Treatment of Primary Axillary Hyperhidrosis with Two Doses of Botulinum Toxin A—Observational Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowe, N.; Naumann, M.; Eadie, N. Treatment of Hyperhidrosis with Botox (onabotulinumtoxinA): Development, Insights, and Impact. Medicine 2023, 102, e32764. [Google Scholar] [CrossRef]
- Cerfolio, R.J.; De Campos JR, M.; Bryant, A.S.; Connery, C.P.; Miller, D.L.; DeCamp, M.M.; Krasna, M.J. The Society of Thoracic Surgeons Expert Consensus for the Surgical Treatment of Hyperhidrosis. Ann. Thorac. Surg. 2011, 91, 1642–1648. [Google Scholar] [CrossRef]
- Doolittle, J.; Walker, P.; Mills, T.; Thurston, J. Hyperhidrosis: An Update on Prevalence and Severity in the United States. Arch. Dermatol. Res. 2016, 308, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hasimoto, E.N.; Cataneo, D.C.; Reis, T.A.D.; Cataneo, A.J.M. Hyperhidrosis: Prevalence and impact on quality of life. J. Bras. Pneumol. 2018, 44, 292–298. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inose, Y.; Nakamura, H.; Kikukawa, Y. Questionnaire-Based Epidemiological Survey of Primary Focal Hyperhidrosis and Survey on Current Medical Management of Primary Axillary Hyperhidrosis in Japan. Arch. Dermatol. Res. 2022, 315, 409–417. [Google Scholar] [CrossRef]
- Wohlrab, J.; Bechara, F.G.; Schick, C.; Naumann, M. Hyperhidrosis: A Central Nervous Dysfunction of Sweat Secretion. Dermatol. Ther. 2023, 13, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Marchese, M.R.; Bussu, F.; Settimi, S.; Scarano, E.; Almadori, G.; Galli, J. Not Only Gustatory Sweating and Flushing: Signs and Symptoms Associated to the Frey Syndrome and the Role of Botulinum Toxin A Therapy. Head Neck 2021, 43, 949–955. [Google Scholar] [CrossRef]
- Safarpour, D.; Jabbari, B. Botulinum Toxin Treatment for Cancer-Related Disorders: A Systematic Review. Toxins 2023, 15, 689. [Google Scholar] [CrossRef] [PubMed]
- Lannan, F.M.; Powell, J.; Kim, G.M.; Hansen, C.R.; Pasquina, P.F.; Smith, D.G. Hyperhidrosis of the Residual Limb: A Narrative Review of the Measurement and Treatment of Excess Perspiration Affecting Individuals with Amputation. Prosthet. Orthot. Int. 2021, 45, 477–486. [Google Scholar] [CrossRef]
- Kisielnicka, A.; Szczerkowska-Dobosz, A.; Purzycka-Bohdan, D.; Nowicki, R. Hyperhidrosis: Disease aetiology, classification and management in the light of modern treatment modalities. Adv. Dermatol. Allergol. 2022, 39, 251–257. [Google Scholar] [CrossRef]
- Marani, A.; Gioacchini, H.; Paolinelli, M.; Bobyr, I.; Martina, E.; Radi, G.; Campanati, A. Pain Control during the Treatment of Primary Palmar Hyperhidrosis with Botulinum Toxin A by a Topical Application of Liposomal Lidocaine: Clinical Effectiveness. Toxins 2024, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Carrasco, D.; de Lucas, R. [Translated Article] Topical Anticholinergics in the Management of Focal Hyperhidrosis in Adults and Children: A Narrative Review. Actas Dermo-Sifiliográficas 2024, 115, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Botox Approved for Severe Underarm Sweating. FDA Consum. 2004, 38, 3.
