Abstract
Maize plays a significant global role as a food source, feed, and as a raw material in industry. However, it is affected by toxin-producing fungi, mainly Fusarium graminearum, Fusarium verticillioides, and Aspergillus flavus, which compromise its quality. This study, conducted in 2022 and 2023 at the Látókép long-term research site of the University of Debrecen, Hungary, investigated the effects of different nitrogen fertilization rates (0, 90 and 150 Kgha−1 N) on mycotoxin contamination (DON vs. FB vs. AFB1) in the kernels of three (3) maize hybrids: DKC4590 (tolerant), GKT376 (sensitive), and P9610 (undefined). The results showed a significant (p = 0.05) influence of nitrogen fertilization and maize genotype on mycotoxin levels. Sole nitrogen impacts were complex and did not define a clear trend, contrary to the hybrids selected, which followed superiority to resistance. Increased nitrogen fertilization was associated with higher DON production, while hybrid selection demonstrated a clearer trend in resistance to mycotoxins. Therefore, to maximize yield and minimize mycotoxin contamination, future research should focus on optimizing nitrogen application rates and breeding for resistance to balance yield and mycotoxin management. These results suggest that while nitrogen fertilization is crucial for maximizing yield, selecting less susceptible maize hybrids remains vital for minimizing mycotoxin contamination.
Keywords:
maize; hybrid; nitrogen fertilization; mycotoxins; Fusarium graminearum; Fusarium verticillioides; Aspergillus flavus Key Contribution:
The effects of nitrogen fertilization on fungal ear rot severity and mycotoxin contamination is complex. The synergistic effect of nitrogen fertilization and hybrid selection is imperative for maize producing farmers suggesting benefits of selecting hybrids with documented resistance traits to enhance food safety; reduce mycotoxin, associated risks in maize production systems, and achieve targeted agronomic goals.
1. Introduction
Maize (Zea mays L.) contributes significantly to global agri-food systems as food, feed, and as a raw material in industry. Over the past decade, maize production has exceeded one billion metric tons annually, surpassing rice and wheat [1], driven by increased demand, technological advancements, area expansion, and yield improvements [2]. It is a significant staple and provides approximately 20% of food calories in many countries, especially in sub-Saharan Africa (SSA), Latin America, and parts of Asia [3]. It is expected to be the most traded cereal in the next few decades [2].
However, maize yield and grain quality is significantly compromised by mycotoxin contamination from toxigenic fungi, such as Aspergillus flavus, Fusarium verticillioides, and Fusarium graminearum, which produce three main mycotoxins, namely aflatoxins, fumonisin and deoxynivalenol (DON), respectively, among others [4]. These mycotoxins, occurring in agricultural commodities before or after harvest [5], pose significant health risks to humans and animals, being mutagenic, carcinogenic, teratogenic, estrogenic, hemorrhagic, immunosuppressive, nephrotoxic, hepatotoxic, and cytotoxic [6]. However, the risk of contamination is influenced by environmental conditions, insect damage, fungal infection during critical developmental stages, and nitrogen management [7,8,9].
Nitrogen is essential for maize growth, affecting yield, quality, and susceptibility to mycotoxin contamination [10]. However, the relationship between nitrogen fertilization and mycotoxin levels is complex, with studies reporting varying effects. In agricultural systems, maize requires nitrogen from external sources, such as organic matter, minerals, or commercial fertilizers [11]. Without supplemental nitrogen, its productivity would decline [12]. As an essential nutrient, nitrogen affects numerous parameters related to the growth and development of the plant, which directly or indirectly influence crop yield and quality [13]. It has been shown to impact the protein and oil content of maize [14], the balance between protein and starch [15], and the extent of mycotoxin contamination and fungal infection [16]. It is widely recognized that nitrogen availability influences maize susceptibility to mycotoxin contamination. The relationship between nitrogen fertilization and mycotoxin contamination is complex; there are reports of increased, decreased, and unaffected toxin contamination in response to added nitrogen [9,13,17]. These inconsistent results suggest that other interacting factors, such as hybrid genetics and environmental conditions, maybe more critical in determining mycotoxin accumulation in maize with varying nitrogen levels. However, most studies have been conducted at relatively high nitrogen levels, highlighting the need to quantify the effects of nitrogen fertilization across a broader range of nitrogen inputs. Understanding these dynamics across different nitrogen levels and maize hybrid genetics is crucial to develop effective management to reduce the risk of mycotoxin exposure to humans and animals.
Hybrid selection could play a significant role in minimizing the extent of mycotoxin contamination. It has been confirmed that resistant hybrids show reduced susceptibility to fungal infections, even though there is no complete resistance [18]. Identifying hybrids with both high yield potential and mycotoxin resistance is critical for sustainable maize production, and finding resistant maize genotypes has been a primary strategy to combat mycotoxin issues [19], with research on fungus-resistant germplasm being a global focus [20,21,22,23,24,25,26,27,28]. However, these hybrids often exhibit reduced susceptibility rather than complete resistance due to the complex selection of multiple traits and the associated genes that contribute collectively to plant resistance. Commercial maize hybrids vary in their sensitivity to infection by toxin-producing fungi, and tolerance information is rarely disclosed to users [3]. The screening of commercial hybrids has shown that tolerance levels can significantly reduce disease severity and subsequent mycotoxin production [4,29,30]. Evidence suggests that some maize hybrids are more resistant to infection by fumonisin-producing fungi and can maintain kernel integrity with less fumonisin accumulation [31]. For DON production, most studies have indicated that hybrids that are more resistant to Gibberella ear rot have lower levels of DON in infected grain [32] and aflatoxin [33]. Despite these findings, hybrids that are resistant to fungi and mycotoxins may have trade-offs in yield potential or other desirable attributes. This necessitates a balanced approach in selecting hybrids that exhibit resistance to mycotoxins and maintain high agronomic performance.
Despite extensive research, how nitrogen affects mycotoxin contamination under varying environmental conditions and in different maize hybrids remains poorly understood. A comprehensive understanding of the complex interactions between nitrogen fertilization and mycotoxin contamination could identify specific scenarios where high grain yields offset the high contamination risks. This would enable growers to achieve their agronomic goals with minimal economic and health costs. It is crucial to identify hybrids that exhibit both good mycotoxin resistance and overall agronomic performance. Given the significance of hybrid selection and nitrogen management in reducing mycotoxin contamination, this study was initiated to evaluate the effects of nitrogen fertilization on a range of pre-defined hybrids on the mycotoxin contamination of maize.
