Metabolome and Its Mechanism Profiling in the Synergistic Toxic Effects Induced by Co-Exposure of Tenuazonic Acid and Patulin in Caco-2 Cells
Abstract
1. Introduction
2. Results
2.1. Combined Toxicity Evaluation Based on Cell Viability
2.2. Cellular Oxidative Stress Assessment
2.3. Cellular Metabolites Analysis
2.4. Cellular Metabolic Pathways Analysis
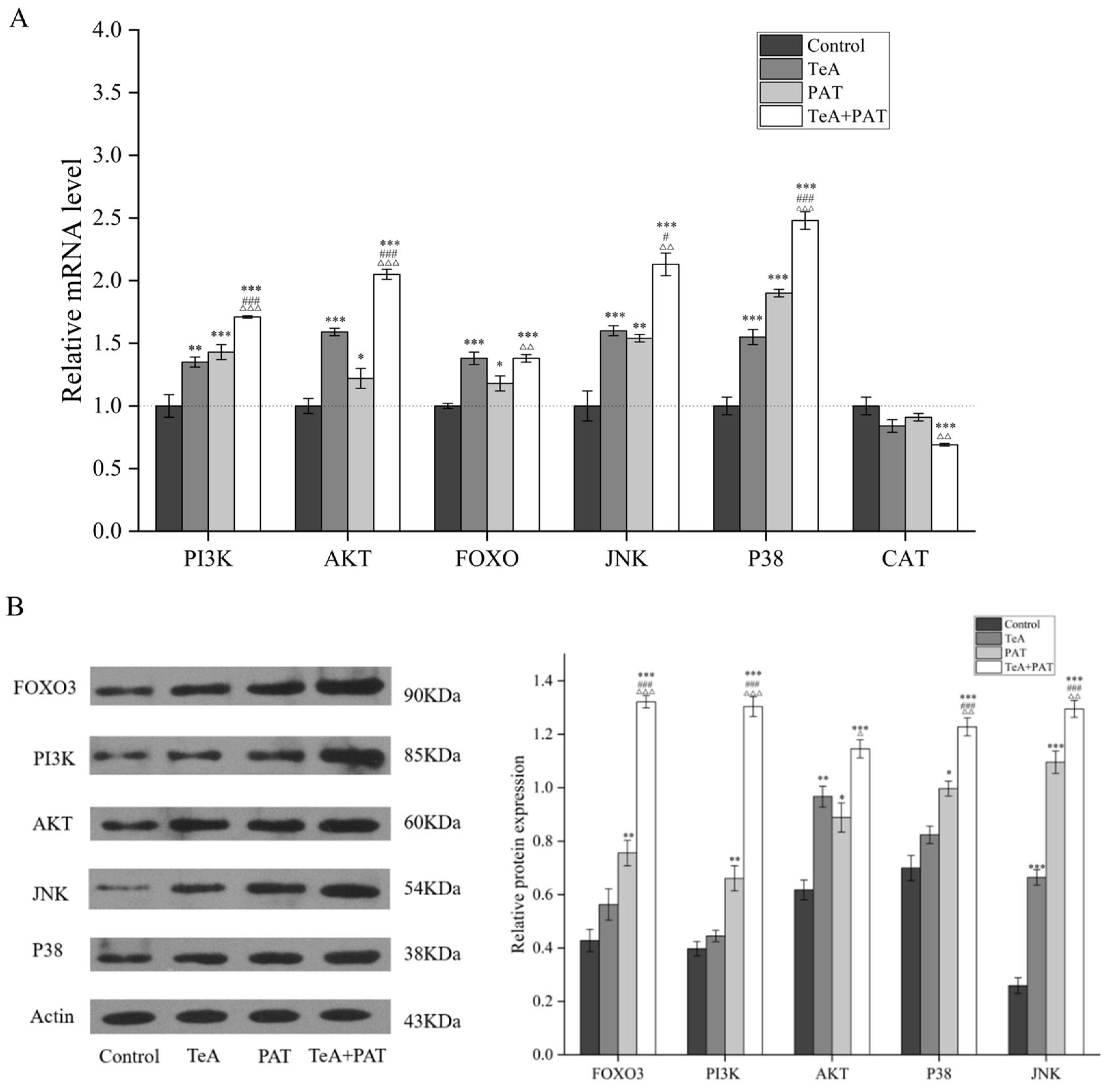
2.5. Validation of Key Metabolic Pathways
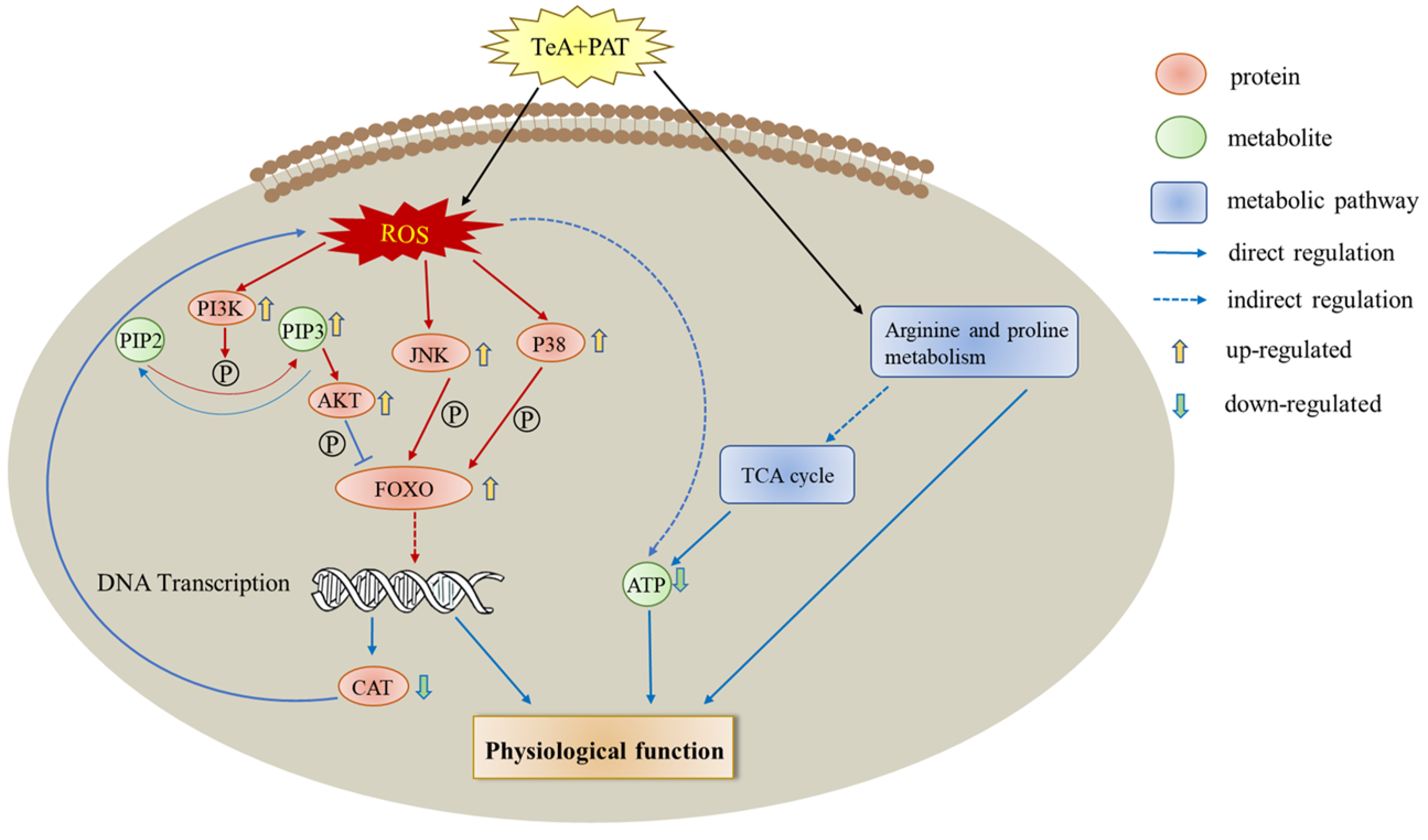
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Mycotoxins Solutions Preparation
5.2. Cell Culture and Treatment for Cytotoxic Determination
5.3. Determination of Reactive Oxygen Species (ROS) Level
5.4. Determination of Catalase (CAT) Activity
5.5. Determination of Adenosine-Triphosphate (ATP) Level
5.6. UHPLC-MS/MS Analysis for Cell Metabolomics
5.7. Real-Time Quantitative PCR (RT-qPCR)
5.8. Western Blotting (WB) Assay
5.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, T.; Bai, J.; Chen, G.; Chen, Z.; Zeng, S.; Yang, Y.; Wu, Z. 3-Acetyldeoxynivalenol induces apoptosis, barrier dysfunction and endoplasmic reticulum stress by inhibiting mTORC1-dependent autophagy in porcine enterocytes. Chem-Biol. Interact. 2023, 384, 110695. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Warth, B.; Sulyok, M.; Marko, D. Non-synergistic cytotoxic effects of fusarium and alternaria toxin combinations in Caco-2 cells. Toxicol. Lett. 2016, 241, 1–8. [Google Scholar] [CrossRef]
- de Oliveira, R.C.; Carnielli-Queiroz, L.; Correa, B. Epicoccum sorghinum in food: Occurrence, genetic aspects and tenuazonic acid production. Curr. Opin. Food Sci. 2018, 23, 44–48. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- EFSA on Contaminants in the Food Chain. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Aichinger, G.; Del Favero, G.; Warth, B.; Marko, D. Alternaria toxins- Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria mycotoxins: An overview of toxicity, metabolism, and analysis in food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magista, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, ausarium and alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018, 11, 159–173. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Verdini, E.; Sdogati, S.; Bibi, R.; Ciasca, B.; Pecorelli, I. Monitoring Alternaria toxins in Italian food to support upcoming regulation. Food Addit. Contam. B 2022, 15, 42–51. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Nie, J.; Bacha, S.A.S.; Yan, Z.; Gao, G. Occurrence and co-occurrence of mycotoxins in apple and apple products from China. Food Control 2020, 118, 107354. [Google Scholar] [CrossRef]
- Lopez, P.; Venema, D.; Mol, H.; Spanjer, M.; de Stoppelaar, J.; Pfeiffer, E.; de Nijs, M. Alternaria toxins and conjugates in selected foods in the Netherlands. Food Control 2016, 69, 153–159. [Google Scholar] [CrossRef]
- Mikusova, P.; Sulyok, M.; Srobarova, A. Alternaria mycotoxins associated with grape berries in vitro and in situ. Biologia 2014, 69, 173–177. [Google Scholar] [CrossRef]
- Zhao, K.; Shao, B.; Yang, D.; Li, F. Natural occurrence of four alternaria mycotoxins in tomato- and citrus-based foods in China. J. Agric. Food Chem. 2015, 63, 343–348. [Google Scholar] [CrossRef]
- Escriva, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- Lin, H.; Jia, B.; Wu, A. Cytotoxicities of co-occurring alternariol, alternariol monomethyl ether and tenuazonic acid on human gastric epithelial cells. Food Chem. Toxicol. 2023, 171, 113524. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, J.; Zhang, J.; Huang, X.; Zhang, Y.; Zhao, Y.; Hong, G. A novel method for predicting the emergence of toxicity interaction in ternary mixtures. Environ. Res. 2024, 240, 117437. [Google Scholar] [CrossRef]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef]
- Wei, D.; Wang, Y.; Jiang, D.; Feng, X.; Li, J.; Wang, M. Survey of alternaria toxins and other mycotoxins in dried fruits in China. Toxins 2017, 9, 200. [Google Scholar] [CrossRef]
