Seed Priming with Rhizospheric Bacillus subtilis: A Smart Strategy for Reducing Fumonisin Contamination in Pre-Harvest Maize
Abstract
1. Introduction
2. Results
2.1. Effects of Bacillus in Reducing Fumonisin Contamination In Vitro
Influence of Different Bacillus Isolates on Reducing Fumonisin Accumulation in Co-Inoculated Detached Cobs under In Vitro Condition
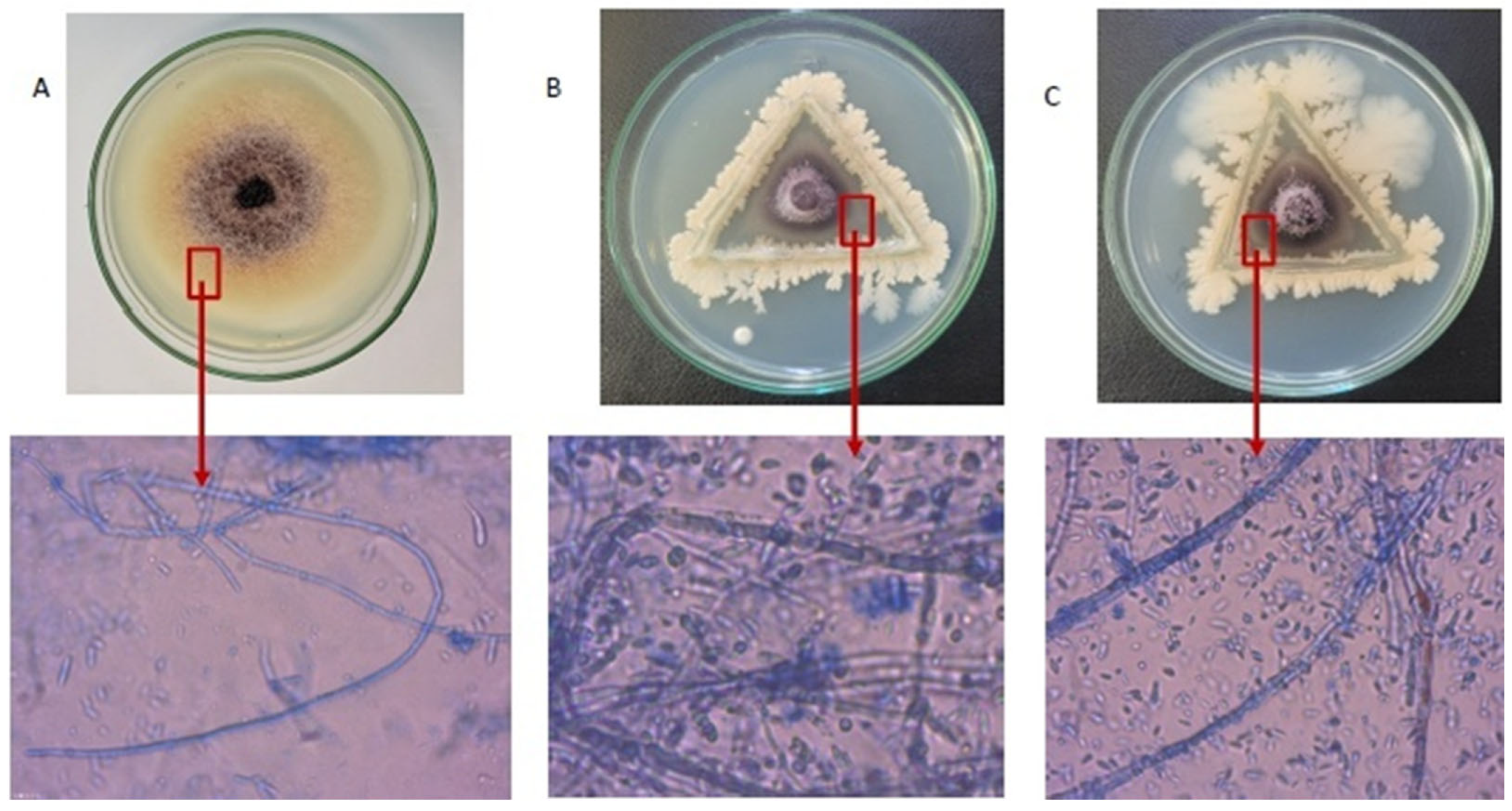
2.2. In Vitro Ultrastructural Changes in F. proliferatum Hyphae by Bacillus Isolates
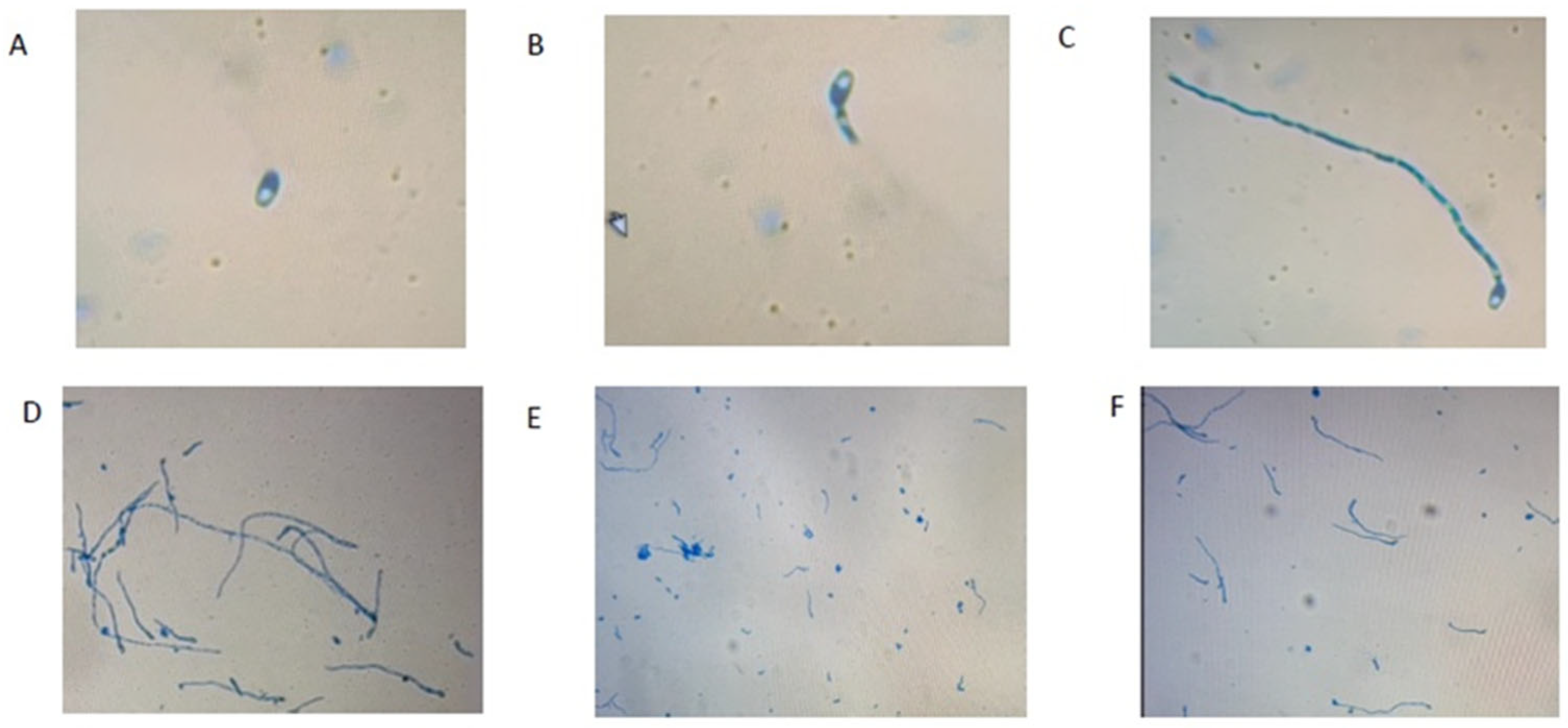
2.3. In Vitro Antagonistic Effect of Bacillus Isolates on F. proliferatum Conidia Germination and Formation
2.4. Effects of Bacillus in Reducing Fumonisin Contamination in Planta
2.4.1. Fumonisin Concentration of Maize Grains Raised from Treated Seed with Bacillus Formulation
2.4.2. Fumonisins Concentration of Maize Grains Obtained from the Co-Inoculated Cob with F. proliferatum and Bacillus Isolates
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Assessment of the Ability of Bacillus spp. in Reducing Fumonisin Contamination in Maize In Vitro
5.1.1. Bacteria and Fungi Culture Conditions
5.1.2. Conidial Suspension Preparation for In Vitro Co-Inoculation of Maize Kernels
5.1.3. In Vitro Co-Inoculation of Maize Kernels (Detached Cob) with F. proliferatum and Different Bacillus Isolates
5.1.4. Conidial Suspension Preparation for In Vitro Mycelial Growth Inhibition of F. proliferatum by Bacillus Isolates
5.1.5. In Vitro Mycelial Growth Inhibition of F. proliferatum by Bacillus Isolates
5.1.6. The Conidia Formation Assay
5.1.7. The Conidia Germination Assay
5.2. Field Efficacy of Different Bacillus Isolates in Reducing Fumonisin Contamination
5.2.1. Crop Husbandry Using Seed Treatment
Formulation of Bacillus Strains
Maize Production with Treated Seeds and Management
5.2.2. Maize Production with Co-Inoculation of Maize Cobs with F. proliferatum and Different Bacillus Isolates in the Field Condition
5.3. Quantification of Total Fumonisin in Inoculated Maize Grain (In Vitro and Field)
5.3.1. Sample Preparation for the Determination of Total Fumonisin