- Lowe, N.J.; Glaser, D.A.; Eadie, N.; Daggett, S.; Kowalski, J.W.; Lai, P.Y.; North American Botox in Primary Axillary Hyperhidrosis Clinical Study Group. Botulinum Toxin Type A in the Treatment of Primary Axillary Hyperhidrosis: A 52-Week Multicenter Double-Blind, Randomized, Placebo-Controlled Study of Efficacy and Safety. J. Am. Acad. Dermatol. 2007, 56, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Vadoud-Seyedi, J.; Simonart, T. Treatment of Axillary Hyperhidrosis with Botulinum Toxin Type A Reconstituted in Lidocaine or in Normal Saline: A Randomized, Side-by-Side, Double-Blind Study. Br. J. Dermatol. 2007, 156, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Solish, N.; Bertucci, V.; Dansereau, A.; Hong, H.C.H.; Lynde, C.; Lupin, M.; Storwick, G. A Comprehensive Approach to the Recognition, Diagnosis, and Severity-Based Treatment of Focal Hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol. Surg. 2007, 33, 908–923. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.; Cha, J. Botulinum Toxin: Pharmacology and Injectable Administration for the Treatment of Primary Hyperhidrosis. J. Am. Acad. Dermatol. 2020, 82, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Wade, R.; Llewellyn, A.; Jones-Diette, J.; Wright, K.; Rice, S.; Layton, A.M.; Woolacott, N. Interventional Management of Hyperhidrosis in Secondary Care: A Systematic Review. Br. J. Dermatol. 2018, 179, 599–608. [Google Scholar] [CrossRef]
- Siri-Archawawat, D.; Tawanwongsri, W. Low-Dose OnabotulinumtoxinA Using Seven-Point Pattern Intradermal Injections in Patients with Moderate-to-Intolerable Primary Axillary Hyperhidrosis: A Single-Blinded, Side-by-Side Randomized Trial. J. Clin. Aesthetic Dermatol. 2023, 16, 37–43. [Google Scholar]
- Grove, G.L.; Togsverd-Bo, K.; Zachariae, C.; Haedersdal, M. Botulinum Toxin A versus Microwave Thermolysis for Primary Axillary Hyperhidrosis: A Randomized Controlled Trial. JAAD Int. 2024, 15, 91–99. [Google Scholar] [CrossRef]