- Mahato, D.K.; Kamle, M.; Sharma, B.; Pandhi, S.; Devi, S.; Dhawan, K.; Selvakumar, R.; Mishra, D.; Kumar, A.; Arora, S.; et al. Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon 2021, 198, 12–23. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Zouaoui, N.; Mallebrera, B.; Berrada, H.; Abid-Essefi, S.; Bacha, H.; Ruiz, M.J. Cytotoxic effects induced by patulin, sterigmatocystin and beauvericin on CHO–K1 cells. Food Chem. Toxicol. 2016, 89, 92–103. [Google Scholar] [CrossRef]
- den Hollander, D.; Holvoet, C.; Demeyere, K.; De Zutter, N.; Audenaert, K.; Meyer, E.; Croubels, S. Cytotoxic effects of alternariol, alternariol monomethyl-ether, and tenuazonic acid and their relevant combined mixtures on human enterocytes and hepatocytes. Front. Microbiol. 2022, 13, 849243. [Google Scholar] [CrossRef]
- Assunção, R.; Pinhão, M.; Loureiro, S.; Alvito, P.; Silva, M.J. A multi-endpoint approach to the combined toxic effects of patulin and ochratoxin a in human intestinal cells. Toxicol. Lett. 2019, 313, 120–129. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Kang, Y.; Guo, T.; Zhou, Y.; Zhang, Y.; Ma, L. Synergistic toxicity induced by the co-exposure of tenuazonic acid and patulin in Caenorhabditis elegans: Daf-16 plays an important regulatory role. Ecotoxicol. Environ. Saf. 2024, 270, 115871. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 5, 621–681. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Yang, X.; Gong, J.; Zhang, X.; Huang, Y.; Zhang, W.; Yang, J.; Lin, J.; Chai, Y.; Liu, J. Evaluation of the combined toxicity of multi-walled carbon nanotubes and cadmium on earthworms in soil using multi-level biomarkers. Ecotox. Environ. Safe 2021, 221, 112441. [Google Scholar] [CrossRef]
- Solhaug, A.; Karlsoen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. Vitro 2016, 36, 120–132. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible mechanisms of oxidative stress-induced skin cellular Senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, X.; Wang, J.; Zhou, Y.; Qi, W.; Chen, H.; Nie, S.; Xie, M. Dendrobium officinale polysaccharide triggers mitochondrial disorder to induce colon cancer cell death via ROS-AMPK-autophagy pathway. Carbohyd. Polym. 2021, 264, 118018. [Google Scholar] [CrossRef]
