Rhein Inhibits Cell Development and Aflatoxin Biosynthesis via Energy Supply Disruption and ROS Accumulation in Aspergillus flavus
Abstract
:1. Introduction
2. Results
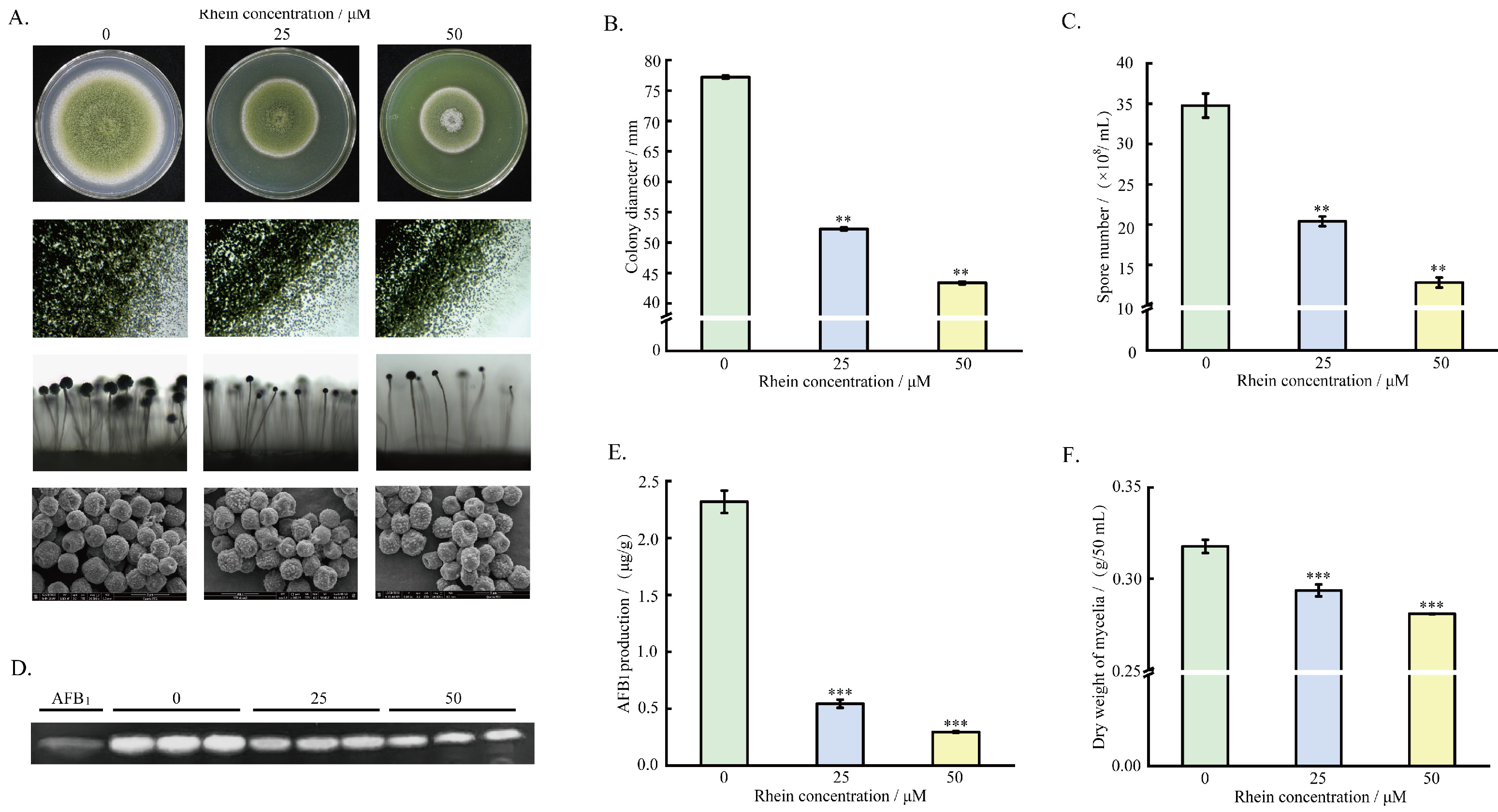
2.1. Effect of Rhein on A. flavus Growth, Spore Formation, and AFB1 Production
2.2. Transcriptomic Analysis
2.3. Effect of Rhein on the Hydrophobicity of A. flavus
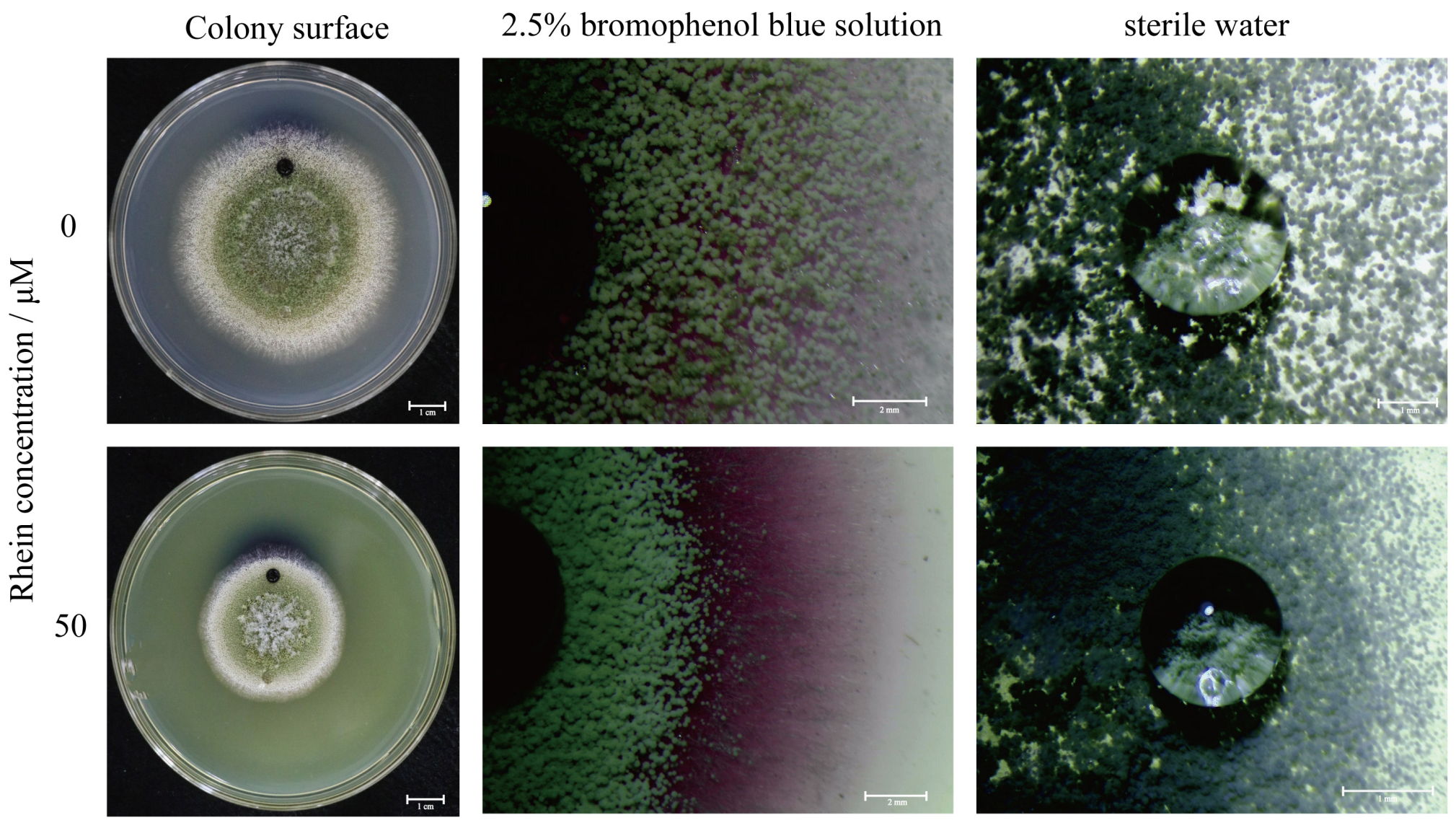
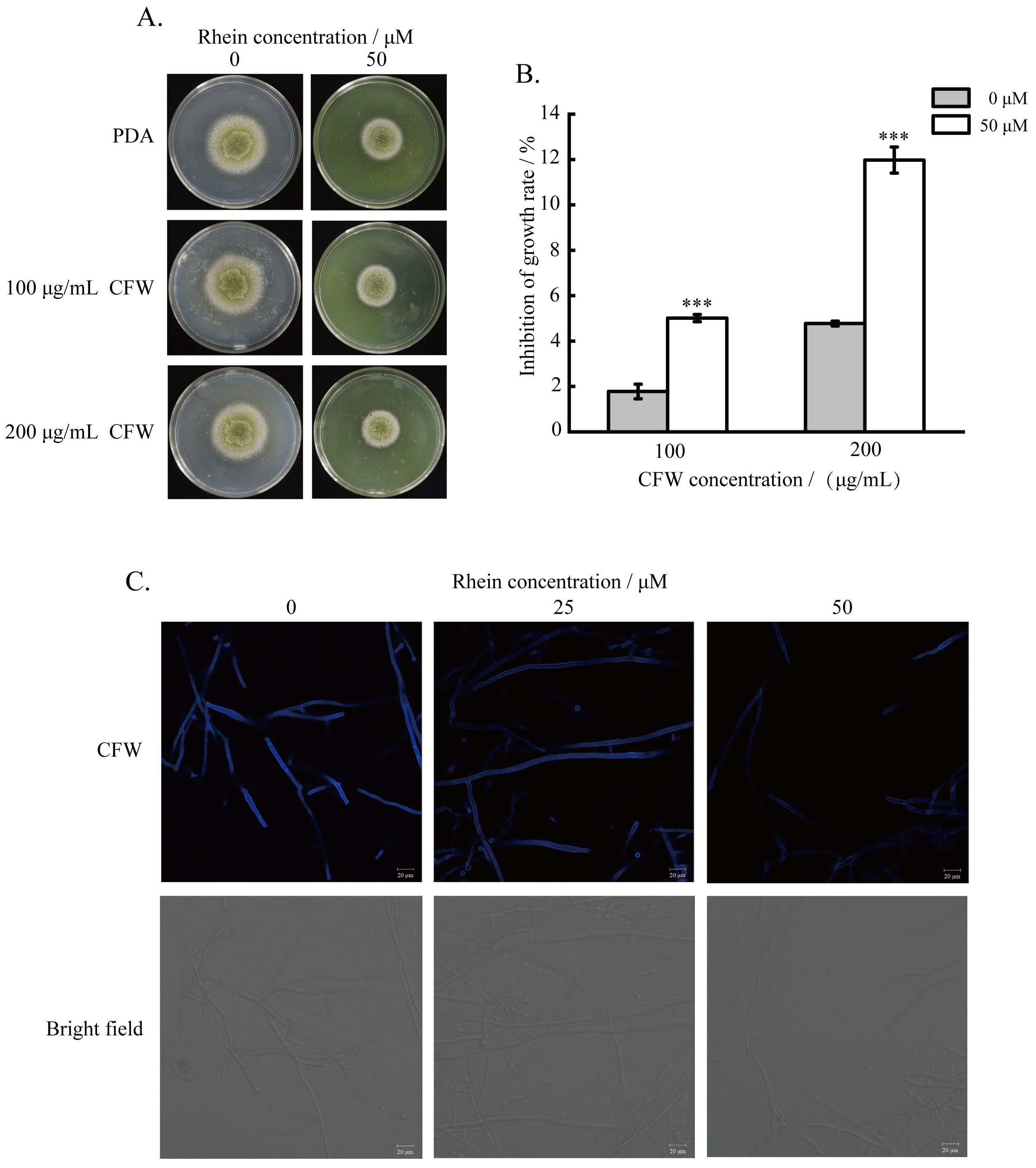
2.4. Effect of the Rhein on the Integrity of A. flavus Cell Wall
2.5. Effect of Rhein on the Integrity of Cell Membrane
2.6. Effect of the Rhein on A. flavus Pathogenicity on Crop Seeds
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains, Chemicals, and Medium
5.2. Effect of Rhein on Phenotype, Spore, and Aflatoxin B1 Production of A. flavus
5.3. RNA-seq Analysis
5.4. Effect of Rhein on Hydrophobicity of A. flavus
5.5. Effect of Rhein on A. flavus Cell Wall
5.6. Effect of Rhein on A. flavus Cell Membrane
5.7. Determination of A. flavus Intracellular ROS, H2O2, and ATP Content
5.8. Effect of Rhein on A. flavus Mycelium Growth and AFB1 Yield on Peanuts and Maize
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Qin, L.; Wang, Y.; Xie, Q.; Li, N.; Wang, S.; Yuan, J. AflSte20 regulates morphogenesis, stress response, and aflatoxin biosynthesis of Aspergillus flavus. Toxins 2019, 11, 730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Han, X.; Guo, Z.; Yang, W.; Liu, Y.; Yang, K.; Zhuang, Z.; Wang, S. Proteomic profile of Aspergillus flavus in response to water activity. Fungal Biol. 2015, 119, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; Khoury, A.A.; Khoury, R.E.; Lorber, S.; Oswald, I.P.; Khoury, A.E.; Atoui, A.; Puel, O.; Bailly, J.D. Aflatoxin biosynthesis and genetic regulation: A review. Toxins 2020, 12, 150. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, G.; Zhang, D.; Liu, Y.; Li, Y.; Lin, G.; Guo, Z.; Wang, S.; Zhuang, Z. The PHD transcription factor Rum1 regulates morphogenesis and aflatoxin biosynthesis in Aspergillus flavus. Toxins 2018, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Aflatoxin biosynthesis, genetic regulation, toxicity, and control strategies: A review. J Fungi 2021, 7, 606. [Google Scholar] [CrossRef]