2. Results
In 2022 and 2023, the effects of the treatments were significant and influenced ear rot severity and mycotoxin contamination, as shown in Table 1.

Table 1.
Effects of nitrogen fertilization, hybrids and inoculation treatments on ear rot severity and mycotoxin contamination (Debrecen, 2022 and 2023).
2.1. Nitrogen Fertilization, Hybrids Tolerance, Ear Rot Severity, and Mycotoxin Contamination
The impact of nitrogen fertilization and hybrid tolerance on ear rot severity and mycotoxin production was evaluated (Table 1). Nitrogen fertilization treatments significantly (p < 0.05) influenced ear rot severity for FV % and FB production by 200 and 121% in 2022 between N0 and the other doses (N90 and N150), while FB production was significantly reduced with N fertilization in 2023. N fertilization was insignificant for the ear rot severity of FG%, as well as the DON toxins produced. On the other hand, hybrid tolerance was significant (p < 0.05) for FV% and DON production in 2022 and FG% and FB production in 2023. The influence depended on the tolerance level of the hybrids following hybrid superiority. Despite AF% being significant (p < 0.05) in all years, AFB1 production was only significant (p < 0.05) in 2022, the only year in which it was recorded. Inoculation treatments significantly affected % ear rot severity and produced mycotoxins across all nitrogen rates and hybrid tolerances in all years.
2.2. Interactions between Nitrogen Fertilization and Hybrids on Ear Rot Severity and Mycotoxin Contamination
2.2.1. Influence of Nitrogen Fertilization and Hybrids on Ear Rot Severity
The treatment combinations significantly impacted ear rot severity and mycotoxin contamination in 2022 and 2023, as presented in Table 2. In artificially inoculated plots, the treatment influence on ear rot severity was dynamic and significantly (p < 0.05) varied from 9.6–40.1%; 0.09–0.66% and 0.04–0.77% for FG, FV, and AF in 2022, and from 22.9–67.3%; 0.23–0.56% and 0.2–0.4% for FG; FV, and AF in 2023. In treated plots, the percentage (%) of ear rot severity for FG was the most elevated, and higher by 51%, from 2023 to 2022. However, the ear rot severity in non-inoculated controls was insignificant across fertilization and selected hybrids in the two years. In all years, the less susceptible hybrid DKC4590 recorded the lowest FG% ear rot severity in all fertilizer rates compared to other hybrids.

Table 2.
Interactive effects of nitrogen fertilizer application rate and hybrid genotypes on-ear and kernel rot severity (Debrecen, 2022, 2023).
2.2.2. Effects of Nitrogen Fertilization and Hybrids on Mycotoxin Contamination
The interactive effect of nitrogen fertilizer application rate and hybrid genotypes on the amount of mycotoxin contamination evaluated is shown in Table 3. Regarding mycotoxin contamination, aflatoxin contamination was significant (p < 0.05) in 2022 across fertilizers rates and hybrids; however, the detected amount was below the allowed limits of contaminations for animal feeds (20 ppm) and foods (4 ppm) [34,35,36]. The addition of nitrogen increased contamination by 89% from the highest detected amount of 0.129 ppm for GKT376 at N150, and the lowest amount of 0.014 ppm for P9610 at N0 in 2022, while no aflatoxin was detected in inoculated plots and non-inoculated plots in 2023.

Table 3.
Interactive effects of nitrogen fertilizer application rate and hybrid genotypes on the amount of mycotoxin contamination (Debrecen, 2022, 2023).
The fusarium mycotoxins, DON and FB, significantly differed (p < 0.05) between all nitrogen rates and hybrids used in the two years. DON contamination in artificially inoculated plots followed the same trend in % ear rot severity for FG, which was higher in 2023 than in 2022. In all years, the hybrid DKC4590 significantly (p < 0.05) recorded the lowest amount of DON than other hybrids, following the trend DKC < GKT376 < P9610 in 2022 and DKC < P9610 < GKT376 in 2023. In uninoculated controls, DON production was insignificant and detected only for the hybrid GKT376 at N150 in 2022, while there was no detection in all hybrids and N rates in 2023. On the other hand, FB toxin production in inoculated maize significantly differed (p < 0.05) between hybrids in all N rates in all years. However, within N rates, it was significant (p < 0.05) at N150 in 2022 and N90 and N150 in 2023. In all years, the highest FB amount was recorded for the hybrid GKT376 (6.59 ppm at N150 in 2022 and 8.8 ppm at N90 in 2023), while the lowest record was DKC4590 across N rates in the two years. In non-inoculated controls, 2022 showed significant (p < 0.05) FB values between hybrids at N150; the detected amount was insignificant in 2023 in all hybrids across all N rates.
2.3. Relationship between Ear Rot and Kernel Severity and Production of Mycotoxins
Regression and correlation analyses were performed to determine how much mycotoxin is produced as a result of the effect of the nitrogen added on selected hybrids to ear rot severity (Figure 1 and Table 4). We used the pooled general means to perform a regression analysis to study the relationship between ear rot severity within fungal species, and a two-tailed Pearson correlation to study the same between fungal isolates.