5.3.2. Total Fumonisin Determination Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, D.A. Cereals. In Food Crops: Evolution, Diversity and Advances; Scientific Publishers: Jodhpur, India, 2019; pp. 17–25. [Google Scholar]
- Bangladesh Bureau of Statistics. Statistical Pocket Book of Bangladesh; Bangladesh Bureau of Statistics, Planning Division, Ministry of Planning, Government of the Peoples Republic of Bangladesh: Dhaka, Bangladesh, 2020. [Google Scholar]
- Alberts, J.F.; Van Zyl, W.H.; Gelderblom, W.C. Biologically based methods for control of fumonisin-producing Fusarium species and reduction of the fumonisins. Front. Microbiol. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Dall’Asta, C.; Battilani, P. Fumonisins and their modified forms, a matter of concern in future scenario? World Mycotoxin J. 2016, 9, 727–739. [Google Scholar] [CrossRef]
- Jannat, M.; Masud, M.M.; Nusrat, M.; Bashar, S.; Mita, M.M.; Hossain, M.I.; Alam, M.Z.; Yeasmin, S.; Islam, M.R. Aflatoxins and Fumonisins Contamination of Maize in Bangladesh: An Emerging Threat for Safe Food and Food Security. In Maize Genetic Resources: Breeding Strategies and Recent Advances; El-Esawi, M.A., Ed.; IntechOpen: London, UK, 2022; pp. 18–69. [Google Scholar]
- Ahangarkani, F.; Rouhi, S.; Gholamour Azizi, I. A review on incidence and toxicity of fumonisins. Toxin Rev. 2014, 33, 95–100. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef] [PubMed]
- Yogendrarajah, P.; Jacxsens, L.; Lachat, C.; Walpita, C.N.; Kolsteren, P.; De Saeger, S.; De Meulenaer, B. Public health risk associated with the co-occurrence of mycotoxins in spices consumed in Sri Lanka. Food Chem. Toxicol. 2014, 74, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wielogorska, E.; Mooney, M.; Eskola, M.; Ezekiel, C.N.; Stranska, M.; Krska, R.; Elliott, C. Occurrence and Human-Health Impacts of Mycotoxins in Somalia. J. Agric. Food Chem. 2019, 67, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H., Jr.; Rothman, K.J.; Hendricks, K.A. Exposure to Fumonisins and the Occurrence of Neural Tube Defects along the Texas–Mexico Border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mitchell, N.J.; Gratz, J.; Houpt, E.R.; Gong, Y.; Egner, P.A.; Groopman, J.D.; Riley, R.T.; Showker, J.L.; Svensen, E.; et al. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environ. Int. 2018, 115, 29–37. [Google Scholar] [CrossRef]
