. Untargeted LC–MS based 13C labelling provides a full mass balance of deoxynivalenol and its degradation products formed during baking of crackers, biscuits and bread. Food Chem. 2019, 279, 303–311. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Berthiller, F.; Häubl, G.; Jaunecker, G.; Adam, G.; Krska, R.; Schuhmacher, R. Stable isotopic labelling-assisted untargeted metabolic profiling reveals novel conjugates of the mycotoxin deoxynivalenol in wheat. Anal. Bioanal. Chem. 2012, 405, 5031–5036. [Google Scholar] [CrossRef]
- Blackwell, B.A.; Edwards, O.E.; Fruchier, A.; ApSimon, J.W.; Miller, J.D. NMR Structural Studies of Fumonisin B1 and Related Compounds from Fusarium moniliforme. In Fumonisins in Food; Springer: Boston, MA, USA, 1996; pp. 75–91. [Google Scholar] [CrossRef]
- Meng-Reiterer, J.; Bueschl, C.; Rechthaler, J.; Berthiller, F.; Lemmens, M.; Schuhmacher, R. Metabolism of HT-2 Toxin and T-2 Toxin in Oats. Toxins 2016, 8, 364. [Google Scholar] [CrossRef]
- Meng-Reiterer, J.; Varga, E.; Nathanail, A.V.; Bueschl, C.; Rechthaler, J.; McCormick, S.P.; Michlmayr, H.; Malachová, A.; Fruhmann, P.; Adam, G.; et al. Tracing the metabolism of HT-2 toxin and T-2 toxin in barley by isotope-assisted untargeted screening and quantitative LC-HRMS analysis. Anal. Bioanal. Chem. 2015, 407, 8019–8033. [Google Scholar] [CrossRef]
- El-Naggar, M.A.; Alkahtani, M.D.; Thabit, T.M.; Sarhan, E.A.; Morsy, K.M. In Vitro Study on Influence of some Streptomyces strains isolated from date palm rhizosphere soil on some toxigenic fungi. Foodborne Pathog. Dis. 2012, 9, 646–654. [Google Scholar] [CrossRef]
- Deroo, W.; De Troyer, L.; Dumoulin, F.; De Saeger, S.; De Boevre, M.; Vandenabeele, S.; De Gelder, L.; Audenaert, K. A Novel In Planta Enrichment Method Employing Fusarium graminearum-Infected Wheat Spikes to Select for Competitive Biocontrol Bacteria. Toxins 2022, 14, 222. [Google Scholar] [CrossRef]
- Dellafiora, L.; Galaverna, G.; Dall’Asta, C.; Cozzini, P. Hazard identification of cis/trans -zearalenone through the looking-glass. Food Chem. Toxicol. 2015, 86, 65–71. [Google Scholar] [CrossRef]
- Vanhoutte, I.; De Mets, L.; De Boevre, M.; Uka, V.; Di Mavungu, J.D.; De Saeger, S.; De Gelder, L.; Audenaert, K. Microbial Detoxification of Deoxynivalenol (DON), Assessed via a Lemna minor L. Bioassay, through Biotransformation to 3-epi-DON and 3-epi-DOM-1. Toxins 2017, 9, 63. [Google Scholar] [CrossRef]
- Lauwers, M.; De Baere, S.; Letor, B.; Rychlik, M.; Croubels, S.; Devreese, M. Multi LC-MS/MS and LC-HRMS methods for determination of 24 mycotoxins including major phase I and II biomarker metabolites in biological matrices from pigs and broiler chickens. Toxins 2019, 11, 171. [Google Scholar] [CrossRef]
- Sanseverino, J.; Gupta, R.K.; Layton, A.C.; Patterson, S.S.; Ripp, S.A.; Saidak, L.; Simpson, M.L.; Schultz, T.W.; Sayler, G.S. Use of Saccharomyces cerevisiae BLYES expressing bacterial bioluminescence for rapid, sensitive detection of estrogenic compounds. Appl. Environ. Microbiol. 2005, 71, 4455–4460. [Google Scholar] [CrossRef] [PubMed]
- Hadley, W. ggplot2-Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016; Available online: http://www.springer.com/series/6991 (accessed on 19 February 2024).