- Glaser, D.A.; Hebert, A.; Pieretti, L.; Pariser, D. Understanding Patient Experience With Hyperhidrosis: A National Survey of 1,985 Patients. J. Drugs Dermatol. 2018, 17, 392–396. [Google Scholar]
- Henning, M.A.; Pedersen, O.B.; Jemec, G.B. Genetic Disposition to Primary Hyperhidrosis: A Review of Literature. Arch. Dermatol. Res. 2019, 311, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Kamikava DY, F.; Wolosker, N.; Silva MF, A.D.; Campos JR, M.D.; Puech-Leão, P. Symptoms of Anxiety and Depression in Patients with Primary Hyperhidrosis and Its Association with the Result of Clinical Treatment with Oxybutynin. Clinics. 2021, 76, e2892. [Google Scholar] [CrossRef]
- Paller, A.S.; Rangel, S.M.; Chamlin, S.L.; Hajek, A.; Phan, S.; Hogeling, M.; Griffith, J.W. Stigmatization and Mental Health Impact of Chronic Pediatric Skin Disorders. JAMA Dermatol. 2024, 160, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Chernyshov, P.V. The Evolution of Quality of Life Assessment and Use in Dermatology. Dermatology 2019, 235, 167–174. [Google Scholar] [CrossRef]
- Şener, S.; Karakoç, Y. Effects of Direct Current Administration on Hyperhidrosis Disease Severity Scale in Patients with Axillary Hyperhidrosis. BioMed Res. Int. 2019, 2019, 3232015. [Google Scholar] [CrossRef]
- Castiglione, L.; Murariu, M.; Boeriu, E.; Enatescu, I. Assessing Botulinum Toxin Effectiveness and Quality of Life in Axillary Hyperhidrosis: A One-Year Prospective Study. Diseases 2024, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Hebert, A.A.; Drew, J.; Quiring, J.; Gopalan, R.; Glaser, D.A. Topical GlycopyrroniumTosylate for the Treatment of Primary Axillary Hyperhidrosis: Patient-Reported Outcomes from the ATMOS-1 and ATMOS-2 Phase III Randomized Controlled Trials. Am. J. Clin. Dermatol. 2019, 20, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Lowe, N.J. Botulinum Toxin Type A in Treatment of Bilateral Primary Axillary Hyperhidrosis: Randomised, Parallel Group, Double Blind, Placebo Controlled. BMJ 2001, 323, 596. [Google Scholar] [CrossRef]