- Shirima, C.P.; Kimanya, M.E.; Routledge, M.N.; Srey, C.; Kinabo, J.L.; Humpf, H.U.; Wild, C.P.; Tu, Y.K.; Gong, Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 2015, 123, 173–178. [Google Scholar] [CrossRef] [PubMed]
- UlHaq, I.; Sarwar, M.K.; Faraz, A.; Latif, M.Z. Synthetic chemicals: Major component of plant disease management. In Plant Disease Management Strategies for Sustainable Agriculture through Traditional and Modern Approaches; Ul Haq, I., Ijaz, S., Eds.; Springer: Cham, Switzerland, 2020; Volume 13, pp. 53–81. [Google Scholar]
- Mitchell, N.J.; Xue, K.S.; Lin, S.; Marroquin-Cardona, A.; Brown, K.A.; Elmore, S.E.; Tang, L.; Romoser, A.; Gelderblom, W.C.A.; Wang, J.; et al. Calcium Montmorillonite Clay Reduces AFB1 and FB1 Biomarkers in Rats Exposed to Single and Co-Exposures of Aflatoxin and Fumonisin. J. Appl. Toxicol. 2014, 34, 795–804. [Google Scholar] [CrossRef]
- Alberts, J.; Schatzmayr, G.; Moll, W.D.; Davids, I.; Rheeder, J.; Burger, H.M.; Shephard, G.; Gelderblom, W. Detoxification of the fumonisin mycotoxins in maize: An enzymatic approach. Toxins 2019, 11, 523. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Abd Allah, E.F. Bacillus subtilis: A plant-growth-promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Morales, A.; Martínez-Peniche, R.A.; Arvizu-Gómez, J.L.; Arvizu-Medrano, S.M.; Rodríguez-Ontiveros, A.; Ramos-López, M.A.; Pacheco-Aguilar, J.R. Production of a mixture of fengycins with surfactant and antifungal activities by Bacillus sp. MA04, a versatile PGPR. Indian J. Microbiol. 2018, 58, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, D.R.; Perkins, J.B. The genus Bacillus. In Practical Handbook of Microbiology; Goldman, E., Green, L.H., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 309–326. [Google Scholar]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Guimarães, R.A.; Zanotto, E.; Perrony, P.E.; Zanotto, L.A.; da Silva, L.J.; Machado, J.D.; Pinto, F.A.; Medeiros, H.N.; von Pinho, R.G.; de Melo, I.S.; et al. Integrating a chemical fungicide and Bacillus subtilis BIOUFLA2 ensures leaf protection and reduces ear rot (Fusarium verticillioides) and fumonisin content in maize. J. Phytopathol. 2021, 169, 139–148. [Google Scholar] [CrossRef]