- Li, W.; Sang, Y.; Zhang, G. Combined cytotoxicity of aflatoxin B1 and deoxynivalenol to hepatoma HepG2/C3A cells. World Mycotoxin J. 2017, 10, 387–399. [Google Scholar] [CrossRef]
- Mitchell, C.T.; Bridgeman, L.; Moyano-López, C.; Penalva-Olcina, R.; Juan, C.; Juan-García, A. Study of cytotoxicity in neuroblastoma cell line exposed to patulin and citrinin. Food Chem. Toxicol. 2024, 186, 114556. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Jin, G.; Yang, X.; Yang, X.; Zhou, H. Regulating dyslipidemia effect of polysaccharides from Pleurotus ostreatus on fat-emulsion-induced hyperlipidemia rats. Int. J. Biol. Macromol. 2017, 101, 107–116. [Google Scholar] [CrossRef]
- Bai, M. Metabonomics study of liver and kidney subacute toxicity induced by garidi-5 in rats. Chin. Herb. Med. 2022, 14, 422–431. [Google Scholar]
- Gan, C.; Huang, X.; Wu, Y.; Zhan, J.; Zhang, X.; Liu, Q.; Huang, Y. Untargeted metabolomics study and pro-apoptotic properties of B-norcholesteryl benzimidazole compounds in ovarian cancer SKOV3 cells. J. Steroid Biochem. 2020, 202, 105709. [Google Scholar] [CrossRef]
- Liu, J.; Litt, L.; Segal, M.R.; Kelly, M.J.S.; Pelton, J.G.; Kim, M. Metabolomics of oxidative stress in recent studies of endogenous and exogenously administered intermediate metabolites. Int. J. Mol. Sci. 2011, 12, 6469–6501. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Gao, Y.; Wang, J.; Zheng, N. Integrated metabolomics and lipidomics analysis reveals lipid metabolic disorder in NCM460 cells caused by aflatoxin B1 and aflatoxin M1 alone and in combination. Toxins 2023, 15, 255. [Google Scholar] [CrossRef]