- Stolman, L.P. Hyperhidrosis: Medical and Surgical Treatment. Eplasty 2008, 8, e22. [Google Scholar]
- Kouris, A.; Armyra, K.; Christodoulou, C.; Karimali, P.; Karypidis, D.; Kontochristopoulos, G. Quality of Life in Patients with Focal Hyperhidrosis before and after Treatment with Botulinum Toxin A. ISRN Dermatol. 2014, 2014, 308650. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, S.E.; Rystedt, A.; Balling, M.; Swartling, C. Hyperhidrosis Substantially Reduces Quality of Life in Children: A Retrospective Study Describing Symptoms, Consequences and Treatment with Botulinum Toxin. Acta Derm. Venereol. 2018, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y.; Khan, G. Dermatology Life Quality Index (DLQI)-a Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Basra MK, A.; Salek, M.S.; Camilleri, L.; Sturkey, R.; Finlay, A.Y. Determining the Minimal Clinically Important Difference and Responsiveness of the Dermatology Life Quality Index (DLQI): Further Data. Dermatology 2015, 230, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.A.; Pariser, D.M.; Hebert, A.A.; Landells, I.; Somogyi, C.; Weng, E.; Brin, M.F.; Beddingfield, F. A Prospective, Nonrandomized, Open-Label Study of the Efficacy and Safety of OnabotulinumtoxinA in Adolescents with Primary Axillary Hyperhidrosis. Pediatr. Dermatol. 2015, 32, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Solish, N. Focal Hyperhidrosis: Diagnosis and Management. Can. Med. Assoc. J. 2005, 172, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Martina, E.; Diotallevi, F.; Radi, G.; Campanati, A.; Offidani, A. Therapeutic Use of Botulinum Neurotoxins in Dermatology: Systematic Review. Toxins 2021, 13, 120. [Google Scholar] [CrossRef]