- Dukare, A.; Paul, S. Biological Control of Fusarium Wilt and Growth Promotion in Pigeon Pea (Cajanus cajan) by Antagonistic Rhizobacteria, Displaying Multiple Modes of Pathogen Inhibition. Rhizosphere 2021, 17, 100278. [Google Scholar] [CrossRef]
- Ju, R.; Zhao, Y.; Li, J.; Jiang, H.; Liu, P.; Yang, T.; Bao, Z.; Zhou, B.; Zhou, X.; Liu, X. Identification and evaluation of a potential biocontrol agent, Bacillus subtilis, against Fusarium sp. in apple seedlings. Ann. Microbiol. 2014, 64, 377–383. [Google Scholar] [CrossRef]
- Kyei, N.N.; Waid, J.L.; Ali, N.; Cramer, B.; Humpf, H.-U.; Gabrysch, S. Maternal exposure to multiple mycotoxins and adverse pregnancy outcomes: A prospective cohort study in rural Bangladesh. Arch. Toxicol. 2023, 97, 1795–1812. [Google Scholar] [CrossRef] [PubMed]
- Kyei, N.N.; Cramer, B.; Humpf, H.-U.; Degen, G.H.; Ali, N.; Gabrysch, S. Assessment of multiple mycotoxin exposure and its association with food consumption: A human biomonitoring study in a pregnant cohort in rural Bangladesh. Arch. Toxicol. 2022, 96, 2123–2138. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Rahman, M.M.; Ali, M.Z.; Yousuf, M.A.; Akther, S.; Rahman, M.H.; Islam, M.A.; Hossen, A. An overview of mycotoxin contamination of animal feeds. Bangladesh J. Livest. Res. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Islam, M.S.; Chakraborty, S.; Antu, S.I.; Ali, M.H.; Khokon, R. Bacillus cereus (OR975563) of maize rhizosphere can promote plant growth and potentially suppress mycotoxin (Fumonisin B1) during storage. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Mita, M.M.; Jannat, M.; Bashar, S.; Protic, I.A.; Saha, P.; Masud, M.M.; Islam, R.; Islam, N.B.; Alam, M.Z.; Islam, M.R. Potential native bacilli reduce fumonisin contamination in maize. Agronomy 2022, 12, 2608. [Google Scholar] [CrossRef]