- Cui, Y.; Han, J.; Ren, J.; Chen, H.; Xu, B.; Song, N.; Li, H.; Liang, A.; Shen, G. Untargeted LC-MS-based metabonomics revealed that aristolochic acid I induces testicular toxicity by inhibiting amino acids metabolism, glucose metabolism, beta-oxidation of fatty acids and the TCA cycle in male mice. Toxicol. Appl. Pharm. 2019, 373, 26–38. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Q.; Wu, H.; Xia, S.; Guo, H.; Blazenovic, I.; Zhang, Y.; Sun, X. Insights into cellular metabolic pathways of the combined toxicity responses of Caco-2 cells exposed to deoxynivalenol, zearalenone and aflatoxin B1. Food Chem. Toxicol. 2019, 126, 106–112. [Google Scholar] [CrossRef]
- Arafat, W.; Kern, D.; Dirheimer, G. Inhibition of aminoacyl-transfer RNA-synthetases by the mycotoxin Patulin. Chem-Biol. Interact. 1985, 56, 333–349. [Google Scholar] [CrossRef]
- Wang, C.; Xia, C.; Zhu, Y.; Zhang, H. Innovative fluorescent probes for in vivo visualization of biomolecules in living Caenorhabditis elegans. Cytom. Part A 2021, 99, 560–574. [Google Scholar] [CrossRef]
- Barthel, A.; Ostrakhovitch, E.A.; Walter, P.L.; Kampkoetter, A.; Klotz, L. Stimulation of phosphoinositide 3-kinase/Akt signaling by copper and zinc ions: Mechanisms and consequences. Arch. Biochem. Biophys. 2007, 463, 175–182. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Storz, P. Forkhead homeobox type o transcription factors in the responses to oxidative stress. Antioxid Redox Sign. 2011, 14, 593–605. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kikuchi, O.; Sasaki, T.; Kim, H.J.; Yokota-Hashimoto, H.; Lee, Y.S.; Kitamura, T. FoxO1 as a double-edged sword in the pancreas: Analysis of pancreas-and β-cell-specific FoxO1 knockout mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E603–E613. [Google Scholar] [CrossRef]
- Wang, B.J.; Zheng, W.L.; Feng, N.; Wang, T.; Zou, H.; Gu, J.; Yuan, Y.; Liu, X.; Liu, Z.; Bian, J. The effects of autophagy and PI3K/AKT/m-TOR signaling pathway on the cell-cycle arrest of rats primary sertoli cells induced by zearalenone. Toxins 2018, 10, 398. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Lu, C.; Zhang, J. Role of insulin signaling pathway in apoptosis induced by food chain delivery of nano-silver under the action of environmental factors. Comp. Biochem. Phys. C 2022, 261, 109429. [Google Scholar] [CrossRef]
