Selection of Candidate Monoclonal Antibodies for Therapy of Botulinum Toxin Type A Intoxications
Abstract
:1. Introduction
2. Results
2.1. Specific Activity of Antibodies against Closely Related Molecules
2.2. Inhibitory Activity of Antibodies against the Proteolytic Action of the Light Chain of Botulinum Toxin Type A
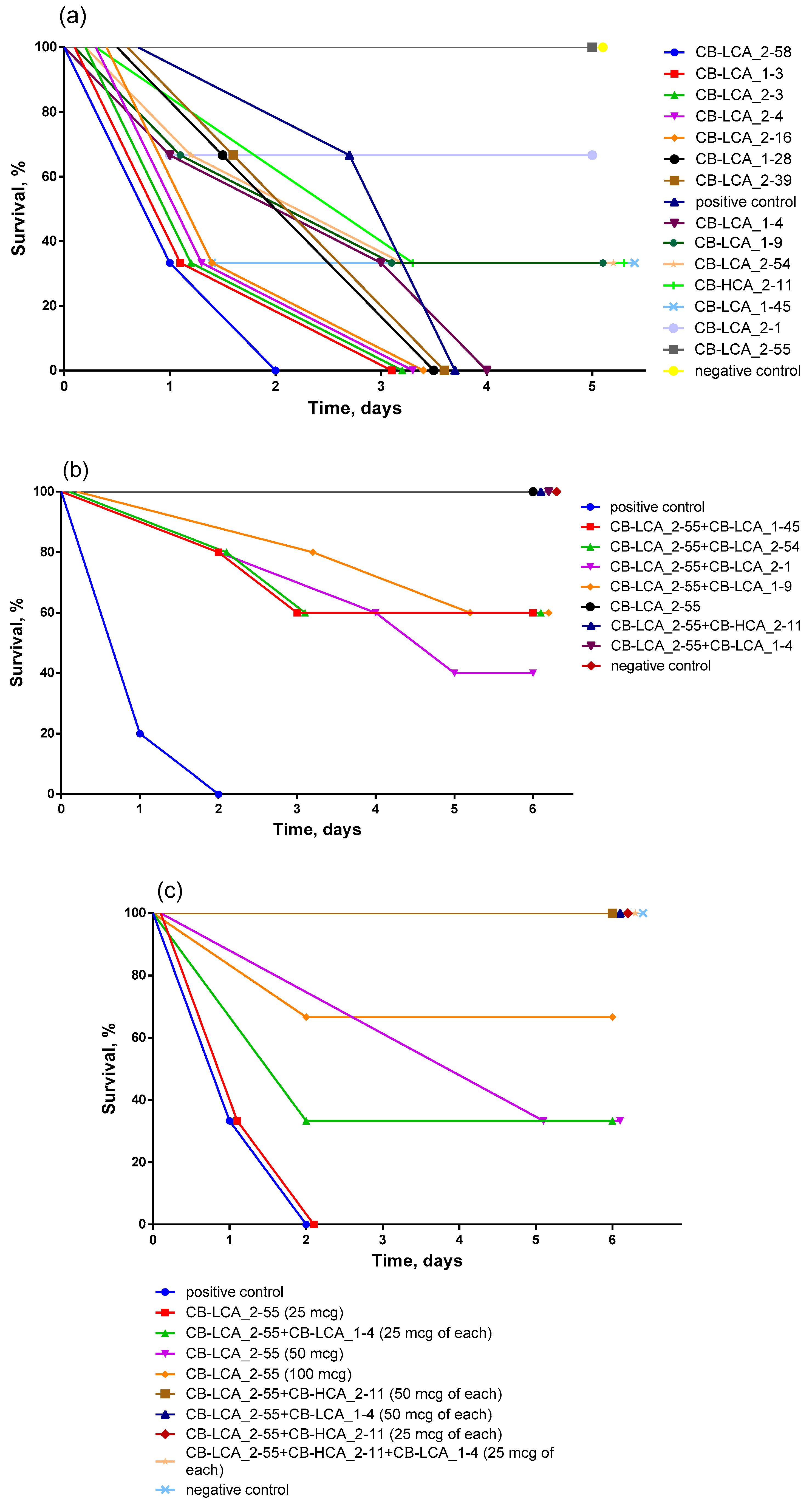
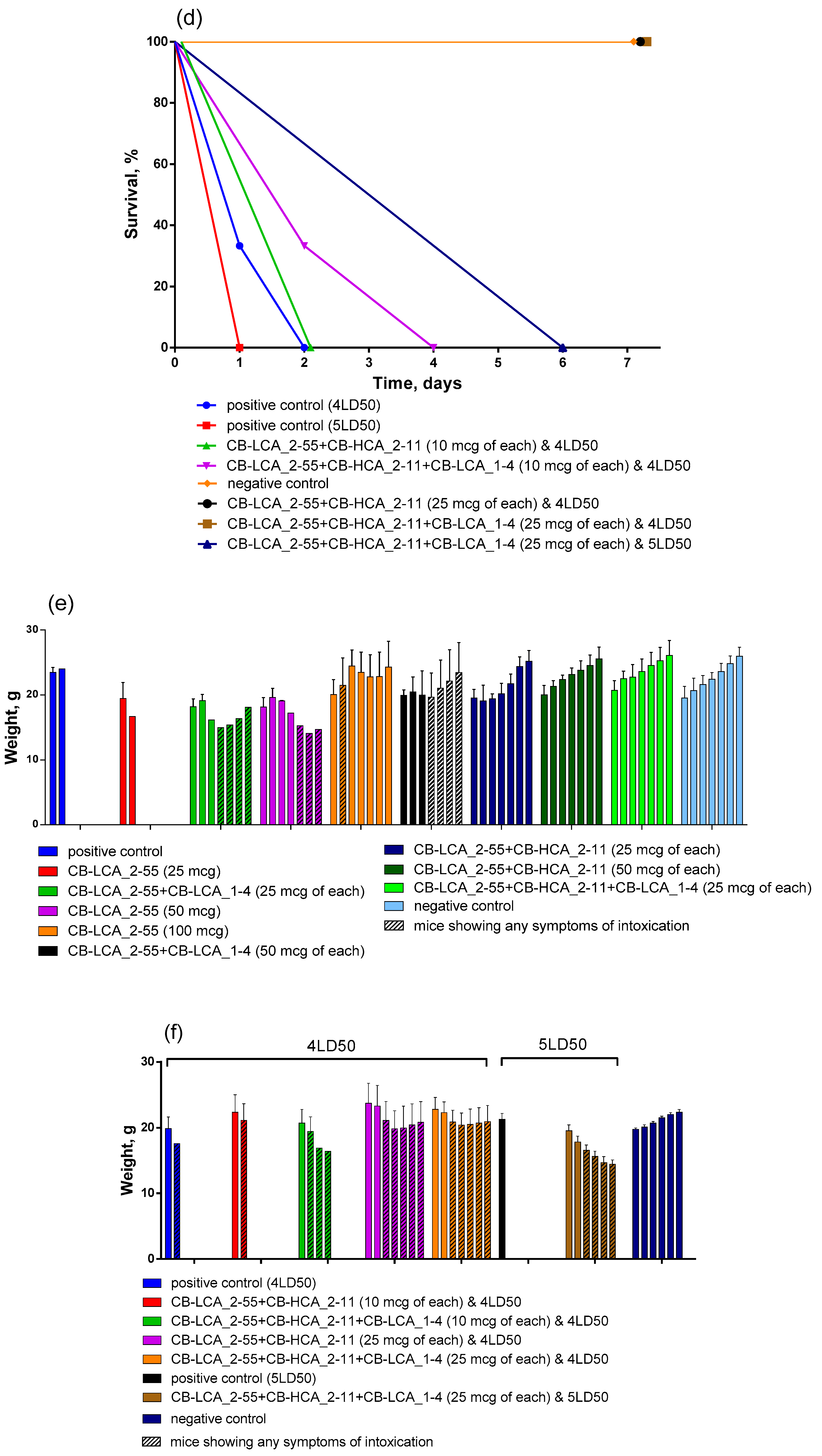
2.3. Antitoxic Activity of Antibodies against Validated Native Toxin and Selection of the Most Effective Combinations of Antibodies by Mouse Bioassay
2.4. Characterization of Selected Antibodies
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Production of Recombinant Proteins
5.3. Preparation of Botulinum Toxin Type A
5.4. Immunization of Animals
5.5. Hybridoma Production
5.6. Obtaining Antibodies from Ascitic Fluid
5.7. Analysis of mAbs Specificity by Immunoblotting
5.8. Determination of the Antibodies Ability to Inhibit Proteolytic Activity of the Light Chain of Botulinum Toxin Type A
5.9. Analysis of the Antitoxic Activity of mAbs Using a Mouse Bioassay
5.10. Determination of Isotypes
5.11. Determination of the Equilibrium Dissociation Constant Values
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rasetti-Escargueil, C.; Popoff, M.R. Antibodies and Vaccines against Botulinum Toxins: Available Measures and Novel Approaches. Toxins 2019, 11, 528. [Google Scholar] [CrossRef]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef]
- Eruslanov, B.V.; Svetoch, E.A.; Mitsevich, I.P.; Fursova, N.K.; Dyatlov, I.A. Botulism: Characterization of the Pathogen and the Laboratory Diagnostic Methods. Bacteriology 2018, 3, 47–59. (In Russian) [Google Scholar]
- Glass, K.; Marshall, K. Clostridium botulinum. In Foodborne Infections and Intoxications, 4th ed.; Morris, J.G., Potter, M.E., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 371–387. [Google Scholar] [CrossRef]
- Singh, B.R. Intimate details of the most poisonous poison. Nat. Struct. Biol. 2000, 7, 617–619. [Google Scholar] [CrossRef]
- Poulain, B.; Lonchamp, E.; Jover, E.; Popoff, M.R.; Molgó, J. Mechanisms of action of botulinum toxins and neurotoxins. Ann. Dermatol. Venereol. 2009, 136 (Suppl. 4), S73–S76. [Google Scholar]