| 50 U TOXIN | 100 U TOXIN | Significance | |
|---|---|---|---|
| N = 18 | N = 13 | ||
| AGE | 33.22 ± 10.55 | 33.62 ± 10.24 | 0.918 |
| SEX | 0.676 | ||
| Male | 3 (16.7%) | 3 (23.1%) | |
| Female | 15 (83.3%) | 10 (76.9%) | |
| CLINIC START | 0.965 | ||
| <10 years | 6 (33.3%) | 4 (30.8%) | |
| 10–15 years | 8 (44.4%) | 4 (30.8%) | |
| 15–20 years | 2 (11.1%) | 3 (23.1%) | |
| >20 years | 2 (11.1%) | 2 (15.3%) | |
| FAMILY HISTORY | 0.897 | ||
| No | 9 (50%) | 6 (46.2%) | |
| Yes | 9 (50%) | 7 (53.8%) | |
| BILATERALITY | |||
| Yes | 18 (100%) | 13 (100%) | 0.999 |
| PALMS | |||
| Yes | 8 (44.4%) | 9 (69.2%) | 0.171 |
| PLANTS | |||
| Yes | 8 (44.4%) | 9 (69.2%) | 0.171 |
| LOCATIONS OTHER THAN PALMS AND PLANTS | |||
| Head and neck | 1 (5.6%) | 0 (0%) | 0.868 |
| Trunk | 2 (11.1%) | 0 (0%) | 0.615 |
| Others | 1 (5.6%) | 2 (15.4%) | 0.765 |
| ASSOCIATED SYMPTOMS | |||
| Coldness | 2 (11.1%) | 5 (38.5%) | 0.173 |
| Itching | 2 (11.1%) | 2 (15.4%) | 0.847 |
| NIGHT SWEATS | |||
| No | 18 (100%) | 13 (100%) | 0.999 |
| TRIGGERING FACTORS | |||
| Heat | 0 (0%) | 0 (0%) | 0.999 |
| Stress | 1 (5.6%) | 1 (7.7%) | 0.615 |
| Heat and stress | 13 (72.2%) | 9 (69.2%) | 0.826 |
| Always/no stimulus | 4 (22.2%) | 3 (23.1%) | 0.704 |
| 50 U N = 40 | 100 U N = 42 | Significance | |
|---|---|---|---|
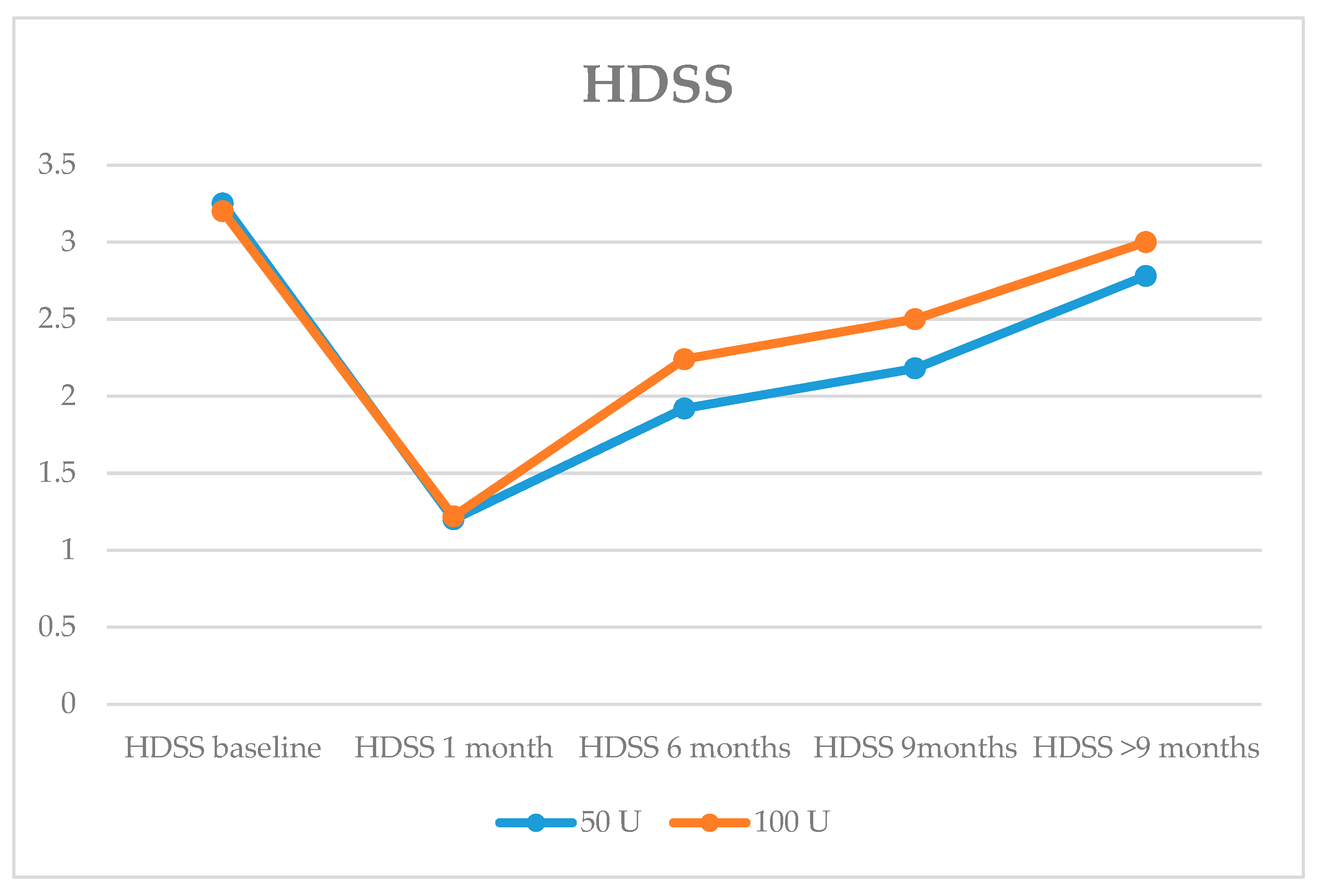
| HDSSMean | 3.25 ± 0.44 | 3.19 ± 0.39 | 0.521 |
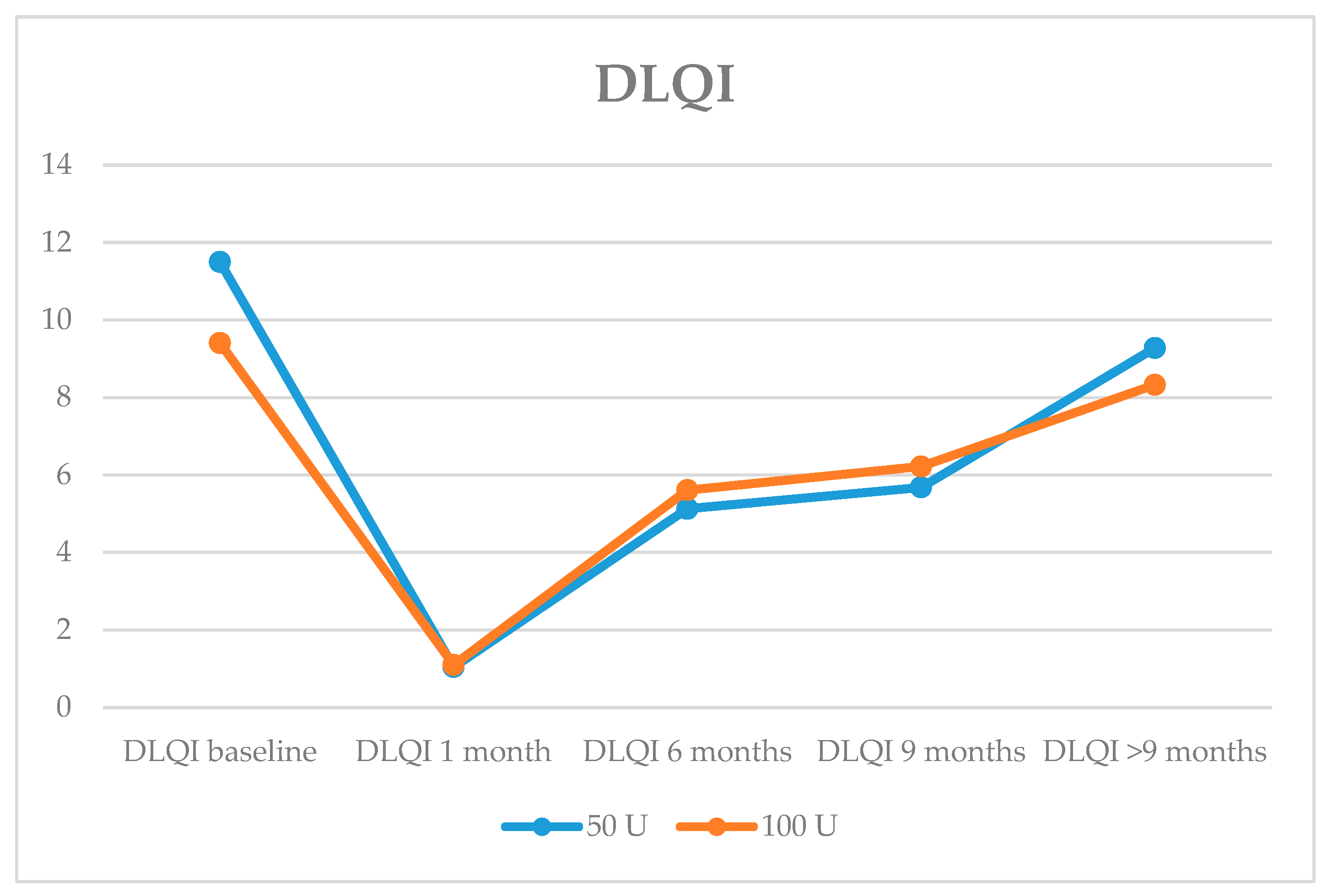
| DLQI Mean | 11.50 ± 5.91 | 9.36 ± 3.11 | 0.280 |
| DOSE | Total N% | p | |||
|---|---|---|---|---|---|
| 50 U N = 40 | 100 U N = 42 | ||||
| N% | N% | ||||
| Itching, Discomfort, Pain | Nothing | 21(52.5%) | 35 (83.3%) | 56 (68.3%) | 0.007 |
| A little | 16 (40.0%) | 5 (11.9%) | 21 (25.6%) | ||
| A lot | 3 (7.5%) | 1 (2.4%) | 4 (4.9%) | ||
| Very much | 0 (0.0%) | 1 (2.4%) | 1(1.2%) | ||
| Embarrassed, Self-Conscious | Nothing | 2 (5.0%) | 2 (4.8%) | 4 (4.9%) | 0.536 |
| A little | 21 (52.5%) | 26 (61.9%) | 47 (57.3%) | ||