- Yang, Y.X.; Yu, S.; Jia, B.X.; Liu, N.; Wu, A. Metabolomic profiling reveals similar cytotoxic effects and protective functions of quercetin during deoxynivalenol-and 15-acetyl deoxynivalenol-induced cell apoptosis. Toxicol. Vitro 2020, 66, 104838. [Google Scholar] [CrossRef]
- Chu, Q.; Chen, W.; Jia, R.; Ye, X.; Li, Y.; Liu, Y.; Jiang, Y.; Zheng, X. Tetrastigma hemsleyanum leaves extract against acrylamide-induced toxicity in HepG2 cells and Caenorhabditis elegans. J. Hazard. Mater. 2020, 393, 122364. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Kang, W.; Lin, Z.; Gan, F.; Huang, K. Non-cytotoxic dosage of fumonisin B1 aggravates ochratoxin A-induced nephrocytotoxicity and apoptosis via ROS-dependent JNK/MAPK signaling pathway. Toxicology 2021, 457, 152802. [Google Scholar] [CrossRef]
- Doppler, M.; Kluger, B.; Bueschl, C.; Schneider, C.; Krska, R.; Delcambre, S.; Hiller, K.; Lemmens, M.; Schuhmacher, R. Stable isotope-assisted evaluation of different extraction solvents for untargeted metabolomics of plants. Int. J. Mol. Sci. 2016, 17, 1017. [Google Scholar] [CrossRef]
- Chou, T.C.; Matin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn: Paramus, NJ, USA, 2005. [Google Scholar]
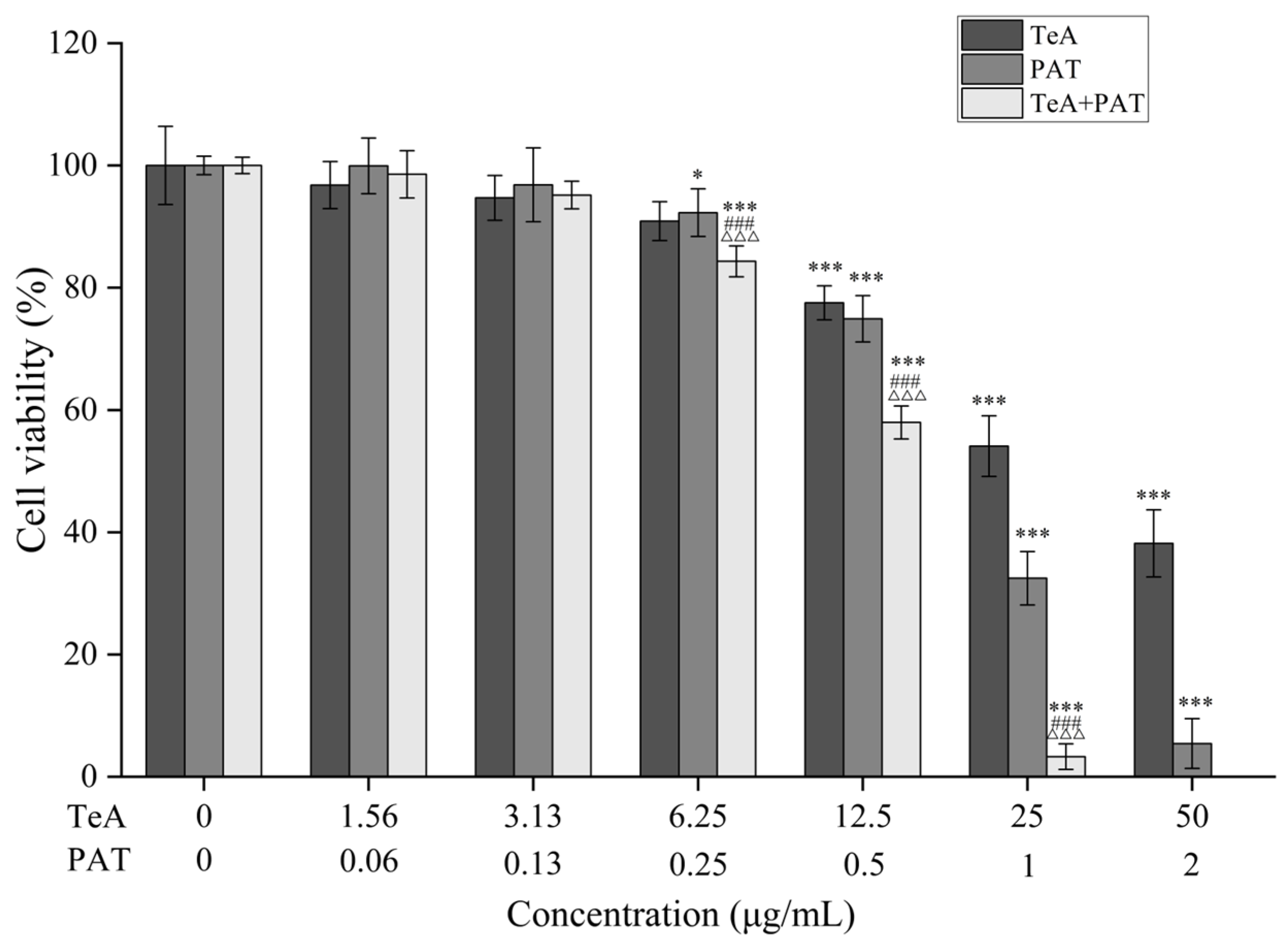
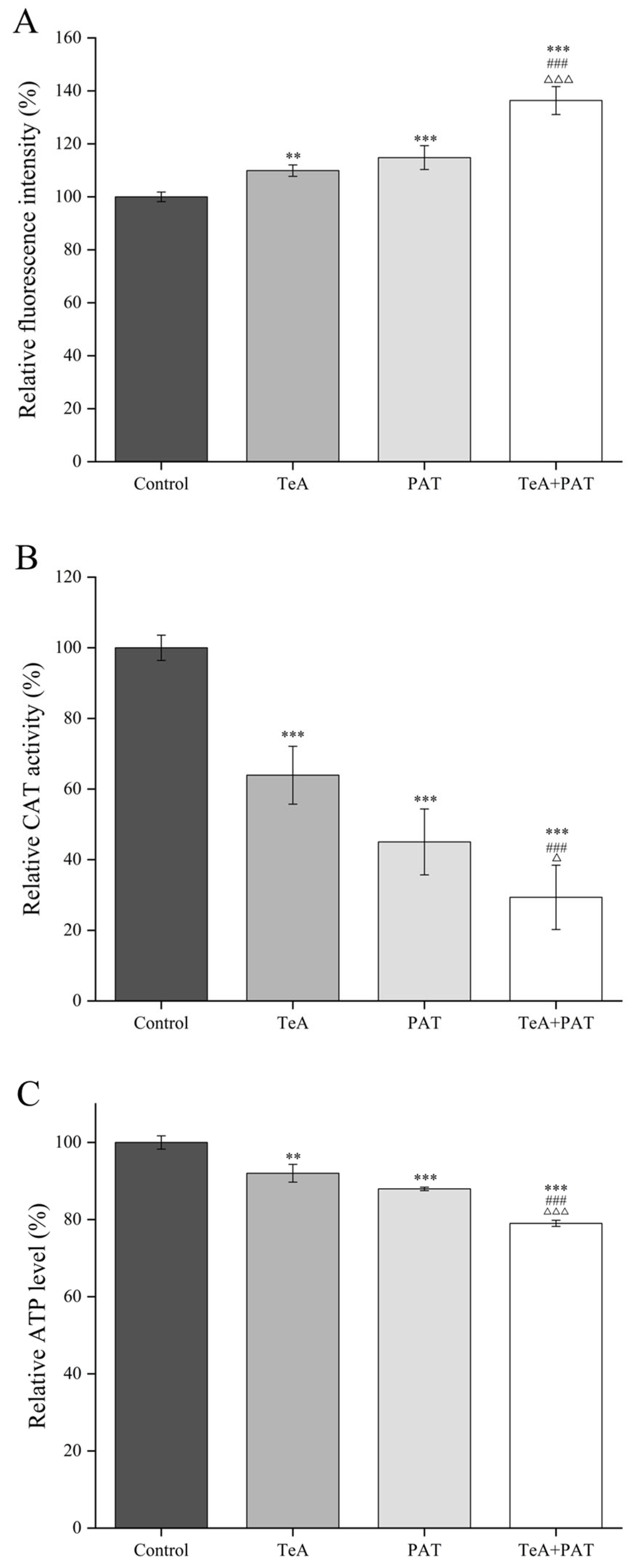
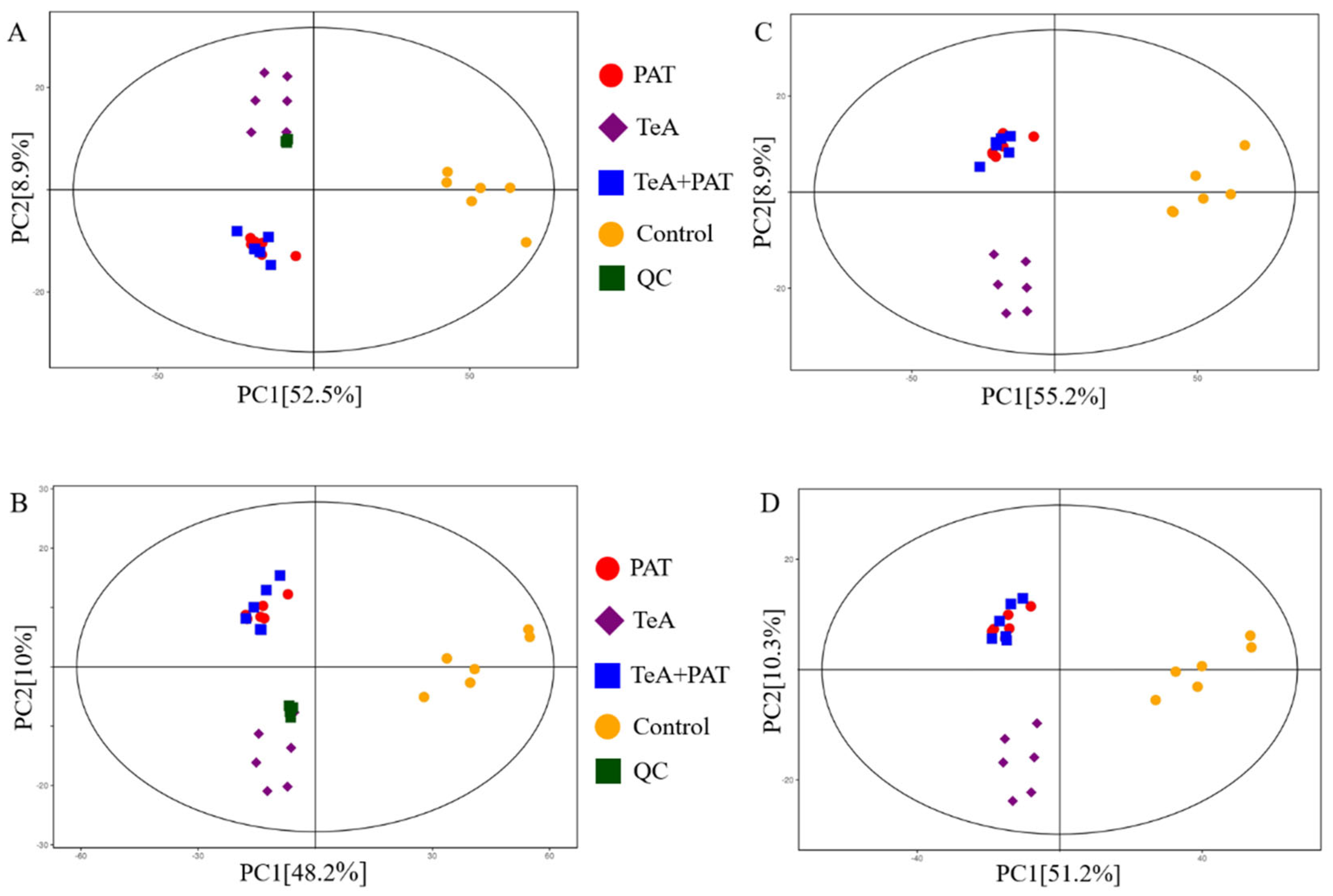
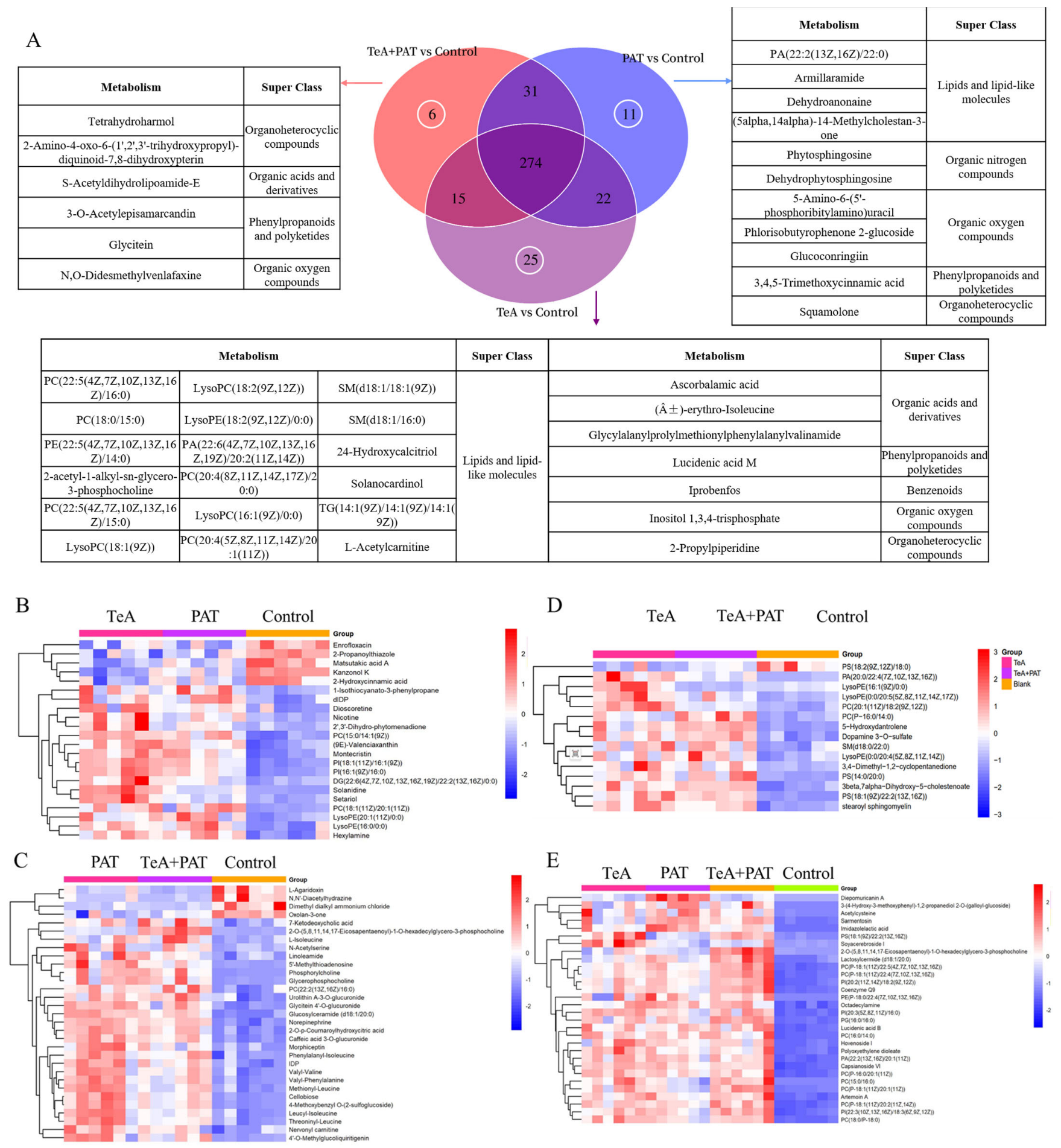


| Mycotoxin | Dose-Effect Parameters | CI Values and Descriptions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dm/(μg/mL) | m | r | IC25 | IC50 | IC75 | ||||
| TeA | 32.46 | 1.28 | 0.99 | ||||||
| PAT | 0.69 | 2.65 | 0.98 | ||||||
| TeA + PAT | 10.21 | 2.58 | 0.96 | 1.03 | Nearly addictive | 0.87 | Slightly synergism | 0.77 | Moderate synergism |
| KEGG Pathways | TeA | PAT | TeA + PAT |
|---|---|---|---|