- Eom, T.J.; Moon, H.; Yu, J.H.; Park, H.S. Characterization of the velvet regulators in Aspergillus flavus. J. Microbiol. 2018, 56, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.; Song, M.; Zhang, J. Current strategies in controlling Aspergillus flavus and aflatoxins in grains during storage: A review. Sustainability 2024, 16, 3171. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; El-Wakil, D.A.; Attia, M.S.; Ali, O.M.; AbdElgawad, H.; Hashem, A.H. Inhibition of Aspergillus flavus growth and aflatoxin production in Zea mays L. using Endophytic Aspergillus fumigatus. J Fungi 2022, 8, 482. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Mansour, C.; Kouidhi, B.; Chaieb, K. Use of lactic acid bacteria for the inhibition of Aspergillus flavus and Aspergillus carbonarius growth and mycotoxin production. Toxicon 2019, 166, 15–23. [Google Scholar] [CrossRef]
- Liang, D.; Xing, F.; Selvaraj, J.N.; Liu, X.; Wang, L.; Hua, H.; Zhou, L.; Zhao, Y.; Wang, Y.; Liu, Y. Inhibitory effect of cinnamaldehyde, citral, and eugenol on aflatoxin biosynthetic gene expression and aflatoxin B1 biosynthesis in Aspergillus flavus. J. Food Sci. 2015, 80, M2917–M2924. [Google Scholar] [CrossRef]
- Wu, C.; Cao, H.; Zhou, H.; Sun, L.; Xue, J.; Li, J.; Bian, Y.; Sun, R.; Dong, S.; Liu, P.; et al. Research progress on the antitumor effects of rhein: Literature review. Anticancer Agents Med. Chem. 2017, 17, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Li, G.M.; Chen, J.R.; Zhang, H.Q.; Cao, X.Y.; Sun, C.; Peng, F.; Yin, Y.P.; Lin, Z.; Yu, L.; Chen, Y.; et al. Update on pharmacological activities, security, and pharmacokinetics of rhein. Evid. Based Complement Altern. Med. 2021, 2021, 4582412. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front. Pharmacol. 