- Thanongsaksrikul, J.; Chaicumpa, W. Botulinum Neurotoxins and Botulism: A Novel Therapeutic Approach. Toxins 2011, 3, 469–488. [Google Scholar] [CrossRef]
- Capek, P.; Dickerson, T.J. Sensing the deadliest toxin: Technologies for botulinum neurotoxin detection. Toxins 2010, 2, 24–53. [Google Scholar]
- Dover, N.; Barash, J.R.; Hill, K.K.; Xie, G.; Arnon, S.S. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 2014, 209, 192–202. [Google Scholar] [CrossRef]
- Smith, T.J.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Tsai, R.; Laporte, S.L.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Smith, L.A.; et al. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect. Immun. 2005, 73, 5450–5457. [Google Scholar] [CrossRef]
- Arndt, J.W.; Jacobson, M.J.; Abola, E.E.; Forsyth, C.M.; Tepp, W.H.; Marks, J.D.; Johnson, E.A.; Stevens, R.C. A structural perspective of the sequence variability within botulinum neurotoxin subtypes A1–A4. J. Mol. Biol. 2006, 362, 733–742. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, C.; Levy, R.; Arndt, J.W.; Forsyth, C.M.; Razai, A.; Lou, J.; Geren, I.; Stevens, R.C.; Marks, J.D. Molecular evolution of antibody cross-reactivity for two subtypes of type A botulinum neurotoxin. Nat. Biotechnol. 2007, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Adekar, S.P.; Takahashi, T.; Jones, R.M.; Al-Saleem, F.H.; Ancharski, D.M.; Root, M.J.; Kapadnis, B.P.; Simpson, L.L.; Dessain, S.K. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS ONE 2008, 3, e3023. [Google Scholar] [CrossRef] [PubMed]
- Nepal, M.R.; Jeong, T.C. Alternative Methods for Testing Botulinum Toxin: Current Status and Future Perspectives. Biomol. Ther. 2020, 28, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Tepp, W.H.; Johnson, E.A. Critical Analysis of Neuronal Cell and the Mouse Bioassay for Detection of Botulinum Neurotoxins. Toxins 2019, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.K.; Casadevall, A. Monoclonal antibodies and toxins--a perspective on function and isotype. Toxins 2012, 4, 430–454. [Google Scholar] [CrossRef]