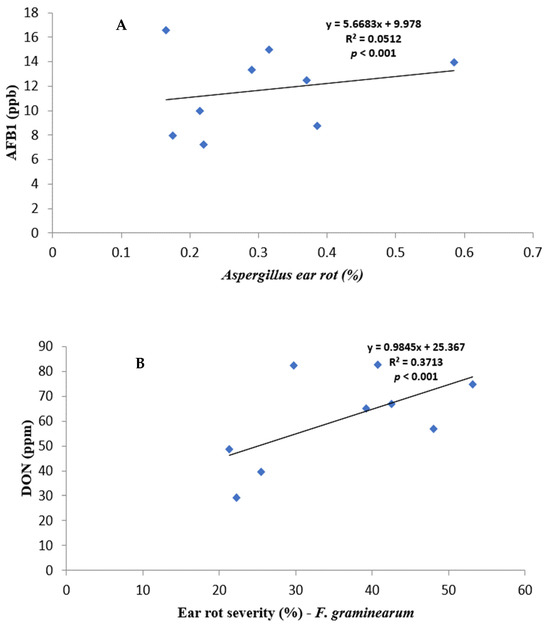
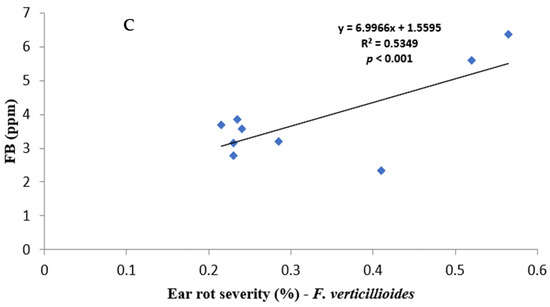
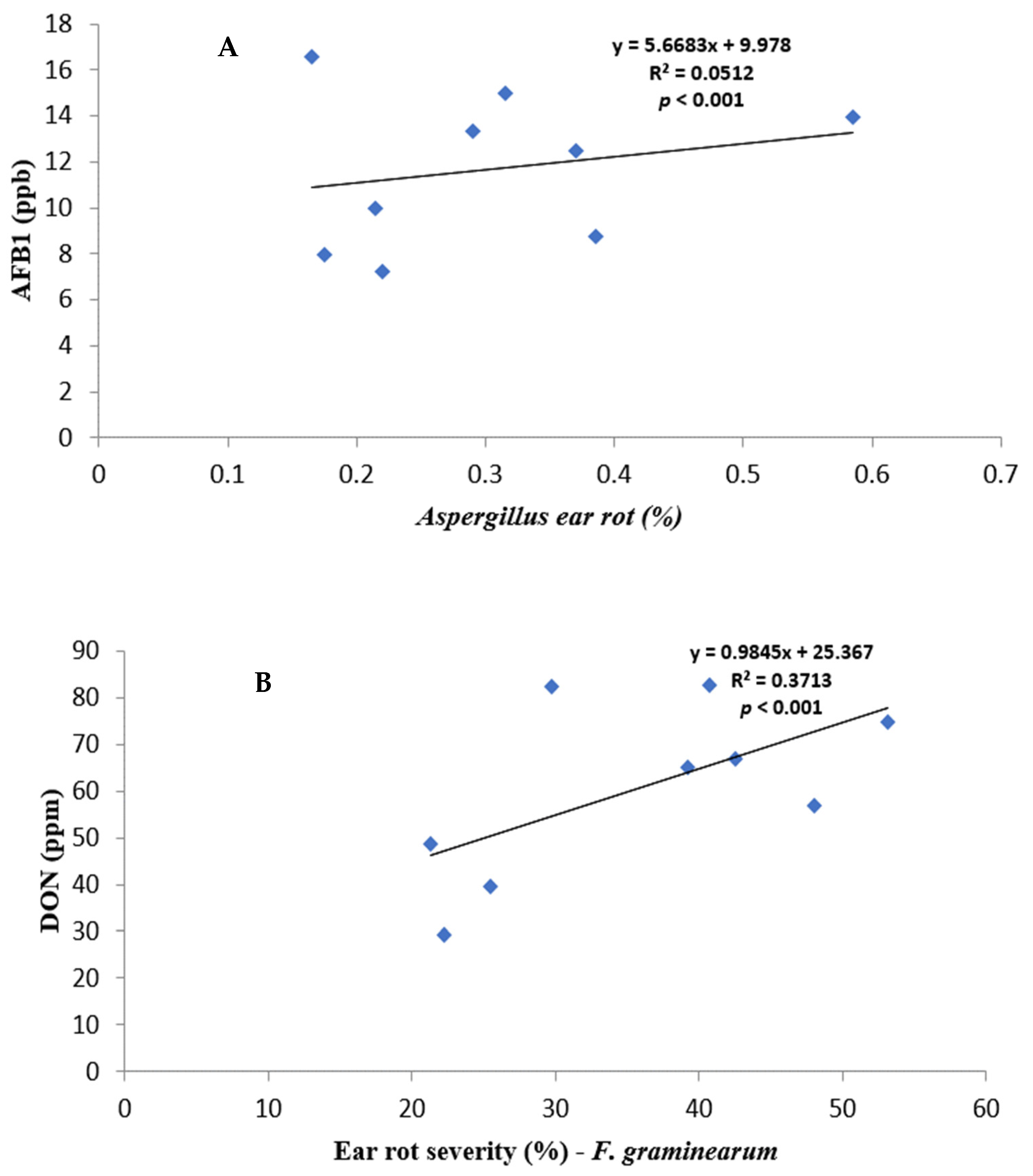
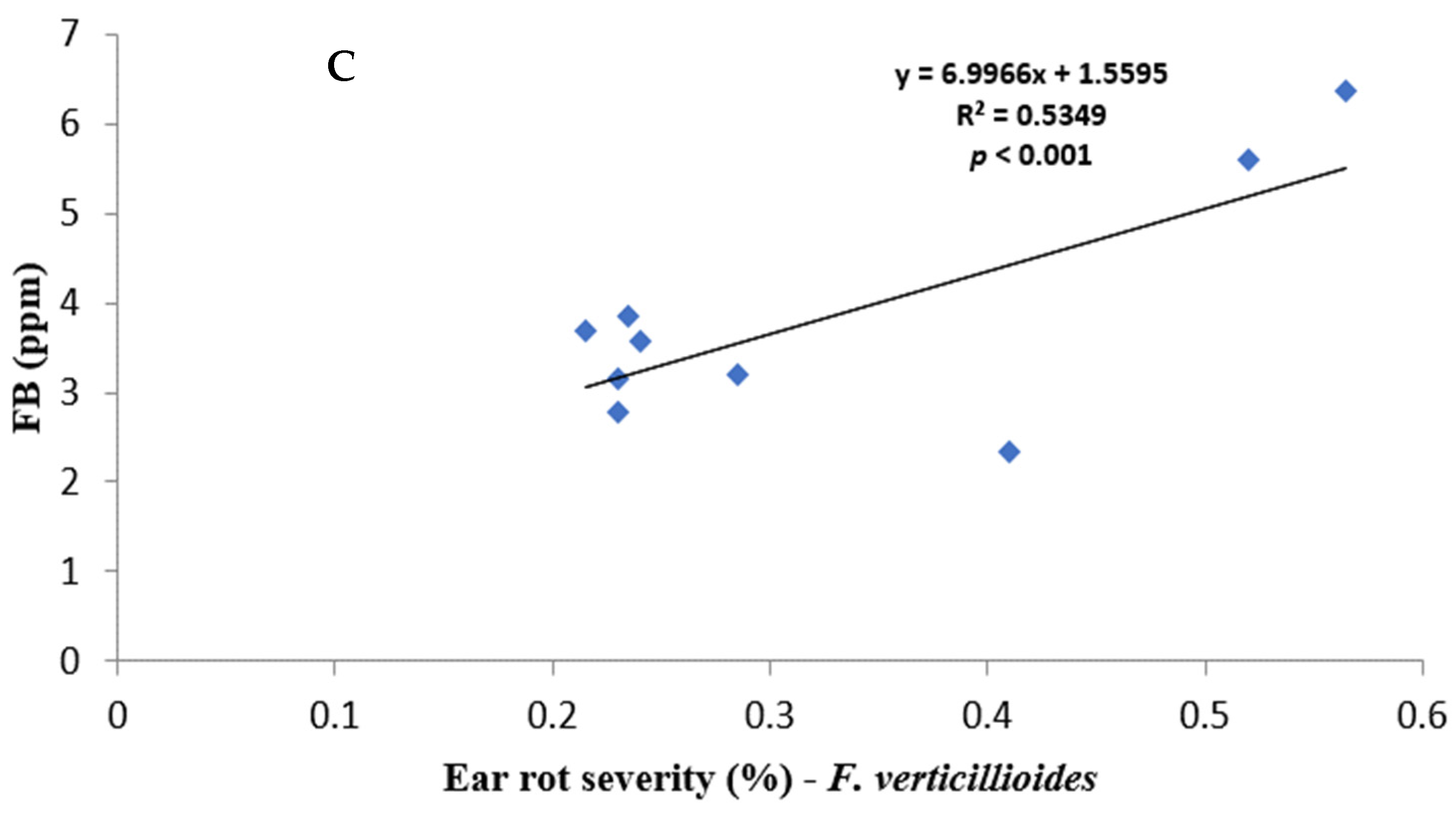
Figure 1.