2016, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, J.K.; Huang, H.; Park, S.; Park, S.; Kwon, W.; Kim, E.; Jang, S.; Kim, S.Y.; Choi, S.K.; et al. Rhein suppresses colorectal cancer cell growth by inhibiting the mTOR pathway in vitro and in vivo. Cancers 2021, 13, 2176. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.L.; Chen, F.; Shi, J. Rhein inhibits malignant phenotypes of human renal cell carcinoma by impacting on MAPK/NF-κB signaling pathways. Onco Targets Ther. 2018, 11, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Kim, K.Y. Rhein inhibits the growth of Propionibacterium acnes by blocking NADH dehydrogenase-2 activity. J. Med. Microbiol. 2020, 69, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.Y.; Lin, L.Y.; Yuan, X.M.; Ying, W.L.; Xu, Y.; Pan, X.Y.; Ge, P.H. Antifungal activity of Rhein and aloe-emodin from Rheum palmatum on fish pathogenic Saprolegnia sp. J. World Aquacult. Soc. 2017, 48, 137–144. [Google Scholar] [CrossRef]
- Wang, B.; Yang, J.; Zhao, X.; Feng, X.; Xu, S.; Li, P.; Li, L.; Chen, Y. Antifungal activity of the botanical compound rhein against Phytophthora capsici and the underlying mechanisms. Pest Manag. Sci. 2024, 80, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Singh, S.S.; Verma, S.; Kumar, S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Chewchinda, S.; Wuthi-udomlert, M.; Gritsanapan, W. HPLC quantitative analysis of rhein and antidermatophytic activity of cassia fistula pod pulp extracts of various storage conditions. Biomed. Res. Int. 2013, 2013, 821295. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, S.; Zheng, Y.; Zhang, Y.; Hsiang, T.; Huang, R.; Qi, J.; Gan, T.; Chang, Y.; Li, J. Antifungal and insecticidal activities of rhein derivatives: Synthesis, characterization and preliminary structure-activity relationship studies. Nat. Prod. Res. 2022, 36, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Zhao, Z.M.; Li, J.C.; Yang, G.Z.; Liu, Y.Q.; Dai, L.X.; Zhang, J.Y. Insecticidal and antifungal activities of Rheum palmatum L. anthraquinones and structurally related compounds. Ind. Crop. Prod. 2019, 137, 508–520. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Yu, L.; Shi, J.; Zhang, Y.; Tian, Y.; Li, L.; Zhu, X.; Li, J. Rhein-amino acid easter caonjugates as potential antifungal agents: Synthesis and biological evaluation. Molecules 2023, 28, 2074. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Son, S.H.; Chen, W.; Son, Y.E.; Lee, I.; Yu, J.H.; Park, H.S. Regulation of conidiogenesis in Aspergillus flavus. Cells 2022, 11, 2796. [Google Scholar] [CrossRef]
- Andrianopoulos, A.; Timberlake, W.E. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell Biol. 1994, 14, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.E.; Borowsky, A.T.; Joehnk, B.; Steenwyk, J.L.; Shen, X.X.; Sil, A.; Rokas, A. Recurrent loss of abaA, a master regulator of asexual development in filamentous fungi, correlates with changes in genomic and morphological traits. Genome Biol. Evol. 2020, 12, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Mead, M.E.; Kim, S.C.; Rokas, A.; Yu, J.H. WetA bridges cellular and chemical development in Aspergillus flavus. PLoS ONE 2017, 12, e0179571. [Google Scholar] [CrossRef]
- Pedersen, M.H.; Borodina, I.; Moresco, J.L.; Svendsen, W.E.; Frisvad, J.C.; Søndergaard, I. High-yield production of hydrophobins RodA and RodB from Aspergillus fumigatus in Pichia pastoris. Appl. Microbiol. Biotechnol. 2011, 90, 1923–1932. [Google Scholar] [CrossRef]
- Grünbacher, A.; Throm, T.; Seidel, C.; Gutt, B.; Röhrig, J.; Strunk, T.; Vincze, P.; Walheim, S.; Schimmel, T.; Wenzel, W.; et al. Six hydrophobins are involved in hydrophobin rodlet formation in Aspergillus nidulans and contribute to hydrophobicity of the spore surface. PLoS ONE 2014, 9, e94546. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.; Wang, J.; Wei, S.; Zhai, H.; Zhang, S.; Hu, Y. Afper1 contributes to cell development and aflatoxin biosynthesis in Aspergillus flavus. Int. J. Food Microbiol. 2022, 377, 109828. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Sant, D.G.; Tupe, S.G.; Ramana, C.V.; Deshpande, M.V. Fungal cell membrane-promising drug target for antifungal therapy. J. Appl. Microbiol. 2016, 121, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal properties and mechanisms of three volatile aldehydes (octanal, nonanal and decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Transcriptomic analysis shows the antifungal mechanism of honokiol against Aspergillus flavus. Int. J. Food Microbiol. 2023, 384, 109972. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhao, L.; Zhao, W.; Xie, Y. (E)-2-Hexenal, as a potential natural antifungal compound, inhibits Aspergillus flavus spore germination by disrupting mitochondrial energy metabolism. J. Agric. Food Chem. 2019, 67, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yao, B.; Meng, X. Inhibitory effect and possible mechanism of phenyllactic acid on Aspergillus flavus spore germination. J. Basic Microbiol. 2022, 62, 1457–1466. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial oxidative stress-a causative factor and therapeutic target in many diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, oxidative stress and innate immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef]
- van Hameren, G.; Campbell, G.; Deck, M.; Berthelot, J.; Gautier, B.; Quintana, P.; Chrast, R.; Tricaud, N. In vivo real-time dynamics of ATP and ROS production in axonal mitochondria show decoupling in mouse models of peripheral neuropathies. Acta Neuropathol. Commun. 2019, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, W.; Hao, J.; Wang, Y.; Wei, S.; Zhang, S.; Hu, Y.; Lv, Y. Estragole inhibits growth and aflatoxin biosynthesis of Aspergillus flavus by affecting reactive oxygen species homeostasis. Microbiol. Spectr. 2023, 11, e0134823. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.Y.; Qin, Y.L.; Zhang, S.B.; Zhai, H.C.; Lv, Y.Y.; Wei, S.; Hu, Y.S. Inhibitory mechanisms of plant volatile 1-octanol on the germination of Aspergillus flavus spores. Food Biophys. 2024, 19, 96–108. [Google Scholar] [CrossRef]