- Rahman, M.M.; Masud, M.M.; Hossain, M.I.; Islam, N.; Alam, M.Z.; Rashid, M.M.; Khan, M.A.I.; Latif, M.A.; Halder, K.P.; Islam, M.R. Potential role of rice plant growth promoting phylloplane and rhizospheric bacteria in controlling Xanthomonas oryzae pv. oryzae. In Integrative Advances in Rice Research; IntechOpen: London, UK, 2022. [Google Scholar]
- Islam, M.R.; Uddin, M.N.; Evana, V.R.; Islam, M.N.; Islam, M.H.; Haque, M.M. Plant growth–promoting rhizobacteria controlling late blight pathogen, Phytophthora infestans. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 105–124. [Google Scholar]
- Ntushelo, K.; Ledwaba, L.K.; Rauwane, M.E.; Adebo, O.A.; Njobeh, P.B. The mode of action of Bacillus species against Fusarium graminearum, tools for investigation, and future prospects. Toxins 2019, 11, 606. [Google Scholar] [CrossRef]
- Zalila-Kolsi, I.; Mahmoud, A.B.; Ali, H.; Sellami, S.; Nasfi, Z.; Tounsi, S.; Jamoussi, K. Antagonist effects of Bacillus spp. strains against Fusarium graminearum for protection of durum wheat (Triticum turgidum L. subsp. durum). Microbiol. Res. 2016, 192, 148–158. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, L.; Bie, X.; Lu, Z.; Liu, H.; Zhang, C.; Lu, F.; Zhao, H. Identification of novel surfactin derivatives from NRPS modification of Bacillus subtilis and its antifungal activity against Fusarium moniliforme. BMC Microbiol. 2016, 16, 31. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.; Sun, J.; Bie, X.; Lu, Z. Membrane disruption and DNA binding of Fusarium graminearum cell induced by C16-Fengycin A produced by Bacillus amyloliquefaciens. Food Cont. 2019, 102, 206–213. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xu, Z.; Zhang, N.; Shen, Q.; Zhang, R. Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 2014, 5, 636. [Google Scholar] [CrossRef]
- Fujita, S.; Yokota, K. Disease suppression by the cyclic lipopeptides Iturin A and Surfactin from Bacillus spp. against fusarium wilt of lettuce. J. Gen. Plant Pathol. 2019, 85, 44–48. [Google Scholar] [CrossRef]