| Treatment | CFU/Spikelet ± SD |
|---|---|
| LMG19352 | 1.22 × 104 ± 1.80 × 103 ** |
| R25614 | 3.43 × 103 ± 3.73 × 103 ns |
| LMG16995 | 6.67 × 101 ± 1.15 × 102 ns |
| Time (Days) | ZEN Concentration (ng/Spikelet) ± SD |
|---|---|
| 0 | 11.04 ± 2.76 |
| 5 | 1.84 ± 1.35 |
| Analyte | ESI Mode | Quantifier Ion | Qualifier Ion | |||||
|---|---|---|---|---|---|---|---|---|
| Precursor Ion > Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | Precursor Ion > Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | |||
| ZEN | + | 319.0 > 283.0 | 40 | 12 | 319.0 > 301.0 | 40 | 12 | |
| ZAN | + | 321.2 > 189.1 | 40 | 12 | 321.2 > 303.3 | 40 | 8 | |
| α-ZEL | + | 321.0 > 285.0 | 40 | 11 | 321.0 > 303.0 | 40 | 7 | |
| β-ZEL | + | 321.0 > 285.0 | 40 | 11 | 321.0 > 303.0 | 40 | 8 | |
| α-ZAL | − | 323.2 > 123.0 | 30 | 23 | 323.2 > 305.2 | 30 | 23 | |
| β-ZAL | − | 323.2 > 189.1 | 30 | 23 | 323.2 > 305.2 | 30 | 23 | |
| Analyte | LOD (µg/L) | LOQ (µg/L) | Accuracy (%) |
|---|---|---|---|
| ZEN | 5 | 9 | 99.60 |
| α-ZEL | 10 | 20 | 106.33 |
| β-ZEL | 14 | 29 | 102.37 |
| α-ZAL | 27 | 55 | 96.49 |
| β-ZAL | 13 | 26 | 109.39 |
| Analyte | ESI Mode | Quantifier Ion | Qualifier Ion | |||||
|---|---|---|---|---|---|---|---|---|
| Precursor Ion > Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | Precursor Ion > Product Ion (m/z) | Cone Voltage (V) | Collision Energy (eV) | |||
| ZEN | - | 317.1 > 175.0 | 15 | 25 | 317.1 > 130.8 | 15 | 30 | |
| ZAN | - | 319.1 > 275.0 | 20 | 22 | 319.1 > 205.0 | 20 | 20 | |
| Analyte | LOD (ng) | LOQ (ng) | Accuracy (%) |
|---|---|---|---|
| ZEN | 0.067 | 0.222 | 100.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Troyer, L.; De Zutter, N.; De Saeger, S.; Dumoulin, F.; Croubels, S.; De Baere, S.; De Gelder, L.; Audenaert, K. Actinobacteria as Promising Biocontrol Agents for In Vitro and In Planta Degradation and Detoxification of Zearalenone. Toxins 2024, 16, 253. https://doi.org/10.3390/toxins16060253
De Troyer L, De Zutter N, De Saeger S, Dumoulin F, Croubels S, De Baere S, De Gelder L, Audenaert K. Actinobacteria as Promising Biocontrol Agents for In Vitro and In Planta Degradation and Detoxification of Zearalenone. Toxins. 2024; 16(6):253. https://doi.org/10.3390/toxins16060253
Chicago/Turabian StyleDe Troyer, Larissa, Noémie De Zutter, Sarah De Saeger, Frédéric Dumoulin, Siska Croubels, Siegrid De Baere, Leen De Gelder, and Kris Audenaert. 2024. "Actinobacteria as Promising Biocontrol Agents for In Vitro and In Planta Degradation and Detoxification of Zearalenone" Toxins 16, no. 6: 253. https://doi.org/10.3390/toxins16060253