- Del Río, L.A.; López-Huertas, E. ROS Generation in Peroxisomes and its Role in Cell Signaling. Plant Cell Physiol. 2016, 57, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Romero-Puertas, M.C.; del Río, L.A. role of peroxisomes as a source of reactive oxygen species (ROS) signaling molecules. Subcell Biochem. 2013, 69, 231–255. [Google Scholar] [CrossRef]
- Pellegrino, E.; Aylan, B.; Bussi, C.; Fearns, A.; Bernard, E.M.; Athanasiadi, N.; Santucci, P.; Botella, L.; Gutierrez, M.G. Peroxisomal ROS control cytosolic Mycobacterium tuberculosis replication in human macrophages. J. Cell Biol. 2023, 222, e202303066. [Google Scholar] [CrossRef] [PubMed]
- Krzywanski, D.M.; Dickinson, D.A.; Iles, K.E.; Wigley, A.F.; Franklin, C.C.; Liu, R.M.; Kavanagh, T.J.; Forman, H.J. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch. Biochem. Biophys. 2004, 423, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, Y.; Wu, M.; Lv, Y.; Zhang, S.; Zhai, H.; Hu, Y. Mechanisms of methyl 2-methylbutyrate suppression on Aspergillus flavus growth and aflatoxin B1 biosynthesis. Int. J. Food Microbiol. 2024, 409, 110462. [Google Scholar] [CrossRef]
- Kenne, G.J.; Gummadidala, P.M.; Omebeyinje, M.H.; Mondal, A.M.; Bett, D.K.; McFadden, S.; Bromfield, S.; Banaszek, N.; Velez-Martinez, M.; Mitra, C.; et al. Activation of aflatoxin biosynthesis alleviates total ROS in Aspergillus parasiticus. Toxins 2018, 10, 57. [Google Scholar] [CrossRef]
- Tai, B.; Chang, J.; Liu, Y.; Xing, F. Recent progress of the effect of environmental factors on Aspergillus flavus growth and aflatoxins production on foods. Food Qual. Safety 2020, 4, 21–28. [Google Scholar] [CrossRef]
- Meneely, J.P.; Kolawole, O.; Haughey, S.A.; Miller, S.J.; Krska, R.; Elliott, C.T. The challenge of global aflatoxins legislation with a focus on peanuts and peanut products: A systematic review. Expo Health 2023, 15, 467–487. [Google Scholar] [CrossRef]