- Nowakowski, A.; Wang, C.; Powers, D.B.; Amersdorfer, P.; Smith, T.J.; Montgomery, V.A.; Sheridan, R.; Blake, R.; Smith, L.A.; Marks, J.D. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 2002, 99, 11346–11350. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, T. Antibody cocktails: Next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 2007, 25, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Lindström, M.; Korkeala, H. Laboratory diagnostics of botulism. Clin. Microbiol. Rev. 2006, 19, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kim, Y.I.; Kang, K.K.; Oh, S.K.; Sung, S.E.; Jung, Y.S.; Cho, J.Y.; Song, H.; Hwang, D.Y.; Park, S.J.; et al. Comparison study of the response with botulinum toxin muscle injection in the ICR mice from three different sources. Lab. Anim. Res. 2019, 35, 11. [Google Scholar] [CrossRef]
- Dembek, Z.F.; Smith, L.A.; Rusnak, J.M. Botulism: Cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med. Public Health Prep. 2007, 1, 122–134. [Google Scholar] [CrossRef]
- Davis, L.E. Botulism. Curr. Treat. Options Neurol. 2003, 5, 23–31. [Google Scholar] [CrossRef]
- Maslanka, S.E. Botulism as a Disease of Humans. In Molecular Aspects of Botulinum Neurotoxin; Foster, K.A., Ed.; Springer: New York, NY, USA, 2014; pp. 259–289. [Google Scholar] [CrossRef]
- Mayers, C.N.; Holley, J.L.; Brooks, T. Antitoxin therapy for botulinum intoxication. Rev. Med. Microbiol. 2001, 12, 29–37. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, C.; Geren, I.N.; Lou, J.; Conrad, F.; Forsyth, C.; Wen, W.; Chakraborti, S.; Zao, H.; Manzanarez, G.; Smith, T.J.; et al. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng. Des. Sel. 2011, 24, 321–331. [Google Scholar] [CrossRef]
- Feltrup, T.M.; Singh, B.R. Development of a fluorescence internal quenching correction factor to correct botulinum neurotoxin type A endopeptidase kinetics using SNAPtide. Anal. Chem. 2012, 84, 10549–10553. [Google Scholar] [CrossRef]
- Cheng, L.W.; Stanker, L.H.; Henderson, T.D.; Lou, J.; Marks, J.D. Antibody protection against botulinum neurotoxin intoxication in mice. Infect. Immun. 2009, 77, 4305–4313. [Google Scholar] [CrossRef]
- Diamant, E.; Lachmi, B.-E.; Keren, A.; Barnea, A.; Marcus, H.; Cohen, S.; David, A.B.; Zichel, R. Evaluating the Synergistic Neutralizing Effect of Anti-Botulinum Oligoclonal Antibody Preparations. PLoS ONE 2014, 29, e87089. [Google Scholar] [CrossRef]