- Lee, T.; Park, D.; Kim, K.; Lim, S.M.; Yu, N.H.; Kim, S.; Kim, H.Y.; Jung, K.S.; Jang, J.Y.; Park, J.C.; et al. Characterization of Bacillus amyloliquefaciens DA12 showing potent antifungal activity against mycotoxigenic Fusarium species. Plant Pathol. J. 2017, 33, 499–507. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.D.; Li, H.P.; Yuan, Q.S.; Song, X.S.; Yao, W.; He, W.J.; Zhang, J.B.; Liao, Y.C. Antagonistic mechanism of Iturin A and Plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS ONE 2015, 10, e0116871. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, B.; Li, K.; Zhou, H.; Lai, W.; Tang, Y.; Yan, Z.; Sun, W.; Liu, N.; Yu, D.; et al. Pre-warning of abiotic factors in maize required for potential contamination of Fusarium mycotoxins via response surface analysis. Food Control 2021, 121, 107570. [Google Scholar] [CrossRef]
- Zhao, Y.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; Song, H.; Tan, X.; Sun, L.; Sangare, L.; Folly, Y.M.E.; et al. Antagonistic action of Bacillus subtilis strain SG6 on Fusarium graminearum. PLoS ONE 2014, 9, e92486. [Google Scholar] [CrossRef] [PubMed]
- Baard, V.; Bakare, O.O.; Daniel, A.I.; Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Biocontrol potential of Bacillus subtilis and Bacillus tequilensis against four Fusarium species. Pathogens 2023, 12, 254. [Google Scholar] [CrossRef]
- Adeniji, A.A.; Aremu, O.S.; Babalola, O.O. Selecting lipopeptide-producing, Fusarium-suppressing Bacillus spp.: Metabolomic and genomic probing of Bacillus velezensis NWUMFkBS10.5. MicrobiologyOpen 2019, 8, e00742. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clement, C.; Barka, E.A. Use of plant growth promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Cawoy, H.; Debois, D.; Franzil, L.; De Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef]
- Khan, N.; Maymon, M.; Hirsch, A.M. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 2017, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Hanif, A.; Zhang, F.; Li, P.; Li, C.; Xu, Y.; Zubair, M.; Zhang, M.; Jia, D.; Zhao, X.; Liang, J.; et al. Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins 2019, 11, 295. [Google Scholar] [CrossRef]