- Tumukunde, E.; Xie, R.; Wang, S. Updates on the functions and molecular mechanisms of the genes involved in Aspergillus flavus development and biosynthesis of aflatoxins. J Fungi 2021, 7, 666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, M.; Bai, Y.; Ge, F.; Wang, S. Antioxidant-related catalase CTA1 regulates development, aflatoxin biosynthesis, and virulence in pathogenic fungus Aspergillus flavus. Environ. Microbiol. 2020, 22, 2792–2810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, Y.; Yao, B.; Hu, T.; Ma, Z.; Shi, W.; Ye, Y. Curcumin inhibits Aspergillus flavus infection and aflatoxin production possibly by inducing ROS burst. Food Res. Int. 2023, 167, 112646. [Google Scholar] [CrossRef]
- Chen, B.; Ye, F.; Yu, L.; Jia, G.; Huang, X.; Zhang, X.; Peng, S.; Chen, K.; Wang, M.; Gong, S.; et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J. Am. Chem. Soc. 2012, 134, 17963–17971. [Google Scholar] [CrossRef]
- Fasoyin, O.E.; Wang, B.; Qiu, M.; Han, X.; Chung, K.R.; Wang, S. Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 2018, 115, 41–51. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Synergistic effects of combined cinnamaldehyde and nonanal vapors against Aspergillus flavus. Int. J. Food Microbiol. 2023, 402, 110277. [Google Scholar] [CrossRef]
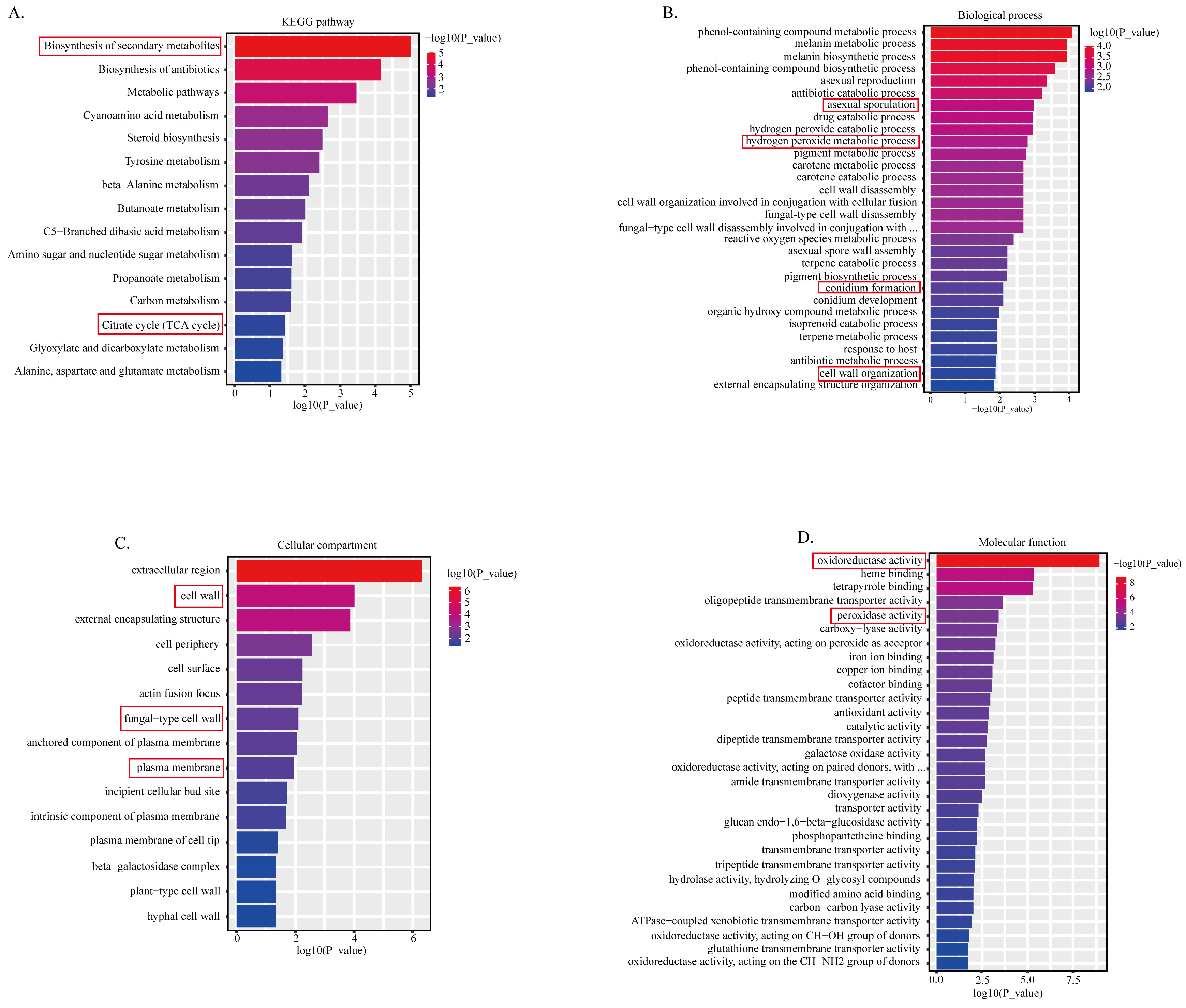

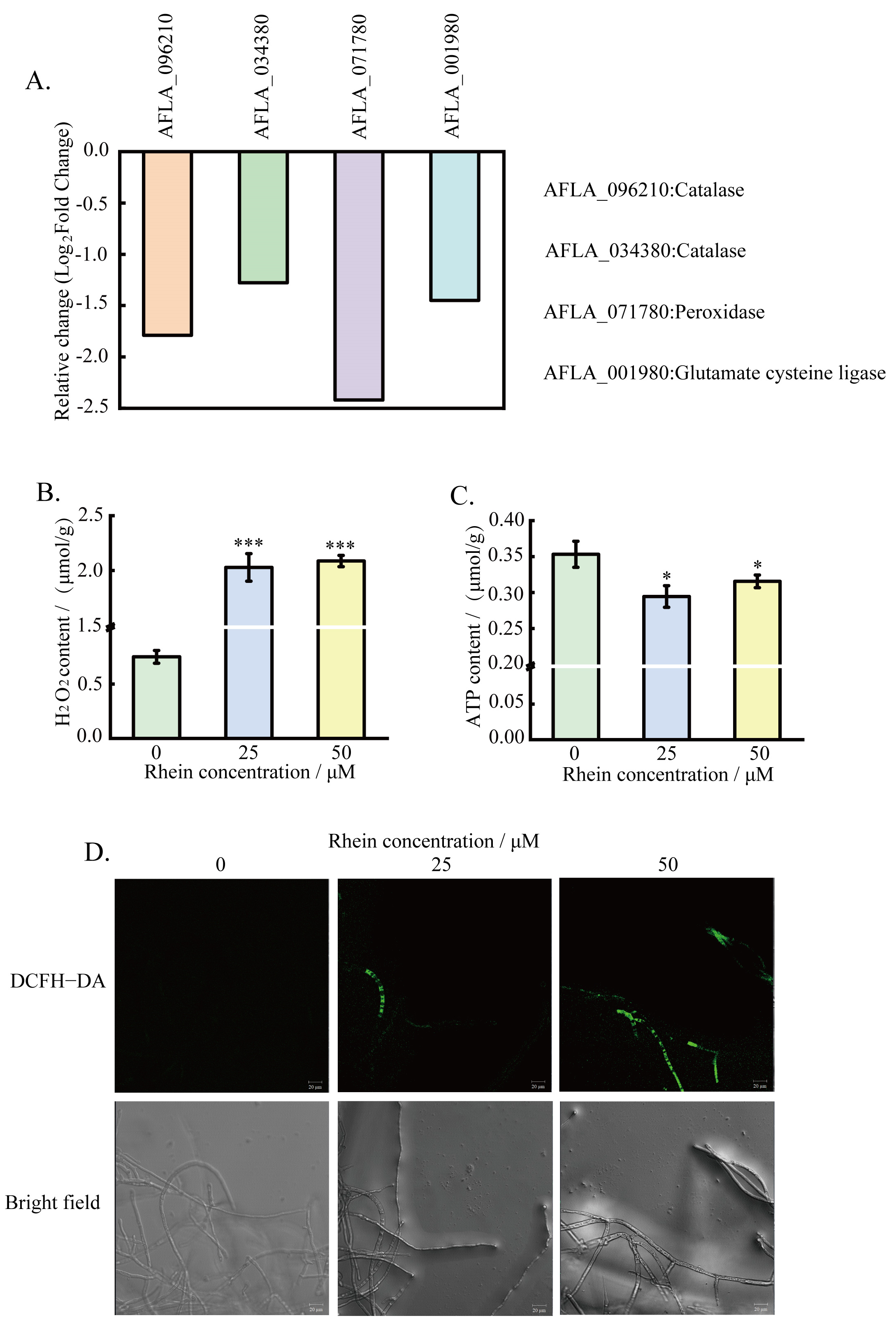
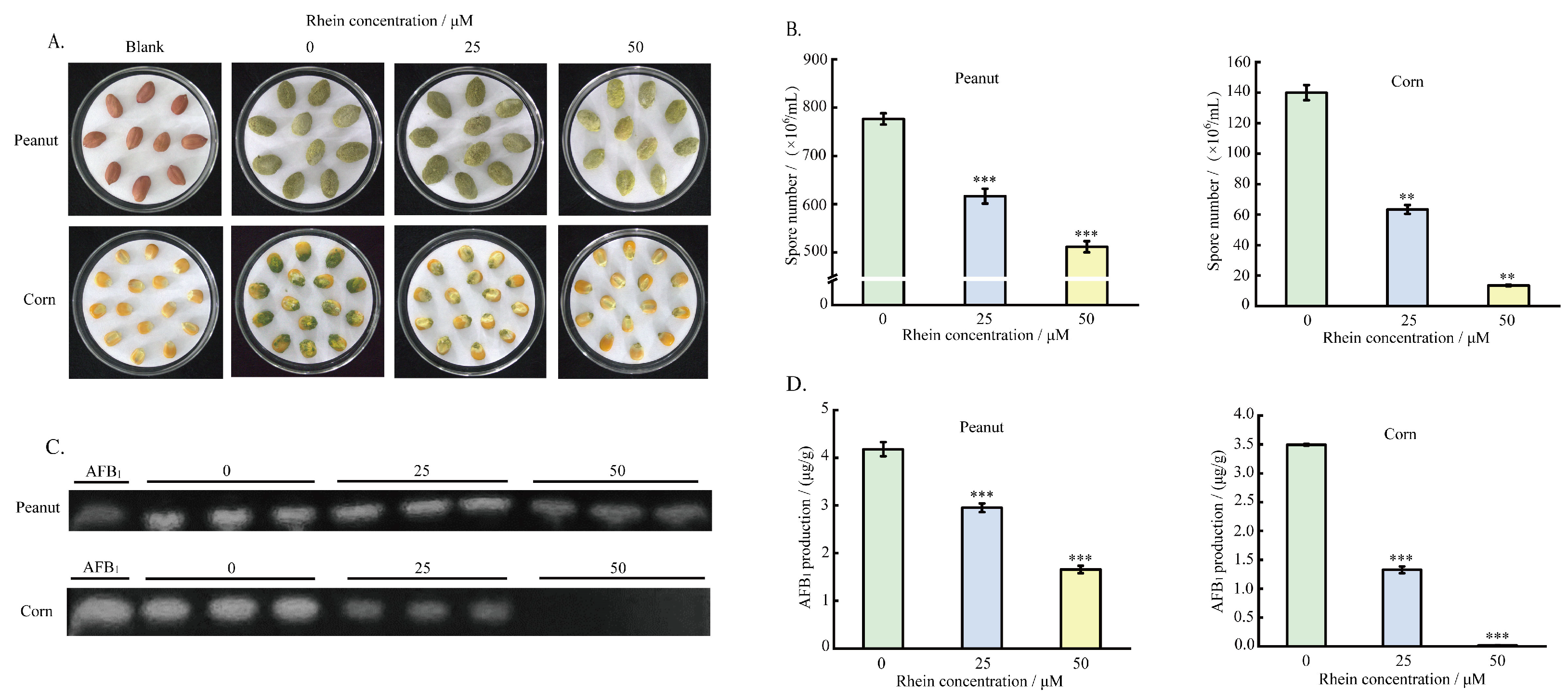
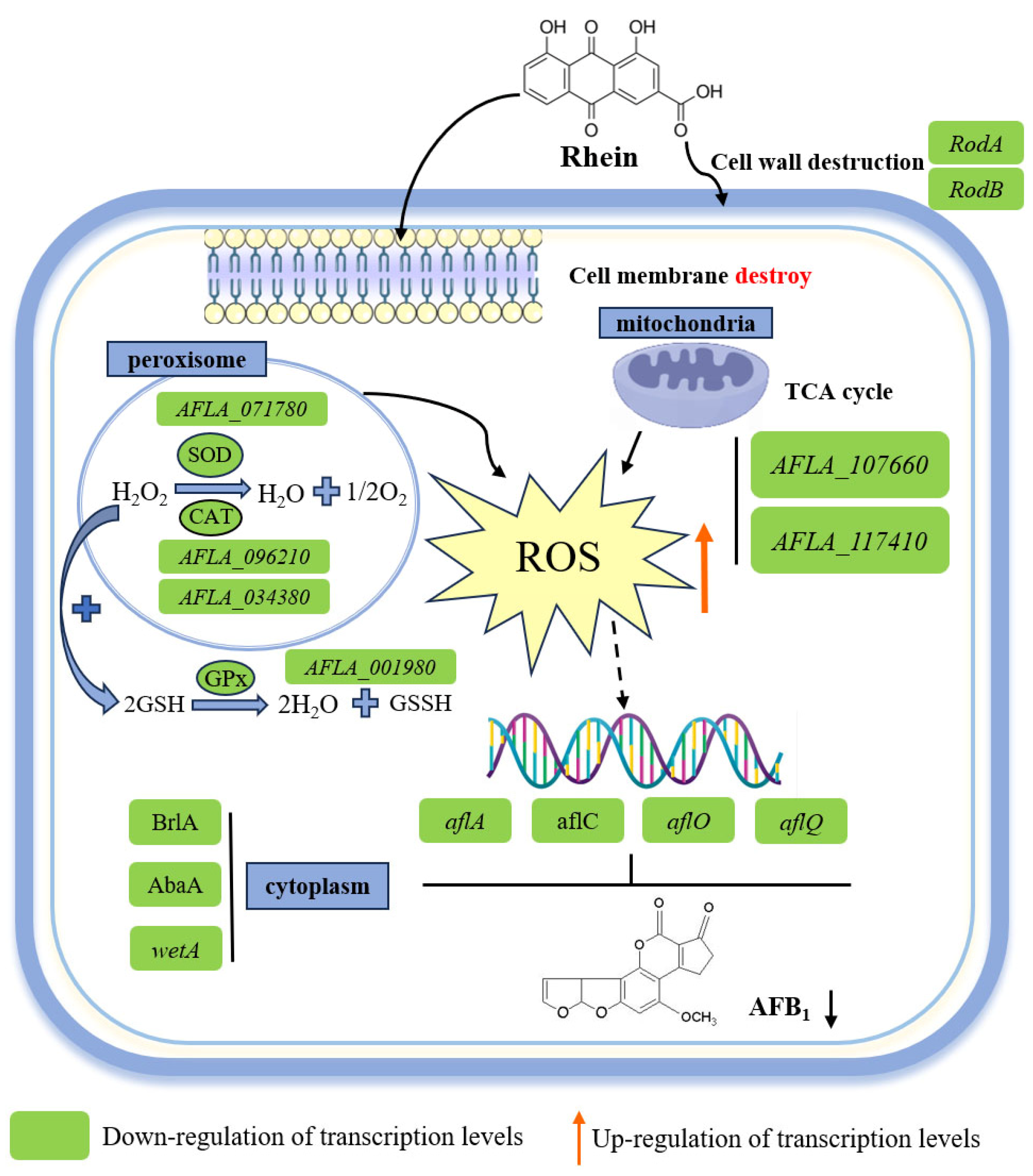
| Gene ID | log2(FC) | Gene Functional Description |
|---|---|---|
| Conidium formation and development | ||
| AFLA_052030 | −1.55 | Developmental regulatory protein wetA |
| AFLA_082850 | −2.47 | C2H2 type conidiation transcription factor BrlA |
| AFLA_029620 | −2.06 | Transcription factor AbaA |
| AFLA_098380 | −1.85 | Conidial hydrophobin RodA |
| AFLA_014260 | −1.44 | Conidial hydrophobin RodB |
| AFLA_044790 | −1.63 | Conidiation-specific family protein |
| Cell wall and membrane | ||
| AFLA_052780 | −1.60 | Cell wall glucanase (Scw4) |
| AFLA_061960 | −1.94 | Cell wall protein |
| AFLA_137200 | 1.56 | Chitin synthase |
| AFLA_025900 | −2.71 | Chitin deacetylase |