- LoBuglio, A.F.; Wheeler, R.H.; Trang, J.; Haynes, A.; Rogers, K.; Harvey, E.B.; Sun, L.; Ghrayeb, J.; Khazaeli, M.B. Mouse/human chimeric monoclonal antibody in man: Kinetics and immune response. Proc. Natl. Acad. Sci. USA 1989, 86, 4220–4224. [Google Scholar] [CrossRef]
- Kolesnikov, A.V.; Zakharova, M.Y.; Kozyr, A.V.; Shemyakin, I.G. Way of Reception Functionally Active Recombinant Protein of Ulcer (LF) Lethal Factor, Recombinant Plasmide DNA pETHIS-LF Coding Active Protein LF; H Strain Escherichia coli BL-HISLF Producing Active Protein of Malignant Anthrax Lethal Factor. RU Patent No. RU 2 361 921 C1, 23 November 2007. (In Russian). [Google Scholar]
- Ryabko, A.K.; Kozyr, A.V.; Kolesnikov, A.V.; Khlyntseva, A.E.; Zharnikova, I.V.; Shemyakin, I.G. Strategies for upgrading analyte detection in immuno-PCR studied on identification of type A botulinum neurotoxin. Appl. Biochem. Microbiol. 2016, 52, 110–120. [Google Scholar] [CrossRef]
- Malizio, C.J.; Goodnough, M.C.; Johnson, E.A. Purification of Clostridium botulinum type A neurotoxin. Methods Mol. Biol. 2000, 145, 27–39. [Google Scholar] [CrossRef]
- Stewart, S.J. Monoclonal Antibody Production. In Basic Methods in Antibody Production and Characterization; Howard, G.C., Bethell, D.R., Eds.; CRC Press: New York, NY, USA, 2000; pp. 51–67. [Google Scholar] [CrossRef]
- Zeninskaya, N.A.; Riabko, A.K.; Marin, M.A.; Kombarova, T.I.; Mitsevich, I.P.; Yeruslanov, B.V.; Firstova, V.V.; Shemyakin, I.G. Production and Characterization of Rat Monoclonal Antibodies against the PAL Antigen of Legionella spp. Mol. Gen. Microbiol. Virol. 2022, 37, 65–70. [Google Scholar] [CrossRef]
- Bradbury, A. Coning Hybridoma cDNA by RACE. In Antibody Engineering. Springer Protocols Handbooks, 2nd ed.; Kontermann, R., Dübel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 15–20. [Google Scholar] [CrossRef]


| # | mAb | Analyzed Antigens | ||||
|---|---|---|---|---|---|---|
| rBoNT/A-LC | rBoNT/A-HC50 | rBoNT/B-LC | rBoNT/B-HC50 | Native BoNT/A * | ||
| 1 | CB-LCA_1-3 | +/+ | +/+ | −/− | +/+ | + |
| 2 | CB-LCA_1-4 | +/+ | +/+ | −/− | +/+ | + |