- Islam, M.H.; Masud, M.M.; Jannat, M.; Hossain, M.I.; Islam, S.; Alam, M.Z.; Serneels, F.J.; Islam, M.R. Potentiality of formulated bioagents from lab to field: A sustainable alternative for minimizing the use of chemical fungicide in controlling potato late blight. Sustainability 2022, 14, 4383. [Google Scholar] [CrossRef]
- Bangladesh Agricultural Research Council. Fertilizer Recommendation Guide-2018; Bangladesh Agricultural Research Council: Dhaka, Bangladesh, 2018. [Google Scholar]
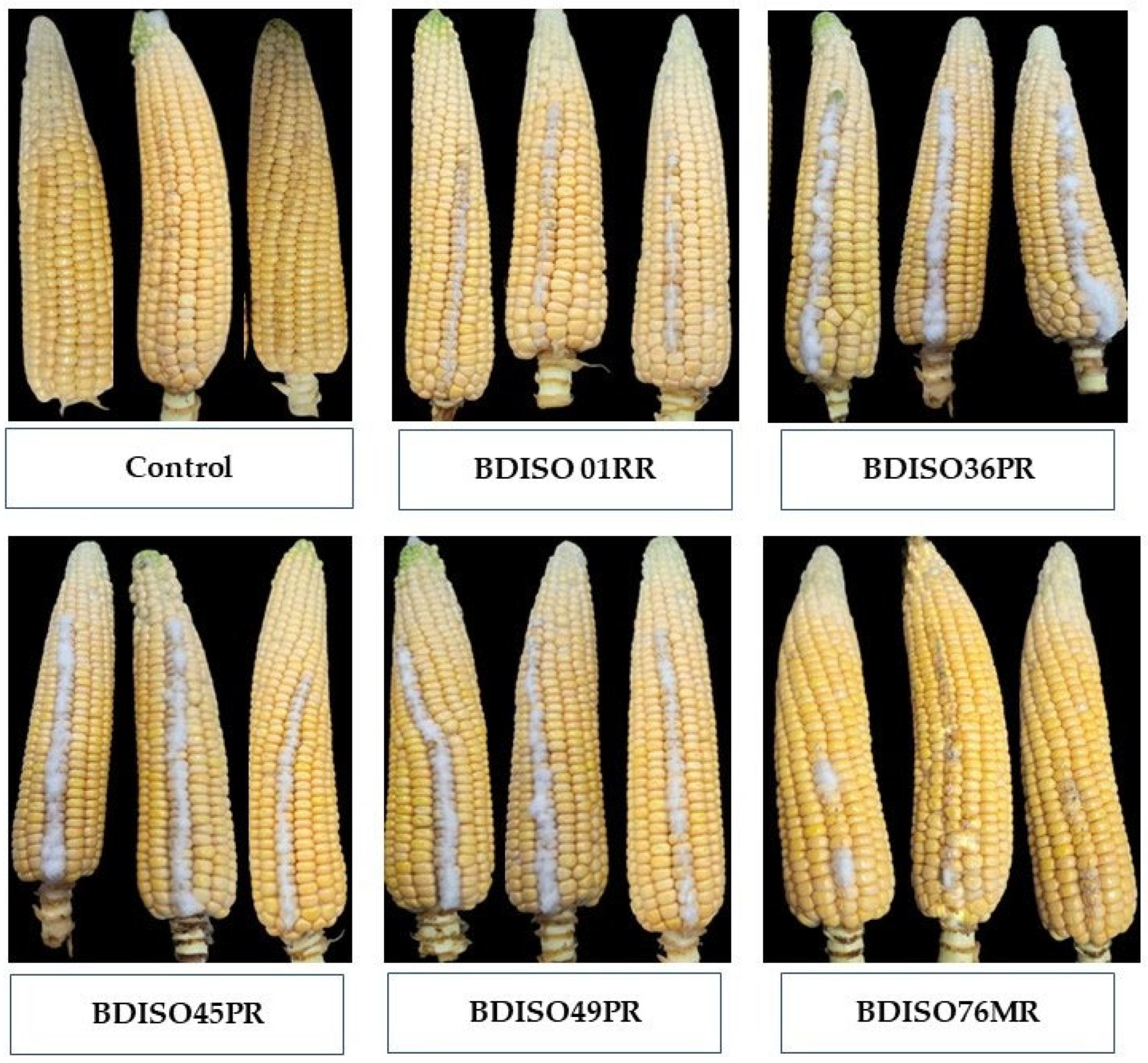
| Treatments | In Vitro | |
|---|---|---|
| Fumonisin Concentration (ppm) | % Reduction over Control * | |
| Untreated Control | 245.57 ± 2.90 a | 0.00 |
| BDISO36PR | 40.10 ± 4.63 b | 83.67 |
| BDISO01RR | 5.06 ± 0.69 b | 97.94 |
| BDISO49PR | 9.55 ± 0.43 b | 96.11 |
| BDISO45PR | 33.10 ± 7.38 b | 86.52 |
| BDISO76MR | 3.63 ± 1.18 b | 98.52 |
| Level of significance | *** | |
| LSD | 39.71 | |
| %CV | 0.23 | |
| Treatments | Conidiation of F. proliferatum (×103/mL) | % Reduction in Conidia Formation over Control * | % Germination of Conidia of F. proliferatum over Control | % Reduction in Conidia Germination over Control ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 12 HPI | 24 HPI | 72 HPI | 12 HPI | 24 HPI | 72 HPI | 12 HPI | 24 HPI | 12 HPI | 24 HPI | |
| Untreated Control | 23.00 ± 1.00 a | 47.00 ± 1.73 a | 140.67 ± 4.93 a | 0 | 0 | 0 | 69.00 ± 3.61 a | 87.84 ± 7.17 a | 0 | 0 |
| BDISO36PR | 16.00 ± 2.65 b | 32.00 ± 5.29 b | 97.00 ± 15.13 b | 30.43 | 31.91 | 31.04 | 59.70 ± 4.73 a | 80.52 ± 7.89 ab | 17.34 | 22.18 |
| BDISO01RR | 7.00 ± 1.73 de | 14.00 ± 2.65 de | 40.67 ± 10.02 d | 69.57 | 70.21 | 71.09 | 49.74 ± 7.02 a | 45.42 ± 4.64de | 34.31 | 48.33 |
| BDISO49PR | 12.00 ± 0.50 c | 24.00 ± 1.00 c | 72.00 ± 3.00 b c | 47.83 | 48.94 | 48.82 | 50.10 ± 7.15 a | 59.49 ± 9.56 cd | 43.02 | 32.09 |
| BDISO45PR | 9.00 ± 1.00 d | 18.00 ± 2.65 d | 55.33 ± 7.57 cd | 60.87 | 61.70 | 60.66 | 54.10 ± 3.65 a | 68.46 ± 1.75 bc | 3.40 | 8.36 |
| BDISO76MR | 5.00 ± 1.00 e | 11.00 ± 1.73 e | 32.67 ± 4.93 d | 78.26 | 76.60 | 77.01 | 45.83 ± 1.89 a | 33.84 ± 3.42 e | 57.03 | 61.56 |
| Level of significance | *** | *** | *** | - | - | - | NS | *** | - | - |
| LSD | 2.65 | 5.08 | 27.121 | - | - | - | 45.27 | 15.55 | - | - |