Regression relationship between ear rot severity and mycotoxin contamination: (A) AF% vs. AFB1; (B) FG% vs. DON, and (C) FV% vs. FB.

Table 4.
Coefficient values of Pearson correlation showing a relationship between fungal species ear rot severity and mycotoxin contamination (Debrecen, 2022, 2023).
All fungal species showed a positive relationship with varying strengths between ear rot severity and respective mycotoxin production. AF% ear rot severity, and AFB1 production, showed a relatively weak correlation of r = 0.2264, but it was significant (p < 0.001). FV% ear rot severity and total FB production indicated a strong correlation of r = 0.7313496 and it was significant (p < 0.001). FG% ear rot severity significantly (p < 0.001) correlated—r = 0.6093—to the production of DON. A relatively weak negative correlation was recorded between FG% ear rot severity and AFB and FB contamination. In contrast, AF% ear rot severity and FUM production, as well as FV% ear rot severity and AFB1 production, showed positive significant correlations.
3. Discussion
The discussion section is divided into three parts. The first and second parts are dedicated to the influence of nitrogen fertilization and hybrids on the mycotoxin contamination of maize; the third part is about the interactions between the two discussed factors and the relationship between fungal ear rot severity and mycotoxin contamination. Studies show the complexity and inconsistency of how the individual nitrogen applied affects fungal ear rot severity and the resulting production of mycotoxin. At the same time, studies indicate a positive relationship between hybrid tolerance and mycotoxin contamination. Nitrogen application is essential to maximizing yield; fertilizing with a suitable hybrid in agronomic traits and tolerance would help quantify the amount of toxin produced in added nitrogen.
3.1. Effects of Nitrogen Fertilization on Mycotoxin Contamination
Nitrogen fertilization plays a significant role in affecting both mycotoxin contamination and maize yield. The observed inconsistencies in mycotoxin levels with varying nitrogen application rates in this study highlight the complexity of this relationship. While some reports indicate an increase in mycotoxin contamination with nitrogen application rate, others report contradictory research results. These disparities highlight the need for a comprehensive understanding of the interaction between nitrogen application, ear rot severity, and mycotoxin production. Our study revealed a general increase in mycotoxin contamination with nitrogen fertilization for AFB1 and FB in 2022. This contradicts the findings by Blandino et al. [15], who reported increased fumonisin contamination in maize due to nitrogen deficiencies.
Similarly, Tubajika et al. [37] reported reduced aflatoxin contamination by 34–45% when 50–250 KgN/ha was applied compared to N0 plots. However, the dynamics shifted in 2023, with no AFB1 recorded, while DON toxin contamination decreased with increasing nitrogen rates when used alone, resulting in the highest DON contamination at N0 in all years (Table 1). These inconsistencies align with previous studies, emphasizing the intricate relationship between nitrogen fertilization, ear rot severity and mycotoxin contamination in maize production [38,39,40,41]. The susceptibility of maize to mycotoxin contamination is dynamically influenced by nitrogen fertilization, with evidence suggesting that applied nitrogen increases and decreases mycotoxin contamination [9,17]. It appears that other interacting factors, such as hybrid genetics and environmental conditions, may be more critical in determining how much mycotoxin accumulates in maize in response to additional nitrogen, given the erratic effects of nitrogen fertilization on mycotoxin contamination. Quantifying the impacts across a range of nitrogen inputs is crucial, as most research has been conducted at relatively high nitrogen levels. This approach could provide a more comprehensive understanding of the complex interactions between nitrogen fertilization and mycotoxin contamination, facilitating the development of targeted agronomic management approaches to reduce mycotoxin risks in maize production.
3.2. Influence of Hybrids on Mycotoxin Contamination
The results revealed a significant effect of hybrid selection on mycotoxin contamination in all years. Consistent with findings by Bocianowski et al. [42], maize hybrids exhibiting tolerance superiority showed reduced levels of mycotoxin contamination. The less susceptible hybrid DKC 4590 consistently exhibited the lowest mycotoxin levels, whereas GKT376 demonstrated elevated mycotoxin levels. The hybrid P9610, with unknown tolerance but higher yields, was not significantly different to GKT376 in most cases for all toxins, except it recorded the lowest and most significant FB than all the others in 2023. With this exception, the overall results agree with other studies, suggesting that hybrids with good tolerance reduce the mycotoxin contamination risks [4,29,30]. Since the pre-defined hybrids have known resistance, this study does not align with Barošević et al. [43], which observed inconsistencies between hybrids in mycotoxin contamination in maize due to unknown resistances. This difference could be attributed to the known resistance characteristics of the maize hybrids used in our study, highlighting the importance of selecting hybrids with documented resistance traits to reduce mycotoxin and associated risks. A key insight of this study is that hybrid susceptibility to mycotoxin contamination depended on the specific mycotoxin and hybrid feature [44]. The different hybrids greatly influenced all the fungi: A. flavus, F. verticillioides, and F. graminearum mycotoxin. These results universally imply that the diverse responses of maize hybrids to different mycotoxins highlight the complexity of the interaction between the genetic landscape of the hybrids used and mycotoxin production. Overall, the different responses of the hybrids to the various mycotoxins and the potential trade-offs between increased resistance and increased susceptibility make hybrid breeding for reduced mycotoxin contamination a critical task. Despite the imperative for selecting maize hybrids with decreased susceptibility to fungal infection, the observed variation suggests that a significant array of maize-producing farmers choose hybrids less susceptible to fungal infection. This generally indicates the need for targeted educational initiatives, and disseminating knowledge about the benefits of selecting hybrids with documented resistance traits to enhance food safety and reduce mycotoxin and associated risks in maize production systems.