| 3 | CB-LCA_1-9 | +/+ | −/− | −/− | −/− | + |
| 4 | CB-LCA_1-28 | +/+ | +/+ | −/− | +/+ | + |
| 5 | CB-LCA_1-45 | +/+ | +/+ | −/− | +/+ | + |
| 6 | CB-LCA_2-1 | +/+ | +/+ | −/− | +/+ | + |
| 7 | CB-LCA_2-3 | +/+ | +/+ | −/− | +/+ | + |
| 8 | CB-LCA_2-4 | +/+ | +/+ | −/− | +/+ | + |
| 9 | CB-HCA_2-11 | +/+ | +/+ | −/− | +/− | + |
| 10 | CB-LCA_2-16 | +/+ | +/+ | −/− | +/+ | + |
| 11 | CB-LCA_2-39 | +/+ | +/+ | −/− | +/− | + |
| 12 | CB-LCA_2-54 | +/+ | +/+ | −/− | +/+ | + |
| 13 | CB-LCA_2-55 | +/+ | +/+ | −/− | +/+ | + |
| 14 | CB-LCA_2-58 | +/+ | +/+ | −/− | +/+ | + |
| mAb | KD, M (to Target Protein) | Heavy Chain Type | Light Chain Type |
|---|---|---|---|
| CB-LCA_1-4 | 5.29·10−7 | G1 | κ |
| CB-HCA_2-11 | 8.97·10−8 | G1 | κ |
| CB-LCA_2-55 | 4.22·10−7 | G1 | κ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeninskaya, N.A.; Ryabko, A.K.; Marin, M.A.; Kombarova, T.I.; Shkuratova, M.A.; Rogozin, M.M.; Silkina, M.V.; Romanenko, Y.O.; Ivashchenko, T.A.; Shemyakin, I.G.; et al. Selection of Candidate Monoclonal Antibodies for Therapy of Botulinum Toxin Type A Intoxications. Toxins 2024, 16, 284. https://doi.org/10.3390/toxins16070284
Zeninskaya NA, Ryabko AK, Marin MA, Kombarova TI, Shkuratova MA, Rogozin MM, Silkina MV, Romanenko YO, Ivashchenko TA, Shemyakin IG, et al. Selection of Candidate Monoclonal Antibodies for Therapy of Botulinum Toxin Type A Intoxications. Toxins. 2024; 16(7):284. https://doi.org/10.3390/toxins16070284
Chicago/Turabian StyleZeninskaya, Natalia A., Alena K. Ryabko, Maksim A. Marin, Tatyana I. Kombarova, Maria A. Shkuratova, Methun M. Rogozin, Marina V. Silkina, Yana O. Romanenko, Tatiana A. Ivashchenko, Igor G. Shemyakin, and et al. 2024. "Selection of Candidate Monoclonal Antibodies for Therapy of Botulinum Toxin Type A Intoxications" Toxins 16, no. 7: 284. https://doi.org/10.3390/toxins16070284
APA StyleZeninskaya, N. A., Ryabko, A. K., Marin, M. A., Kombarova, T. I., Shkuratova, M. A., Rogozin, M. M., Silkina, M. V., Romanenko, Y. O., Ivashchenko, T. A., Shemyakin, I. G., & Firstova, V. V. (2024). Selection of Candidate Monoclonal Antibodies for Therapy of Botulinum Toxin Type A Intoxications. Toxins, 16(7), 284. https://doi.org/10.3390/toxins16070284