| Amino acid metabolism | Arginine and proline metabolism; D-Arginine and D-ornithine metabolism; Glycine, serine and threonine metabolism; Glutathione metabolism; D-Glutamine and D-glutamate metabolism; Cysteine and methionine metabolism; Selenoamino acid metabolism; Alanine, aspartate and glutamate metabolism; Valine, leucine and isoleucine degradationvbeta-Alanine metabolism; Tryptophan metabolism; Histidine metabolis; Phenylalanine metabolism; Tyrosine metabolism | Arginine and proline metabolism; D-Arginine and D-ornithine metabolism; Glycine, serine and threonine metabolism; Glutathione metabolism; D-Glutamine and D-glutamate metabolism; Cysteine and methionine metabolism; Selenoamino acid metabolism; Alanine, aspartate and glutamate metabolism; Valine, leucine and isoleucine degradation; beta-Alanine metabolism; Tryptophan metabolism; Histidine metabolism; Phenylalanine metabolism; Tyrosine metabolism; Valine, leucine and isoleucine degradation | Arginine and proline metabolism; D-Arginine and D-ornithine metabolism; Glycine, serine and threonine metabolism; Glutathione metabolism; D-Glutamine and D-glutamate metabolism; Cysteine and methionine metabolism; Selenoamino acid metabolism; Alanine, aspartate and glutamate metabolism; Valine, leucine and isoleucine degradation; beta-Alanine metabolism; Tryptophan metabolism; Histidine metabolism; Tyrosine metabolism; Valine, leucine and isoleucine degradation |
| Lipid metabolism | Glycerophospholipid metabolism; Primary bile acid biosynthesis; Steroid hormone biosynthesis | Glycerophospholipid metabolism; Primary bile acid biosynthesis; Steroid hormone biosynthesis; Ether lipid metabolism; Sphingolipid metabolism | Glycerophospholipid metabolism; Primary bile acid biosynthesis; Steroid hormone biosynthesis; Ether lipid metabolism |
| Metabolism of cofactors and vitamins | Thiamine metabolism; Pantothenate and CoA biosynthesis; Vitamin B6 metabolism; Porphyrin and chlorophyll metabolism | Thiamine metabolism; Pantothenate and CoA biosynthesis; Vitamin B6 metabolism; Porphyrin and chlorophyll metabolism; Riboflavin metabolism | Thiamine metabolism; Pantothenate and CoA biosynthesis; Vitamin B6 metabolism; Porphyrin and chlorophyll metabolism |