3.3. Interactions between Nitrogen Fertilization and Hybrids
The results reveal that the nitrogen application complex pattern of individual mycotoxins was influenced by changes resulting from the impact of the hybrid. While the interaction between nitrogen application rate and maize hybrids was evident in the changes in mycotoxin concentration in the kernels of the three maize hybrids, the dependence was different. There was an additive interaction in the trend in DON contamination with nitrogen fertilization rate, with a reversed impact following the hybrid’s responses. The tolerant hybrid DKC4590 showed less contamination compared to the susceptible hybrid GKT376. Interestingly, the medium nitrogen rate (N90) demonstrated relatively better results in 2022 than in 2023. However, there were no discernible differences between GKT376 and the hybrid P9610 with unknown tolerance features across all nitrogen doses, suggesting the impact of hybrid genetics irrespective of nitrogen dose. The effects of the FB accumulation interaction between nitrogen fertilization and hybrids were inconclusive. In 2022, significantly higher FB levels were observed at N150, while in 2023, significantly higher levels were recorded at the control (N0), with lower levels at the highest nitrogen rate (N150). This variability suggests that manipulating these two factors can yield similar or dissimilar mycotoxin levels in different maize hybrids, creating two environments with high and low mycotoxin risk.
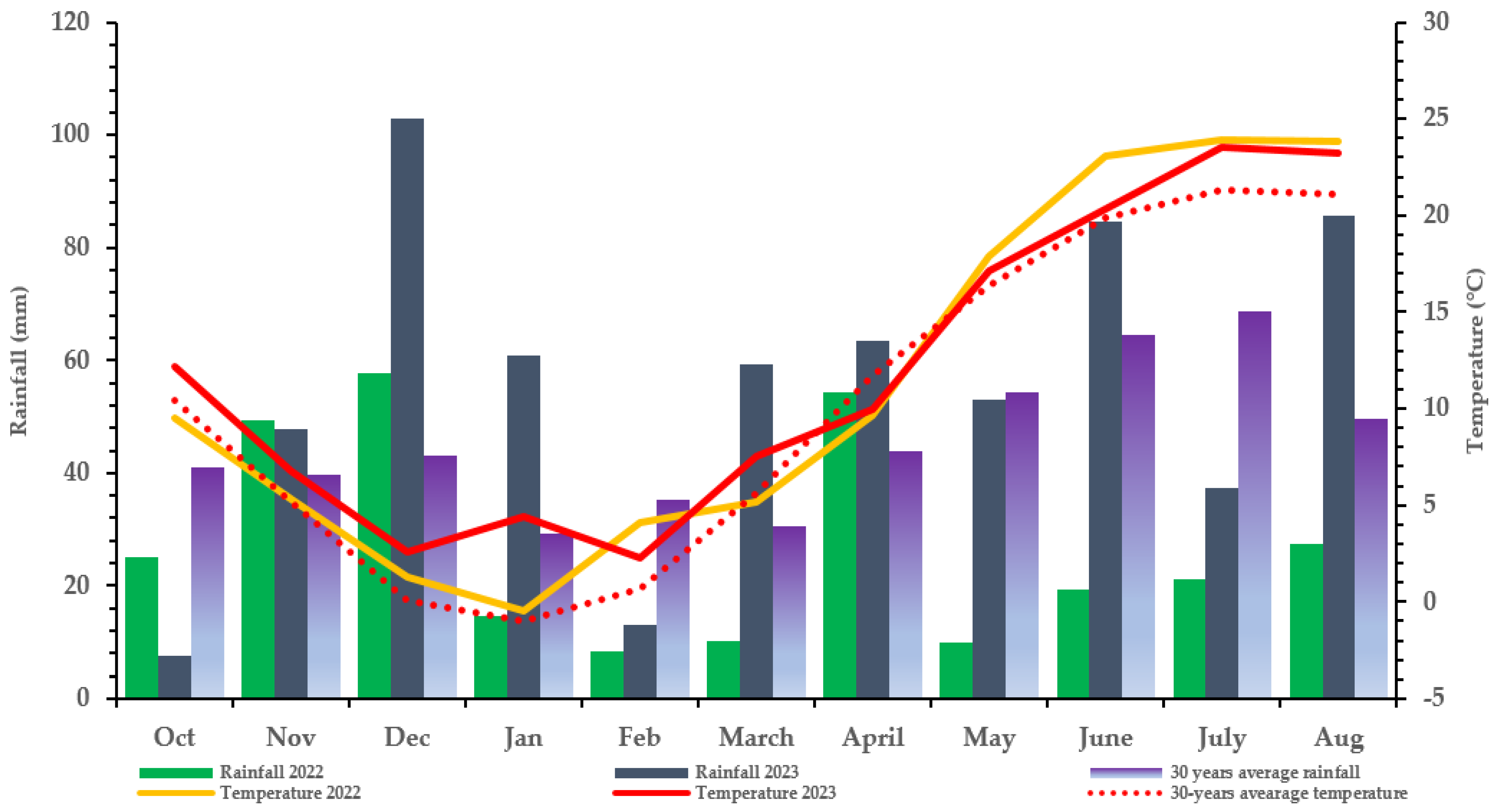
Despite the % average aspergillus ear rot being higher in 2023 (0.32%) than in 2022 (0.28%), only 2022 recorded AFB, which indicated a significantly higher AFB1 level (128.56 ppb) at N150 and the lowest level at N0. This stresses that the visual assessment of AF% (mere existence of fungal mycelium) does not reflect the amount of aflatoxin produced. This is further proved by A. flavus-inoculated treatment with an average mean ear rot of 0.05–0.28% in 2022, which resulted in an elevated AFB1 toxin for the hybrid than the AF% of 0.03–0.32% in non-inoculated and inoculated controls, with no AFB1 produced compared to 2022. This could be because mycotoxins arise from intricate metabolic processes brought on by the fungal adaptation to varying environmental and climatic conditions and stressors [45]. The conditions and stressors include the availability of nutrients, temperature, moisture content, water activity, relative humidity, substrate, and fungicide use, which are crucial in identifying the types of fungi that grow and influence crop mycotoxin production [46]. In this study, rainfall (moisture content) and humidity, in particular, could relatively influence the ear rot severity and mycotoxin contamination of A. flavus and F. graminearum, which vary significantly between the two study years (Figure 2).