| AFLA_007630 | −1.52 | Integral membrane protein |
| AFLA_039810 | −1.23 | DUF1212 domain membrane protein |
| Oxidative stress | ||
| AFLA_096210 | −1.79 | Catalase |
| AFLA_034380 | −1.28 | Catalase |
| AFLA_071780 | −2.42 | Peroxidase family protein |
| AFLA_001980 | −1.45 | Glutamate cysteine ligase |
| Glycolysis, citrate cycle, and aflatoxin biosynthesis | ||
| AFLA_101470 | 1.05 | Glyceraldehyde-3-phosphate dehydrogenase |
| AFLA_036370 | −1.29 | Phosphoenolpyruvate carboxykinase |
| AFLA_107660 | −2.13 | Succinyl-CoA synthetase |
| AFLA_117410 | −4.26 | Citrate synthase |
| AFLA_117420 | −1.75 | Fatty acid synthase subunit alpha (aflA) |
| AFLA_081850 | −1.23 | Norsolorinic acid synthase (aflC) |
| AFLA_065570 | −1.35 | O-methyltransferase (aflO) |
| AFLA_073130 | 1.28 | O-methylsterigmatocystin oxidoreductase ordA-like (aflQ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sahibzada, K.I.; Du, R.; Lei, Y.; Wei, S.; Li, N.; Hu, Y.; Lv, Y. Rhein Inhibits Cell Development and Aflatoxin Biosynthesis via Energy Supply Disruption and ROS Accumulation in Aspergillus flavus. Toxins 2024, 16, 285. https://doi.org/10.3390/toxins16070285
Wang X, Sahibzada KI, Du R, Lei Y, Wei S, Li N, Hu Y, Lv Y. Rhein Inhibits Cell Development and Aflatoxin Biosynthesis via Energy Supply Disruption and ROS Accumulation in Aspergillus flavus. Toxins. 2024; 16(7):285. https://doi.org/10.3390/toxins16070285
Chicago/Turabian StyleWang, Xiaoyan, Kashif Iqbal Sahibzada, Ruibo Du, Yang Lei, Shan Wei, Na Li, Yuansen Hu, and Yangyong Lv. 2024. "Rhein Inhibits Cell Development and Aflatoxin Biosynthesis via Energy Supply Disruption and ROS Accumulation in Aspergillus flavus" Toxins 16, no. 7: 285. https://doi.org/10.3390/toxins16070285
APA StyleWang, X., Sahibzada, K. I., Du, R., Lei, Y., Wei, S., Li, N., Hu, Y., & Lv, Y. (2024). Rhein Inhibits Cell Development and Aflatoxin Biosynthesis via Energy Supply Disruption and ROS Accumulation in Aspergillus flavus. Toxins, 16(7), 285. https://doi.org/10.3390/toxins16070285