| %CV | 0.12 | 0.12 | 21.12 | - | - | - | 0.48 | 0.14 | - | - |
| Treatments | Seed Treatment | Co-Inoculation | ||
|---|---|---|---|---|
| Fumonisin Concentration (ppm) | %Reduction over Control * | Fumonisin Concentration (ppm) | %Reduction over Control * | |
| Untreated Control | 164.90 ± 13.06 a | 0.00 | 164.9 ± 13.06 a | 0.00 |
| BDISO01RR | 4.68 ± 0.75 b | 97.16 | 9.87 ± 1.60 b | 94.01 |
| BDISO36PR | 7.77 ± 1.25 b | 95.28 | 11.03 ± 2.70 b | 93.31 |
| BDISO49PR | 33.27 ± 10.48 b | 79.82 | 10.26 ± 2.40 b | 93.78 |
| BDISO45PR | 14.26 ± 1.10 b | 91.35 | 22.94 ± 6.35 b | 86.09 |
| BDISO76MR | 4.50 ± 1.32 b | 97.27 | 7.50 ± 2.50 b | 95.45 |
| Level of significance | *** | *** | ||
| LSD | 20.00 | 17.83 | ||
| %CV | 0.24 | 0.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jannat, M.; Auyon, S.T.; Tushar, A.S.M.; Tonny, S.H.; Hasan, M.H.; Shahi, M.; Singha, U.R.; Sultana, A.; Akter, S.; Islam, M.R. Seed Priming with Rhizospheric Bacillus subtilis: A Smart Strategy for Reducing Fumonisin Contamination in Pre-Harvest Maize. Toxins 2024, 16, 337. https://doi.org/10.3390/toxins16080337
Jannat M, Auyon ST, Tushar ASM, Tonny SH, Hasan MH, Shahi M, Singha UR, Sultana A, Akter S, Islam MR. Seed Priming with Rhizospheric Bacillus subtilis: A Smart Strategy for Reducing Fumonisin Contamination in Pre-Harvest Maize. Toxins. 2024; 16(8):337. https://doi.org/10.3390/toxins16080337
Chicago/Turabian StyleJannat, Muhtarima, Shah Tasdika Auyon, Abu Sina Md. Tushar, Sadia Haque Tonny, Md. Hasibul Hasan, Mangal Shahi, Uday Rana Singha, Ayesha Sultana, Sabera Akter, and Md. Rashidul Islam. 2024. "Seed Priming with Rhizospheric Bacillus subtilis: A Smart Strategy for Reducing Fumonisin Contamination in Pre-Harvest Maize" Toxins 16, no. 8: 337. https://doi.org/10.3390/toxins16080337
APA StyleJannat, M., Auyon, S. T., Tushar, A. S. M., Tonny, S. H., Hasan, M. H., Shahi, M., Singha, U. R., Sultana, A., Akter, S., & Islam, M. R. (2024). Seed Priming with Rhizospheric Bacillus subtilis: A Smart Strategy for Reducing Fumonisin Contamination in Pre-Harvest Maize. Toxins, 16(8), 337. https://doi.org/10.3390/toxins16080337