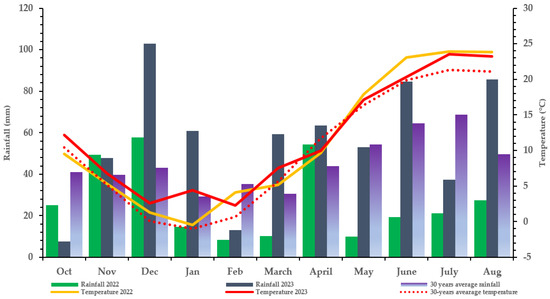
Figure 2.
Meteorological data at the Látókép experimental station of the University of Debrecen (Debrecen: 2022, 2023, Hungary).
Mycotoxins have a direct correlation with fungal infections [46]; this is in line with our findings, where each respective fungal severity positively correlated to the toxin produced (Figure 1); however, the results indicate the co-occurrence of aflatoxin and FB (Table 4), in line with Casu et al. [47]. This co-occurrence poses a further worry as the co-contamination of agricultural commodities results from the simultaneous infection of crops by mycotoxigenic fungal strains and the synthesis of multiple mycotoxins by the same strain, which could result from synergistic interactions. Therefore, the influence of environmental factors is crucial to further determining the additive interaction of various factors.
4. Materials and Methods
4.1. Experimental Site and Treatments
A field experiment was conducted in 2022 and 2023 at the Látókép long-term research site of the University of Debrecen, located at 47°33′42″ N; 21° 27′02″ E, Debrecen, Hungary. The soil conditions are homogenous calciferous Chernozem formed on the Hajdúság loess ridge, with an upper layer humus content average of 2.7–2.8% and a thickness of around 0.8 m. The acidity of the upper soil layers is almost neutral (pHKCl = 6.46–6.6). The phosphorus supply of the calcareous soil is average (AL-soluble P2O5 133 mg kg−1), while its potassium supply is average–good (AL-soluble K2O 240 mg kg−1). The soil plasticity index (KA) was between 43 and 47.6. The experiment point contained sunflowers as the previous crop. The soil moisture content during sowing was suitable for germination in both years. However, during the 2022 season, May, June, and July were dry. Therefore, two instances of (2) supplementary irrigation were performed in May and early July during the critical fertilization, silking, and grain-filling periods. The meteorological data at the Látókép experimental station of the University of Debrecen for 2022 and 2023 are as presented in Figure 2.
The experiment was carried out in a quadruplicate using a split–split plot design. The reason for the design was that hybrids were assumed to be fixed effects and that the relationship between N and mycotoxin production was more of interest than a direct comparison between the effects of various levels of N fertilizer on mycotoxin contamination. The main plots were nitrogen rates (0, 90, and 150 KgNha−1), the sub-plot treatment was the three (3) commercial maize hybrids Table 5, and the sub-subplot treatment was the fungal species ear inoculation. The subplot size was 5 m × 3.04 m (15.2 m2) with a spacing of 76 cm between rows, making four rows of plots consisting of approximately 25 plants each and a distance of 1 m between blocks. Three rows were exposed to artificial inoculation; according to international guidelines, one inoculum was employed for a single pathogen. One with a strain of F. graminearum, one with a strain of F. verticillioides and the third raw sample was inoculated with a strain of A. flavus. The untreated check was the fourth raw.

Table 5.
Characteristics of selected maize hybrids.
4.2. Isolates and Inoculation
All strains of F. graminearum, F. verticillioides, and A. flavus included in this trial were isolated in Hungary from naturally infected grain and were part of the microorganism collection of the Cereal Research Nonprofit Ltd. (Gabonakutató Nonprofit Kft: Szeged, Hungary) GK- Szeged, Hungary. Inocula preparation was performed according to the protocol of Szabó et al. [30]. Inoculation was conducted by toothpick [3] to evaluate kernel resistance (Figure 3). It was performed six days after 50% mid-silking by inserting infested toothpicks into the middle of the upper ear in a hole made by an awl, 15 mm long and 1.5 mm wide, and left until the harvesting period.

Figure 3.
Illustrates the toothpick method inoculation process, depicting the sequence of steps: (1) infested toothpick, (2) creating an aperture, (3) punctured ear, (4) toothpick inserted.
4.3. Evaluation of Ear and Kernel Rot Severity
To ensure improved sampling, the evaluation considered only the ears on which the toothpick’s mark was identified, using an average of 15 inoculated cobs per plot with each fungal isolate. At maturity, with an allowable harvesting moisture content of around 15–17%, the cobs were manually harvested and dehusked to evaluate the severity of the ear and kernel rot. The severity of fungus-induced ear rot was calculated using the percentage scale [3]. Based on this procedure, it was given as the percentage of ear coverage of an average regular ear (700–800 grains). It explains that a 1% infection is obtained when the ear contains 7–8 kernels with visible infection, and a 0.15% is obtained when only one kernel shows visible infection. The recording of severity involved two values, one directly from toothpick infection and the other independent infection as natural.
4.4. Sample Preparations and Measurement of Mycotoxins
For the toxin study, five ears with an average prevalence of ear rot without insect damage were selected per row. The ears were collected in a mesh-lined Rashel bag, stored under dry conditions until dry for three weeks, and then hand-shelled to avoid potential contamination. The grains were rough ground and carefully mixed, then finely milled for toxin measurement. The milling was carried out using a Perten laboratory mill (T: 3310, PI, 126 53 Hagersten, Sweden).
Mycotoxin contamination was measured using an enzyme-linked immunosorbent assay (ELISA) test. The amount of total fumonisin (FUM), DON, and AFB1 was measured by AgraQuant Fumonisin 0.25/5.0 ELISA kit, AgraQuant Deoxynivalenol 0.25/5.0 ELISA kit and AgraQuant Aflatoxin B1 2/50 ELISA kit, manufactured by Romer Labs, Tulln, Austria, respectively. According to the manufacturer’s instructions, the assays were carried out on ground maize kernel samples using a direct competitive assay. The samples were measured at 450 nm using a microtiter plate reader (Thermo Scientific, Waltham, MA, USA). Measurements were repeated four times with CV% < 15%. The ELISA kit detection limits were 0.2 ppm for DON and FB and 2 ppb for AFB1, respectively.