| A lot | 10 (25.0%) | 11 (26.2%) | 21 (25.6%) | ||
| Very much | 7 (17.5%) | 3 (7.1%) | 10 (12.2%) | ||
| Shopping/Housework Problems | Nothing | 8 (20.0%) | 13 (31.0%) | 21 (25.6%) | 0.110 |
| A little | 20 (50.0%) | 25 (59.5%) | 45 (54.9%) | ||
| A lot | 10 (25.0%) | 3 (7.1%) | 13 (15.9%) | ||
| Very much | 2 (5.0%) | 1 (2.4%) | 3 (3.7%) | ||
| Clothes | Nothing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.867 |
| A little | 5 (12.5%) | 7 (16.7%) | 12 (14.6%) | ||
| A lot | 25 (62.5%) | 25 (59.5%) | 50 (61.0%) | ||
| Very much | 10 (25.0%) | 10 (23.8%) | 20 (24.4%) | ||
| Social Activities | Nothing | 1 (2.5%) | 0 (0.0%) | 1 (1.2%) | 0.243 |
| A little | 20 (50.0%) | 29 (69.0%) | 49 (59.8%) | ||
| A lot | 14 (35.0%) | 10 (23.8%) | 24 (29.3%) | ||
| Very much | 5 (12.5%) | 3 (7.1%) | 8 (9.8%) | ||
| Sport | Nothing | 12 (30.0%) | 12 (28.6%) | 24 (29.3%) | 0.006 |
| A little | 11 (27.5%) | 25 (59.5%) | 36 (43.9%) | ||
| A lot | 13 (32.5%) | 4 (9.5%) | 17 (20.7%) | ||
| Very much | 4 (10.0%) | 1 (2.4%) | 5 (6.1%) | ||
| Work Study | Nothing | 10 (25.0%) | 9 (21.4%) | 19 (23.2%) | 0.821 |
| A little | 22 (55.0%) | 27 (64.3%) | 49 (59.8%) | ||
| A lot | 6 (15.0%) | 4 (9.5%) | 10 (12.2%) | ||
| Very much | 2 (5.0%) | 2 (4.8%) | 4 (4.9%) | ||
| Partner/Friends | Nothing | 14 (35.0%) | 11 (26.2%) | 25 (30.5%) | 0.042 |
| A little | 14 (35.0%) | 25 (59.5%) | 39 (47.6%) | ||
| A lot | 9 (22.5%) | 6 (14.3%) | 15 (18.3%) | ||
| Very much | 3 (7.5%) | 0 (0.0%) | 3 (3.7%) | ||
| Sexual Difficulties | Nothing | 22 (55.0%) | 29 (69.0%) | 51 (62.2%) | 0.013 |
| A little | 11 (27.5%) | 13 (31.0%) | 24 (29.3%) | ||
| A lot | 5 (12.5%) | 0 (0.0%) | 5 (6.1%) | ||
| Very much | 2 (5.0%) | 0 (0.0%) | 2 (2.4%) | ||
| Take Away Time | Nothing | 24 (60.0%) | 22 (52.4%) | 46 (56.1%) | 0.025 |
| A little | 9 (22.5%) | 19 (45.2%) | 28 (34.1%) | ||
| A lot | 5 (12.5%) | 1 (2.4%) | 6 (7.3%) | ||