| Carbohydrate metabolism | Inositol phosphate metabolism; Galactose metabolism; Amino sugar and nucleotide sugar metabolism | Inositol phosphate metabolism; Galactose metabolism; Amino sugar and nucleotide sugar metabolism | Inositol phosphate metabolism; Galactose metabolism; Amino sugar and nucleotide sugar metabolism; Citrate cycle (TCA cycle); Glycolysis or Gluconeogenesis; Pyruvate metabolism |
| Nucleotide metabolism | Pyrimidine metabolism; Purine metabolism | Pyrimidine metabolism; Purine metabolism | Pyrimidine metabolism; Purine metabolism |
| Energy metabolism | Nitrogen metabolism | Nitrogen metabolism | Nitrogen metabolism |
| Translation | Aminoacyl-tRNA biosynthesis | Aminoacyl-tRNA biosynthesis | Aminoacyl-tRNA biosynthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhou, H.; Yang, Y.; Guo, T.; Zhou, Y.; Zhang, Y.; Ma, L. Metabolome and Its Mechanism Profiling in the Synergistic Toxic Effects Induced by Co-Exposure of Tenuazonic Acid and Patulin in Caco-2 Cells. Toxins 2024, 16, 319. https://doi.org/10.3390/toxins16070319
Qin Y, Zhou H, Yang Y, Guo T, Zhou Y, Zhang Y, Ma L. Metabolome and Its Mechanism Profiling in the Synergistic Toxic Effects Induced by Co-Exposure of Tenuazonic Acid and Patulin in Caco-2 Cells. Toxins. 2024; 16(7):319. https://doi.org/10.3390/toxins16070319
Chicago/Turabian StyleQin, Yuxian, Hongyuan Zhou, Yulian Yang, Ting Guo, Ying Zhou, Yuhao Zhang, and Liang Ma. 2024. "Metabolome and Its Mechanism Profiling in the Synergistic Toxic Effects Induced by Co-Exposure of Tenuazonic Acid and Patulin in Caco-2 Cells" Toxins 16, no. 7: 319. https://doi.org/10.3390/toxins16070319
APA StyleQin, Y., Zhou, H., Yang, Y., Guo, T., Zhou, Y., Zhang, Y., & Ma, L. (2024). Metabolome and Its Mechanism Profiling in the Synergistic Toxic Effects Induced by Co-Exposure of Tenuazonic Acid and Patulin in Caco-2 Cells. Toxins, 16(7), 319. https://doi.org/10.3390/toxins16070319