4.5. Statistical Data Analysis
The influence of N rates and maize hybrids on the severity of ear and kernel coverage and mycotoxin contamination levels was determined by variance analysis after subjecting the data to a normality test using the Shapiro–Wilk test. The treatment means were compared using LSD and considered significant when p < 0.05. The statistical software Genistat 18th edition, version 18.2 (64 bit) registered for Plant Research International, was used. Additionally, the built-in Excel function (analysis toolpack) was used to perform Pearson correlation analysis (two-tailed) and regression analysis, investigating the relationships between the severity of toxin production within and between toxigenic fungal species.
Author Contributions
Conceptualization, M.S.N. and P.P.; methodology, M.S.N. and P.P.; software, M.S.N.; validation, M.S.N. and P.P.; formal analysis, M.S.N.; investigation, M.S.N. and P.P.; resources, M.S.N. and P.P.; data curation, M.S.N. and P.P.; writing—original draft preparation, M.S.N.; writing—review and editing, M.S.N. and P.P.; visualization, P.P.; supervision, P.P.; project administration, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Tempus Public Foundation (TPF) in the framework of stipendium hungaricum scholarship and the open access support managed by the University of Debrecen and the national library, Hungary.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data for the findings of the current study are available from the corresponding author upon request.
Acknowledgments
We thank all Látókép research site staff for their technical guidance throughout the experimental period. We thank the Cereal Research Nonprofit Ltd. (Gabonakutató Nonprofit Kft) GK-Szeged, Hungary, for providing the inoculum of all fungal strains used in the experiment. We also highly cherish the funding support from the Tempus Public Foundation within the framework of the Stipendium Hungaricum Scholarship Programme.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Food and Agriculture Organization of the United Nations Statistics (FAOstat). Available online: https://www.fao.org/faostat/en/#home (accessed on 12 February 2023).
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Szieberth, D.; Toldine, E.T.; Nagy, Z.; Szabó, B.; Herczig, B.; Bors, I.; Tóth, B. Updating the Methodology of Identifying Maize Hybrids Resistant to Ear Rot Pathogens and Their Toxins—Artificial Inoculation Tests for Kernel Resistance to Fusarium graminearum, F. verticillioides, and Aspergillus flavus. J. Fungi 2022, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Geary, P.A.; Chen, G.; Kimanya, M.E.; Shirima, C.P.; Oplatowska-Stachowiak, M.; Elliott, C.T.; Routledge, M.N.; Gong, Y.Y. Determination of multi-mycotoxin occurrence in maize-based porridges from selected regions of Tanzania by liquid chromatography tandem mass spectrometry (LC-MS/MS), a longitudinal study. Food Control 2016, 68, 337–343. [Google Scholar] [CrossRef]
- CAST-Council for Agriculture, Science and Technology. Mycotoxins: Risks in Plant, Animal, and Human Systems Task Force Reports—R139. 2003. Available online: https://www.cast-science.org/wp-content/uploads/2002/11/CAST_R139_Mycotoxins_Risks_Plant_Animal_Health_Systems.pdf (accessed on 30 April 2024).
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. The status of Fusarium mycotoxins in sub-Saharan Africa: A review of emerging trends and post-harvest mitigation strategies towards food control. Toxins 2017, 9, 19. [Google Scholar] [CrossRef]
- Borràs-Vallverdú, B.; Ramos, A.J.; Cantero-Martínez, C.; Marín, S.; Sanchis, V.; Fernández-Ortega, J. Influence of agronomic factors on mycotoxin contamination in maize and changes during a 10-day harvest-till-drying simulation period: A different perspective. Toxins 2022, 14, 620. [Google Scholar] [CrossRef] [PubMed]
- Mosier, A.; Syers, J.K.; Freney, J.R. (Eds.) Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Island Press: Washington, DC, USA, 2013; Volume 65. [Google Scholar]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of nitrogen use in maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Lei, Q.; Luo, J.; Lindsey, S.; Zhang, J.; Zhai, L.; Wu, S.; Zhang, J.; Liu, X.; et al. Optimizing the nitrogen application rate for maize and wheat based on yield and environment on the Northern China Plain. Sci. Total Environ. 2018, 618, 1173–1183. [Google Scholar] [CrossRef]
- Souza, T.M.; Bernd, L.P.; Okumura, R.S.; Takahashi, H.W.; Ono, E.Y.S.; Hirooka, E.Y. Nitrogen fertilization effect on chemical composition and contamination by fungal-fumonisin of maize kernels. Rev. Bras. Ciências Agrárias 2016, 11, 218–233. [Google Scholar] [CrossRef]
- Shahid, M.R. Effects of nitrogen fertilization rate and harvest time on maize (Zea mays L.) fodder yield and its quality attributes. Asian J. Pharm. Biol. Res. 2012, 2, 19–26. [Google Scholar]
- Holou, R.A.Y.; Kindomihou, V. Impact of nitrogen fertilization on the oil, protein, starch, and ethanol yield of corn (Zea mays L.) grown for biofuel production. J. Life Sci. 2011, 5, 1013–1021. [Google Scholar]
- Blandino, M.; Reyneri, A.; Vanara, F. Influence of nitrogen fertilization on mycotoxin contamination of maize kernels. Crop Prot. 2008, 27, 222–230. [Google Scholar] [CrossRef]
- Scarpino, V.; Sulyok, M.; Krska, R.; Reyneri, A.; Blandino, M. The role of nitrogen fertilization on the occurrence of regulated, modified and emerging mycotoxins and fungal metabolites in Maize Kernels. Toxins 2022, 14, 448. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Ferrarini, A.; Maschietto, V.; Delledonne, M.; Marocco, A.; Bellin, D. Functional genomic analysis of constitutive and inducible defense responses to Fusarium verticillioides infection in maize genotypes with contrasting ear rot resistance. BMC Genom. 2014, 15, 710. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, L.K.; Mylroie, J.E.; Oliveira, D.A.; Smith, J.S.; Ozkan, S.; Windham, G.L.; Williams, W.P.; Warburton, M.L. Characterization of the maize chitinase genes and their effect on Aspergillus flavus and aflatoxin accumulation resistance. PLoS ONE 2015, 10, e0126185. [Google Scholar] [CrossRef] [PubMed]