| Very much | 2 (5.0%) | 0 (0.0%) | 2 (2.4%) | ||
| N | Mean/Standard Deviation | Significance | |
|---|---|---|---|
| Differenceat 1 month | 0.553 | ||
| HDSS | 40 | 2.1 ± 0.6 | |
| 100 U | 42 | 1.9 ± 0.5 | |
| DLQI 50 U | 40 | 10.4 ± 5.9 | |
| 100 U | 42 | 8.3 ± 2.9 | |
| Difference at 6 months | 0.066 | ||
| HDSS 50 U | 39 | 1.3 ± 0.9 | |
| 100 U | 42 | 0.9 ± 0.7 | |
| DLQI 50 U | 39 | 6.3 ± 6.7 | |
| 100 U | 42 | 3.8 ± 3.8 | |
| Difference at 9 months | 0.115 | ||
| HDSS 50 U | 28 | 1 ± 0.9 | |
| 100 U | 24 | 0.7 ± 0.7 | |
| DLQI 50 U | 28 | 5.7 ± 6.2 | |
| 100 U | 24 | 2.7 ± 3.7 | |
| Difference at >9 months | 0.085 | ||
| HDSS 50 U | 18 | 0.3 ± 0.6 | |
| 100 U | 12 | 0 ± 0.4 | |
| DLQI 50 U | 18 | 1.8 ± 4.4 | |
| 100 U | 12 | 0.2 ± 3.5 |
| 50 U | 100 U | Total | Significance | |
| Very much better | 0.441 | |||
| N | 33 | 34 | 67 | |
| % | 82.5% | 81.0% | 81.7% | |
| Much better | 0.441 | |||
| N | 6 | 8 | 14 | |
| % | 15.0% | 19.0% | 17.1% | |
| A little better | 0.441 | |||
| N | 1 | 0 | 1 | |
| % | 2.5% | 0.0% | 1.2% | |
| Total | 0.441 | |||
| N | 40 | 42 | 82 | |
| % | 100.0% | 100.0% | 100.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antón Andrés, M.J.; Candau Pérez, E.D.; Bermejo de la Fuente, M.P. Treatment of Primary Axillary Hyperhidrosis with Two Doses of Botulinum Toxin A—Observational Study. Toxins 2024, 16, 320. https://doi.org/10.3390/toxins16070320
Antón Andrés MJ, Candau Pérez ED, Bermejo de la Fuente MP. Treatment of Primary Axillary Hyperhidrosis with Two Doses of Botulinum Toxin A—Observational Study. Toxins. 2024; 16(7):320. https://doi.org/10.3390/toxins16070320
Chicago/Turabian StyleAntón Andrés, María Jesús, Ernesto Domingo Candau Pérez, and María Pilar Bermejo de la Fuente. 2024. "Treatment of Primary Axillary Hyperhidrosis with Two Doses of Botulinum Toxin A—Observational Study" Toxins 16, no. 7: 320. https://doi.org/10.3390/toxins16070320