- Logrieco, A.; Battilani, P.; Leggieri, M.C.; Jiang, Y.; Haesaert, G.; Lanubile, A.; Mahuku, G.; Mesterházy, A.; Ortega-Beltran, A.; Pasti, M.; et al. Perspectives on global mycotoxin issues and management from the MycoKey Maize Working Group. Plant Dis. 2021, 105, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Williams, W.P.; Windham, G.L.; Menkir, A.; Chen, Z.-Y. Evaluation of African-bred maize germplasm lines for resistance to aflatoxin accumulation. Agronomy 2016, 6, 24. [Google Scholar] [CrossRef]
- Williams, W.P.; Krakowsky, M.D.; Scully, B.T.; Brown, R.L.; Menkir, A.; Warburton, M.L.; Windham, G.L. Identifying and developing maize germplasm with resistance to accumulation of aflatoxins. World Mycotoxin J. 2015, 8, 193–209. [Google Scholar] [CrossRef]
- Mayfield, K.; Betrán, F.J.; Isakeit, T.; Odvody, G.; Murray, S.C.; Rooney, W.L.; Landivar, J.C. Registration of maize germplasm lines Tx736, Tx739, and Tx740 for reducing preharvest aflatoxin accumulation. J. Plant Regist. 2012, 6, 88–94. [Google Scholar] [CrossRef]
- Williams, W.; Krakowsky, M.D.; Windham, G.L.; Balint-Kurti, P.; Hawkins, L.K.; Henry, W. Identifying maize germplasm with resistance to aflatoxin accumulation. Toxin Rev. 2008, 27, 319–345. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Registration of maize germplasm line Mp717. Crop Sci. 2006, 46, 1407. [Google Scholar] [CrossRef]
- Williams, W.P.; Windham, G.L. Registration of maize germplasm line Mp715. Crop Sci. 2001, 41, 1374. [Google Scholar] [CrossRef]
- Scott, G.E.; Zummo, N. Registration of Mp420 germplasm line of maize. Crop Sci. 1992, 32, 1296. [Google Scholar] [CrossRef]
- Scott, G.E.; Zummo, N. Registration of Mp313E parental line of maize. Crop Sci. 1990, 30, 1378. [Google Scholar] [CrossRef]
- Zafar, S.; Iqbal, N.A.E.E.M.; Haidar, M.Z.; Rafique, M.; Ali, M. Responses of spring sown maize (Zea mays L.) genotypes to Aspergillus flavus inoculation: Grain yield and quality attributes. Pak. J. Bot. 2021, 53, 1507–1513. [Google Scholar] [CrossRef]
- Szabo, B.; Toth, B.; Toth Toldine, E.; Varga, M.; Kovacs, N.; Varga, J.; Kocsube, S.; Palagyi, A.; Bagi, F.; Budakov, D.; et al. A new concept to secure food safety standards against Fusarium species and Aspergillus flavus and their toxins in maize. Toxins 2018, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P.; Windham, G.L. Diallel analysis of fumonisin accumulation in maize. Field Crops Res. 2009, 114, 324–326. [Google Scholar] [CrossRef]
- Giomi, G.M.; Kreff, E.D.; Iglesias, J.; Fauguel, C.M.; Fernandez, M.; Oviedo, M.S.; Presello, D.A. Quantitative trait loci for Fusarium and Gibberella ear rot resistance in Argentinian maize germplasm. Euphytica 2016, 211, 287–294. [Google Scholar] [CrossRef]
- Mideros, S.X.; Warburton, M.L.; Jamann, T.M.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Quantitative trait loci influencing mycotoxin contamination of maize: Analysis by linkage mapping, characterization of near-isogenic lines, and meta-analysis. Crop Sci. 2014, 54, 127–142. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 20, 364–365. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 3 July 2024).
- Commission Regulation (EC). No 1126/2007 of 28 September 2007, amending regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 2007, L255, 14–17. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:255:0014:0017:EN:PDF (accessed on 3 July 2024).
- Commission regulation (EC). Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. OJL 2002, 30, 10. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2002L0032:20061020:EN:PDF (accessed on 3 July 2024).
- Tubajika, K.M.; Mascagni, H.J.; Damann, K.E.; Russin, J.S. Nitrogen fertilizer influence on aflatoxin contamination of corn in Louisiana. J. Agric. Food Chem. 1999, 47, 5257–5260. [Google Scholar] [CrossRef] [PubMed]
- Madege, R.R.; Audenaert, K.; Kimanya, M.; Tiisekwa, B.; De Meulenaer, B.; Bekaert, B.; Landschoot, S.; Haesaert, G. Control of Fusarium verticillioides (Sacc.) nirenberg and fumonisins by using a combination of crop protection products and fertilization. Toxins 2018, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Simões, D.; Carbas, B.; Soares, A.; Freitas, A.; Silva, A.S.; Brites, C.; Andrade, E.D. Assessment of agricultural practices for controlling Fusarium and mycotoxins contamination on maize grains: Exploratory study in maize farms. Toxins 2023, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Krnjaja, V.; Mandić, V.; Bijelić, Z.; Stanković, S.; Obradović, A.; Petrović, T.; Vasić, T.; Radović, Č. Influence of nitrogen rates and Fusarium verticillioides infection on Fusarium spp. and fumonisin contamination of maize kernels. Crop Prot. 2021, 144, 105601. [Google Scholar] [CrossRef]
- Santos, F.C.; Figueiredo, J.E.F.; Pinheiro, R.B.; Cota, L.V.; Vasconcelos, A.A.; Albuquerque Filho, M.R.; Costa, R.V.; Pastina, M.M.; Silva-Araújo, D.D. Effects of maize genotypes, nitrogen rates and sources in yield, nutritional status, and fumonisins incidence. Braz. J. Biol. 2023, 83, e274081. [Google Scholar] [CrossRef]
- Bocianowski, J.; Szulc, P.; Waśkiewicz, A.; Cyplik, A. The effect of agrotechnical factors on Fusarium mycotoxins level in maize. Agriculture 2020, 10, 528. [Google Scholar] [CrossRef]
- Barošević, T.; Bagi, F.; Savić, Z.; Ljubičić, N.; Ivanović, I. Assessment of maize hybrids resistance to Aspergillus ear rot and aflatoxin production in environmental conditions in Serbia. Toxins 2022, 14, 887. [Google Scholar] [CrossRef]
- Leite, M.; Freitas, A.; Silva, A.S.; Barbosa, J.; Ramos, F. Maize food chain and mycotoxins: A review on occurrence studies. Trends Food Sci. Technol. 2021, 115, 307–331. [Google Scholar] [CrossRef]
- Scarpino, V.; Reyneri, A.; Sulyok, M.; Krska, R.; Blandino, M. Impact of the insecticide application to maize cultivated in different environmental conditions on emerging mycotoxins. Field Crops Res. 2018, 217, 188–198. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 461889. [Google Scholar] [CrossRef] [PubMed]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).